Abstract
Arcobacter spp. are emerging enteropathogens and potential zoonotic agents that can be transmitted by food and water, being considered a public health risk. The high isolation rate of these bacteria from poultry products suggests that it may be a major source of human infections. One hallmark for differentiating the genus Arcobacter fromCampylobacter includes their growing capacity at low temperatures (15-30 °C) under aerobic conditions. However, little is known about the population density variation of these bacteria at different refrigeration temperatures. The aim of this study was to determine the survival behavior of two different Arcobacter butzleri concentrations (104 CFU/mL and 107 CFU/mL) inoculated on chicken legs and held at two different refrigeration temperatures (4 and 10 °C) throughout storage time. Results have shown that A. butzleri had growing capacity both at 4 and 10 °C. No statistical difference between the survival trends was found for both bacterial concentrations and temperatures tested. This study shows that A. butzleri is a robust species with regard to storage temperature, and represents a potential health risk for poultry meat consumers.
Keywords: Arcobacter, Survival, Poultry, Refrigeration temperature
Arcobacter spp. are emerging enteropathogens and potential zoonotic agents that can be transmitted by food and water1 , 2. Since its first isolation in 1977, from aborted bovine and porcine fetuses, it has been implicated in mastitis, infertility, miscarriages and gastrointestinal disorders in animals, and cases of gastroenteritis, bacteremia, endocarditis, and peritonitis in humans3 - 5.
Nowadays, this genus consists of 21 species6 of which Arcobacter butzleri is the most prevalent one isolated worldwide from environmental samples, water, different animal species, and retailed meats including beef, pork, lamb, and poultry. Also, it has been found in seafood, unpasteurized milk, and even in cheese samples7 - 9. There have been three reports about this genus in Costa Rica, all in poultry products10 - 12.
It is remarkable that in absence of a standard isolation and identification method, the true incidence of this potential pathogen is largely unknown12. Yet, the high isolation rate in poultry meat and sub-products suggests that it should be considered a major source of human infections13. Moreover, it has been ranked as the fourth most common Campylobacter organism isolated from human fecal samples in two independent studies14. Nevertheless, its pathogenic properties, virulence factors, and its clinical significance remain to be defined, even though some attempts have been made to know their adhesive properties14 - 15 as well as the virulence genes associated14.
One hallmark for differentiating the genus Arcobacter andCampylobacter has been its growth capacity at low temperatures (15-30 °C), under aerobic conditions16 . However, little is known about it in terms of survival, particularly at low temperatures and population density variability. This study aimed to determine the effects on two different refrigeration temperatures over two different Arcobacter butzleri concentrations inoculated into poultry meat during its storage time in order to evaluate the potential risk for public health of this product.
On ten different occasions, fifteen raw chicken legs were collected from retail markets in the metropolitan area of San José, Costa Rica, from January to June 2013, for a total of 150 samples. Samples were transported at temperatures of 4-6 °C to the Food Microbiology Laboratory, University of Costa Rica, and analyzed within 24 h.
The control strain A. butzleri (UACH 001), gently provided by theUniversidad Austral de Chile, and previously purified, was used for inoculating chicken leg samples. A high (107-108 CFU/mL) and a low (103-104CFU/mL) bacterial concentration inoculum was prepared using sterile peptone water at 0.1%.
Upon arrival, the chicken legs for inoculation were washed with soap and water, and submerged in a chlorine solution (3 mg/L) for 30 min in order to diminish original bacterial load. Chicken legs used as negative controls were processed immediately without being subjected to any disinfection procedure.
In each occasion, chicken legs were individually packed in sterile plastic bags. Seven chicken legs were inoculated with the high concentration suspension; three of them were incubated at 4 ± 1 °C and the other three at 10 ± 1 °C; the last one was used in order to determine the Arcobacter load inoculated. Inoculation procedure was performed by adding 1 mL of the Arcobacter suspension prepared to each leg. Extensive massage was done for at least one min. to each leg in order to have a homogenous inoculum. The same process was performed for the other seven legs inoculated with the low concentration suspension. A non-inoculated leg was used as a negative control. Refrigeration temperature was recorded every 10 min with data loggers.
A chicken piece from each incubation group was tested on days 3, 6, and 9 to estimate the number of A. butzleri colony forming units (CFU) present.
For determination of the number of colony-forming units present in each sample, decimal dilutions were prepared using sterile peptone water at 0.1% and streaked on blood agar plates, which were incubated aerobically at 35 °C for 48 h. The same procedure was performed for the negative control leg on day 0. From each agar plate, at least five typical Arcobacter colonies were counted and confirmed by Gram staining, morphology and oxidase reaction.
Student t test was used in order to compare both groups (high and low bacterial concentration and incubation at 4 or 10 °C). Data loggers' registers were strictly checked for assuring that temperatures during assays were always between 4 ± 1 °C and 10 ± 1 °C.
