Abstract
Salmonella spp. causes diseases in fowls, when species-specific serovars (Salmonella Pullorum and S.Gallinarum) are present in flocks, and public health problems, when non-typhoid serovars are isolated, as well as possible bacterial resistance induced by the preventive and therapeutic use of antimicrobials in animal production. This study describes the serovars and bacterial resistance of 280Salmonella spp. strains isolated from turkey and broiler carcasses in Southern Brazil between 2004 and 2006. SalmonellaEnteritidis was the most prevalent serovar (55.7%), followed by Heidelberg (5.0%), Agona (4.3%), Bredeney (3.9%), Hadar (3.2%), and Typhimurium (2.9%). Tennessee and S. Enterica subspecies enterica(O: 4.5) were isolated only in turkeys, and Hadar (18.6%) was the most prevalent serovar in this species. Antimicrobial susceptibility tests were performed in 178 isolates (43 from turkeys and 135 from broilers). All isolates were sensitive to amoxicillin + clavulanic acid, polymyxin B, ciprofloxacin, and norfloxacin, and were resistant to bacitracin and penicillin. Broiler carcass isolates showed resistance to nalidixic acid (48.9%), nitrofurantoin (34.3%), neomycin (9.6%), tetracycline (5.2%), and kanamycin (8.9%); and turkey carcass isolates were resistant to nalidixic acid (62.8%), tetracycline (34.9%), and neomycin (30.2%), with a significant difference in turkeys when compared to broiler carcass isolates. These results indicate the need for judicious use of antimicrobials in livestock production, given that the serovars identified are potential causes of food poisoning.
Keywords: Salmonella, Turkeys, Broiler, Slaughterhouse, Antimicrobial
INTRODUCTION
Poultry production is one of the major sectors of the agribusiness complex in Brazil, which ranks as the largest exporter and the third largest producer of chicken meat, and third largest producer and second largest exporter of turkey meat1. The commercialization of poultry products must meet sanitary requirements so as to guarantee that food of animal origin are free of pathogens such as Salmonella spp. Salmonella spp. causes diseases in fowls when species-specific serovars (Salmonella Pullorum and Salmonella Gallinarum) infect flocks, and public health problems occur when non-typhoid serovars are isolated, showing a correlation between poultry products and food poisoning, caused mainly by the Enteritidis, Typhimurium, Agona, Hadar, and Heidelberg serovars2 , 3 , 4 , 5.
The prevalence of Salmonella spp. in poultry carcasses has decreased since the implementation of programs such as Hazard Analysis and Critical Control Point (HACCP)6, but its occurrence is still common in poultry production7, which led the Brazilian Ministry of Agriculture, Livestock and Food Supply to introduce the Pathogen Reduction Program (PRP) for poultry carcasses (broilers and turkeys)8. PRP is targeted at monitoring carcasses in all slaughterhouses under the Federal Inspection Service in Brazil, gathering epidemiological data on the agent and aiding future decisions regarding the control of salmonellosis in poultry.
The use of antimicrobials for therapeutic purposes or as growth promoters had led to the isolation of Salmonella strains that are resistant to these drugs9 , 10 , 11, with implications for public health, as the intake of food with antibiotic residues increases bacterial resistance12 and hinders or precludes the treatment of intestinal diseases in humans and animals.
This study reports on the Salmonella serovars identified in broiler and turkey carcasses under the PRP in Southern Brazil between 2004 and 2006, and the antimicrobial resistance profile of these isolates.
MATERIALS AND METHODS
The study was conducted at SENAI's teaching laboratory - Chapecó, in the State of Santa Catarina, Brazil, with 541 Salmonella samples isolated from broiler and turkey carcasses belonging to 18 broiler farms included in the PRP, in the Southern region of the country between 2004 and 2006. Fifteen random samples were selected from each one of the 18 selected slaughterhouses, totaling 270 samples for serological identification, at the Osvaldo Cruz Foundation (FIOCRUZ), by means of the Kauffmann-White classification of serovars, on the basis of serologic identification of O (somatic) and H (flagellar) antigens. Of those, 178 (43 from turkeys and 135 from broilers) were submitted to antimicrobial susceptibility tests for nalidixic acid (30 µg), ampicillin (10 µg), amoxicillin + clavulanic acid (20 and 10 µg), cephalothin (30 µg), ceftiofur (30 µg), ciprofloxacin (10 µg), chloramphenicol (30 µg), colistin (50 µg), clotrimoxazole - sulfamethoxazole + trimethoprim (25 µg), enrofloxacin (10 µg), spectinomycin (100 µg), streptomycin (10 µg), gentamicin (10 µg), neomycin (30 µg), nitrofurantoin (25 µg), norfloxacin (10 µg), penicillin G (10 U.I.), polymyxin B (300 U.I.), sulfonamide (300 µg), tetracycline (30 nalµg), aztreonam (30 µg), bacitracin (10 U.I.), kanamycin (30 µg), and fosfomycin (25 µg). After 24 hours of incubation at a 36 °C ± 1 °C, the clear zones (circles) formed in response to a given antibiotic when the isolate is inhibited in vitro by a certain concentration of this drug, were assessed and compared with the standard performance table for susceptibility tests13. The results were analyzed by the chi square test of independence and/or by Fisher's exact test, and by SPSS for Windows version 13.0.
RESULTS AND DISCUSSION
Twenty-five Salmonella serovars were identified among 280 isolates from broiler and turkey carcasses (Table 1).S. enteritidis was the most prevalent serovar in broiler carcass isolates and Hadar was the most prevalent one in turkeys (Fig. 1). Note that the identified serovars are non-typhoid and associated with food poisoning in humans14.
Table 1. Salmonella serovars isolated from turkey and poultry carcasses in Southern Brazilian between 2004 and 2006.
| Turkeys | Poultry |
|---|---|
| Salmonella enterica subsp. enterica (O:4,5) | |
| Salmonella Agona | Salmonella Agona |
| S. Anatum | S. Anatum |
| S. Braenderup | |
| S. Brandenburg | |
| S. Bredeney | |
| S. Cerro | |
| S. Corvallis | |
| S. Cubana | |
| S. Derby | S. Derby |
| S. Enteritidis | S. Enteritidis |
| S. Hadar | S. Hadar |
| S. Heidelberg | S. Heidelberg |
| S. Infantis | |
| S. Mbandaka | |
| S. Minnesota | |
| S. Montevideo | |
| S. Ohio | |
| S. Panama | S. Panama |
| S. Rissen | S. Rissen |
| S. Saintpaul | S. Saintpaul |
| S. Schwarzengrund | S. Schwarzengrund |
| S. Senftenberg | S. Senftenberg |
| S. Tennessee | |
| S. Typhimurium | S. Typhimurium |
Fig. 1. - Salmonella serovars isolated from turkey and broiler carcasses between 2004 and 2006.
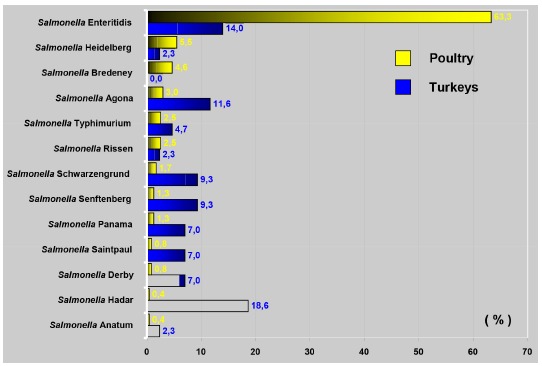
The Enteritidis serovar was isolated in 14% of samples collected from turkeys, a result that is not in agreement with data from other studies2. We did not detect this serovar in turkey carcasses, but only Hadar, Saintpaul, Indiana, and Blockley. The Salmonella enterica subspecies enterica (O: 4.5) and Tennessee serovars were isolated only from turkey carcasses. Although Hadar (18.6%) was the most prevalent serovar in turkeys, it yielded the lowest rate among broiler carcass isolates (0.4%). The presence of this serovar in both species may indicate cross-contamination at the meat packing plant, as both were slaughtered at the same location, but on different slaughter lines. On the other hand, the fowls might have eaten a diet including vegetable-origin raw materials contaminated by these serovars15 , 16. Salmonella has been found to be a source of contamination in soybean bran after detecting 5.45% of samples from this raw material as positive.
Of the 25 identified serovars, only 14 were isolated from turkey carcasses. The distribution and number of serovars may be attributed to the smaller volume in turkey production compared to that of broilers, causing less cross-contamination and, consequently, less dissemination of the serovars. This difference between serovars has already been described in an E.U. report indicating that Enteritidis was prevalent in broilers (11.0%) while Typhimurium was prevalent in turkeys. Describing the presence of Salmonella in Canadian turkeys17, 52 serovars were identified among 2,690 samples (14% Anatum, 12% Hadar, and 10% Agona).Salmonella O: 4.5, Panama, Rissen, and Enteritidis were not reported in Canada, and Enteritidis is considered to be rare in turkeys in that country.
Also in contrast with our findings, 11 serovars (chiefly San Diego 60%, Ohio 10%, Montevideo 6.7%, and Indiana 5%) were identified in turkey carcasses in two slaughterhouses submitted to federal inspection in the USA18, but in this study Enteritidis and Hadar were not isolated. Another U.S. study detected 15 Salmonella serovars, without mentioning their prevalence, but indicated Seftenberg followed by Hadar, Agona, and Heidelberg as major serovars19.
In Brazil, there are few reports on Salmonella in turkey carcasses because production was restricted to a single company for several years.S. Chester was found in turkeys in a clinical case in southern Brazil20. Nowadays, there are many turkey slaughterhouses in Brazil, which explains the PRP rules for this species, that used to be a much underrated and under consumed option, but is now included in sanitary and food safety programs.
The occurrence of the Enteritidis serovar in our study amounted to 55.7% (63.3% in broilers). The serovar Enteritidis was found in 51% of broiler carcasses21 and in 60.4% of frozen chicken22.
After three decades of outbreak reports in humans attributed to the Enteritidis serovar, and given the technical and sanitary changes in Brazilian poultry production, the fact that S. Enteritidis has had high rates of isolation is worrisome, especially because a sanitary program for the control of grandparent and parent breeding stocks already exists (National Program for Poultry Health - PNSA23). The PNSA actively monitors flocks for Pullorum, Gallinarum, Enteritidis, and Typhimurium serovars, but notifications of positive flocks for Enteritidis are infrequent. However, cross-contamination in slaughterhouses may be overlooked as contaminated broilers and are the major source of dissemination of Salmonella in meat packing plants11.
The management of salmonellosis is associated with the prophylactic and therapeutic use of antimicrobials and thus antimicrobial susceptibility tests are essential for the design of effective control programs. Our results indicate that the detected serovars are sensitive to amoxicillin + clavulanic acid, polymyxin B, ciprofloxacin and norfloxacin. Among bird species, ceftiofur and chloramphenicol had a broader action on turkey isolates, enrofloxacin and fosfomycin showed better effects on broiler isolates (Fig. 2).
Fig.2. - Resistance profile of Salmonella samples isolated from turkey and broiler carcasses in Southern Brazil between 2004 and 2006.
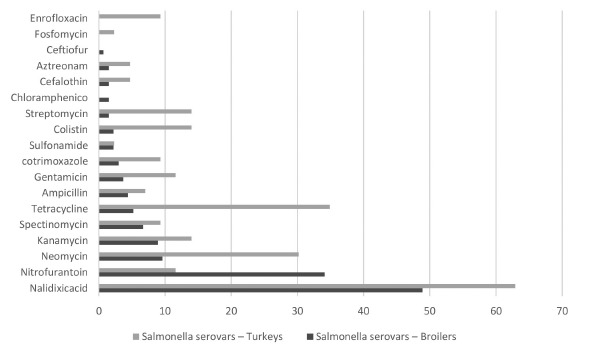
Resistance to bacitracin and penicillin amounted to 100%. The results for bacitracin are in agreement those from other studies24since in the European Union this drug used to be administered as a growth promoter, having been banned later on and is currently considered to be a non-tariff barrier for exporting countries such as Brazil16. The results for penicillin resistance are corroborated by reports of 100% resistance ofSalmonella Weltevreden in chicken and eggs25 , 26.
In the specific case of turkey carcass samples, resistance rates showed some significant differences from those serovars isolated from broilers. Turkey isolates were sensitive to ceftiofur and to chloramphenicol, and resistant to fosfomycin and to enrofloxacin, which was not observed in broiler isolates. Conflicting results were described2 after assessing samples isolated from broilers and turkeys, in which enrofloxacin yielded 50% of resistance in broiler isolates.
The comparison of resistance profiles for the years 2004 to 2006 showed a significant difference only for nalidixic acid (p = 0.029). In Southern Brazil, enrofloxacin is frequently used in poultry production, due to its low cost and efficiency against enteritis, which explains the increase in resistance ofSalmonella to nalidixic acid7, especially in turkey isolates. Other authors19 reported 70% of bacterial resistance to sulfamethoxazole and 65% to tetracycline in two turkey slaughterhouses in the USA, unlike our findings, according to which there was more frequent resistance to nalidixic acid (62.8%).
The samples isolated during the PRP provided essential data on the occurrence ofSalmonella spp. in broiler and turkey carcasses in Southern Brazil, whereas the identification of non-typhoid serovar demonstrates the potential of this pathogen as a causative agent of food poisoning, as well as an important trade barrier to exports. Antimicrobial results indicated the need for judicious use of antimicrobials in animal production, as resistance to these drugs is a potential threat to public and animal health.
REFERENCES
- 1.União Brasileira de Avicultura - UBABEF Relatório anual 2013. 2014 http://www.ubabef.com.br/files/publicacoes/732e67e684103de4a2117dda9ddd280a.pdf
- 2.Antunes P, Réu C, Souza J, Peixe L, Pestana N. Incidence of Salmonella from poultry products and their susceptibility antimicrobial agents. Int J Food Microbiol. 2003;82:97–1003. doi: 10.1016/s0168-1605(02)00251-9. [DOI] [PubMed] [Google Scholar]
- 3.Oliveira VL, Taham T. Pesquisa de Salmonella sp. em ovos comercializados na região do Distrito Federal. Uberaba: Faculdade Associadas de Uberaba; 2013. http://www.fazu.br/ojs/index.php/posfazu/article/viewFile/416/308 [Google Scholar]
- 4.Silva MCD, Ramalho LS, Figueiredo ET. Salmonella spp. em ovos e carcaças de frangos "in natura" comercializadas em Maceió, AL. Hig Aliment. 2004;18:80–84. [Google Scholar]
- 5.WHO . The medical impact of antimicrobial use in food animals. Report of a WHO Meeting. Berlin: WHO/EMC/ZOO/97.4; 1997. [Google Scholar]
- 6.United States Department of Agriculture (USDA) United States Department of Agriculture (USDA) 2011. http://www.usdabrazil.org.br
- 7.Oliveira SD, Siqueira Flores FS, dos Santos LR, Brandelli A. Antimicrobial resistance in Salmonella Enteritidis strains isolated from broiler carcasses, food human and poultry-related samples. Int J Food Microbiol. 2005;97:297–305. doi: 10.1016/j.ijfoodmicro.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 8.Brasil. Ministério da Agricultura . Instrução normativa nº 70, de 06 de outubro de 2003. p. 9, 10 de outubro de 2003. Seção 1. Programa de redução de patógenos monitoramento microbiológico e controle de Salmonella spp. em carcaças de frangos e perus. Brasília: Ministério da Agricultura, Pecuária e Abastecimento - MAPA / Secretaria de Defesa Agropecuária; 2003. [Google Scholar]
- 9.Cardoso MO, Ribeiro AR, Santos RL, Pilotto F, Moraes HLS, Salle CTP, et al. Antibiotic resistance in Salmonella Enteritidis isolated from broiler carcasses. Braz J Microbiol. 2006;37:299–302. [Google Scholar]
- 10.Carramiñana JJ, Rota C, Agustín I, Herrera A. High prevalence of multiple resistance to antibiotics in Salmonella serovars isolated from a poultry slaughterhouse in Spain. Vet Microbiol. 2004;104:133–139. doi: 10.1016/j.vetmic.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Cortez AAL, Carvalho ACFB, Ikuno AA, Bürger KP, Vidal-Martins A. M. C. 2006;73:157–163. [Google Scholar]
- 12.Mantilla SPS, Franco RM, Oliveira LAT, Santos EBS, Gouvêa R. Resistência antimicrobiana de bactérias do gênero Listeria spp. isoladas de carne moída bovina. Braz J Vet Res Anim Sci. 2008;45:116–121. [Google Scholar]
- 13.NCCLS. National Committee for Clinical Laboratory Standards . Padronização dos testes de sensibilidade antimicrobianos por disco-difusão: norma aprovada M2-A8. Wayne: NCCLS; 2003. http://www.anvisa.gov.br [Google Scholar]
- 14.World Health Organization Global foodborne infections network (GFN) country databank. 2013. http://thor.dfvf.dk/pls/portal/GSS.YEAR_RANK_REP.showparms
- 15.Palmeira ALB. Incidência de Salmonella sp. em farelo de soja utilizado na fabricação de rações para aves [CD-ROM]; 22º Congresso Brasileiro de Microbiologia ; Florianópolis: Sociedade Brasileira de Microbiologia; 2003. pp. MV054–MV054. [Google Scholar]
- 16.Neto JP. Uso de medicamentos veterinários: impactos na moderna avicultura; 7º Simpósio Brasil sul de avicultura. ; Chapecó: 2006. pp. 70–78. [Google Scholar]
- 17.Poppe C, Kolar JJ, Demczuk WH, Harris JE. Drugs resistance and biochemical characteristics of Salmonella from turkeys. Can J Vet Res. 1995;59:241–248. [PMC free article] [PubMed] [Google Scholar]
- 18.Trampel DW, Hasiak RJ, Hoffman LJ, Debey MC. Recovery of Salmonella from water, equipment, and carcasses in turkeys processing plants. J Appl Poultry Res. 2000;9:29–34. [Google Scholar]
- 19.Logue CM, Sherwood JS, Olah PA, Elijah LM, Dockter MR. The incidence of antimicrobial-resistant Salmonella spp. on freshly processed poultry from U.S. J Appl Microbiol. 2003;94:16–24. doi: 10.1046/j.1365-2672.2003.01815.x. [DOI] [PubMed] [Google Scholar]
- 20.Hofer E, Silva SJ, Filho, Reis EMF. Prevalência de sorovares de Salmonella isolados de aves no Brasil. Pesq Vet Bras. 1997;17:55–62. http://www.ubabef.com.br/files/publicacoes/732e67e684103de4a2117dda9ddd280a.pdf [Google Scholar]
- 21.Nascimento VP, Cardoso MO, Ribeiro AR, Santos LR, Silva AB, Pontes AP. Prevalência e perfis de resistência de Salmonella isoladas de carcaças de frango frente a antimicrobianos e desinfetantes selecionados.; 19º Congresso Brasileiro de Microbiologia ; Rio de Janeiro: 1997. pp. 291–291. [Google Scholar]
- 22.Santos DMS, Berchieri A Jr, Fernandes SA, Tavechio AT, Amaral LA. Salmonella em carcaças de frango congeladas. Pesq Vet Bras. 2000;20:39–42. [Google Scholar]
- 23.Brasil. Ministério da Agricultura, Pecuária e Abastecimento . Programa Nacional de Sanidade Avícola. Secretária de Defesa Agropecuária; 1995. [Google Scholar]
- 24.Shaheen N, Fatima N, Sajid SU, Gandapur AS. Antibiogram studies of Salmonella enteritidis phage type 4 isolates from poultry and meat. J Ayub Med Coll Abbottabad. 2004;16:55–59. [PubMed] [Google Scholar]
- 25.Bau AC, Carvalhal JB, Aleixo JAG. Prevalence of Salmonella in chicken products and hen's eggs from Pelotas, RS, Brazil. Ciênc Rural. 2001;31:303–307. [Google Scholar]
- 26.Radu S, Mutalib SA, Rusul G, Hassan Z, Yeang LK. Molecular characterization of Salmonella weltevreden isolated from poultry: evidence of conjugal transfer of plasmid and antibiotic resistance. Microbios. 2001;104:39–47. [PubMed] [Google Scholar]