Data obtained on day 0 was used as the base line to define the strains' growth trends. The media obtained after ten replicates for the low bacterial concentration suspension was 8 x 104 CFU/mL, and for the high bacterial concentration suspension 1 x 107 CFU/mL. No typical colonies were determined in the chicken legs used as negative controls.
Bacterial counts analyzed in time showed an initial increase in number followed by a decrease (Fig. 1). A reduction of one or two logarithms was noted at both temperatures tested, being more pronounced at 10 ± 1 °C than at 4 ± 1 °C, and independently of the bacterial strain dose used. It is important to highlight that, independently of the refrigeration temperature used, bacterial counts tend to stabilize after day 6 of storage for high bacterial concentration samples , and day 9 for low bacterial concentration ones ; complete reduction has never been achieved during the study period.
Fig. 1. - Average of 10 different growth counts of Arcobacter butzleri (UACH 001) inoculated in chicken legs at different temperatures. Initial bacterial inocula of 1x107 CFU/mL were used for high load and of 8x104CFU/mL for low load. No significant difference was observed for both bacterial inocula (critic t value = 3.18 with three degrees of freedom and a two-tail analysis).
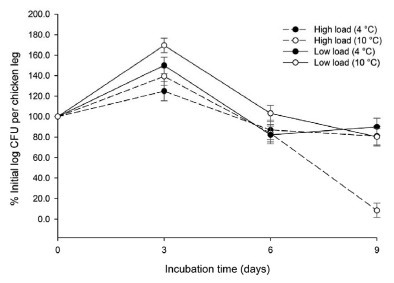
No significant differences in the survival trends for both bacterial concentrations and temperatures tested were detected.
Currently, emerging enteropathogens as Arcobacter are receiving more attention because of their possible consequences for public health. Water and food products of animal origin have been considered as the main transmission routes of these bacteria1 , 17 so that efforts should be made to reduce its presence in the food chain.
The main objective of this work was to determine the behavior of A. butzleri at two different refrigeration temperatures frequently used for temporary storage of poultry products.
Results have shown that in all samples evaluated, there was an initial increase in the bacterial count during the first three days of storage, demonstrating Arcobacter's growth capacity at refrigeration temperatures. Similar results have been described by different authors, including HILTON et al. who described the recovery of these bacteria after 21 days of storage at 4 °C18. KJELDGAARD et al.have also reported the ability of A. butzleri to multiply at 10 °C, and an extended viability, but not growth, at 5 °C in chicken meat juice medium13. In contrast, VAN DRIESSCHE & HOUF, in 2008, have reported that some A. butzleri strains grew for short periods at 4 and 7 °C in pure drinking water before the bacteria number decreased10. Moreover, their results suggest that strain origin does not define its survival capacity, and the presence of organic material influences positively the Arcobacter growth at low incubation temperatures (4-20 °C)17. The multiplication of A. butzleri observed in this work, even at 4 °C on chicken legs, corroborates the observations made by BROWN et al., who have described the contribution of chicken juice to enhance Campylobacter jejunibiofilm formation, and as a source of nutrients, a behavior that had not been described for simple media such as Brucella broth19.
A decrease in the number of CFU was observed after day 3 of storage, at both temperatures tested. Aging, nutrients' consumption, and even the accumulation of metabolites can explain this behavior20.
Nevertheless, bacteria have been detected throughout the period and did not disappear completely. Similar results have been reported by VAN DRIESCHE & HOUF10 and these authors have reported that A. butzleri can be still isolated up to 200 days of incubation in water enriched with organic material at 4 °C and 7 °C.
The survival capacity of A. butzleri at low temperatures supports the fact that it can form biofilms. Several researches affirm that biofilms could be formed at temperatures ranging from 5 to 37 °C, and that these adhesive matrices will protect the bacteria during food processing13 , 21.
Taken together, the results of the present study have confirmed that A. butzleri is capable of growing at 4 and 10 °C, the storage refrigeration temperatures used for meat products as poultry. Also, that there is no statistically significant difference between the survival trends of the two bacterial inocula tested at both temperatures. Therefore, monitoring of raw meat products for the presence of this emerging pathogen will be of interest to reinforce good agricultural and manufacturing practices through food chain.
ACKNOWLEDMENTS
This work was supported by Project 803-B4-053, Universidad de Costa Rica.
REFERENCES
- 1.Collado L, Figueras MJ. Taxonomy, epidemiology and clinical relevance of the genus Arcobacter. Clin Microbiol Rev. 2011;24:174–192. doi: 10.1128/CMR.00034-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levican A, Figueras MJ. Performance of five molecular methods for monitoring Arcobacter spp. BMC Microbiol. 2013;13:220–220. doi: 10.1186/1471-2180-13-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rice EW, Rodgers MR, Wesley IV, Johnson CH, Tanner SA. Isolation of Arcobacter butzleri from ground water. Lett Appl Microbiol. 1999;28:31–35. doi: 10.1046/j.1365-2672.1999.00483.x. [DOI] [PubMed] [Google Scholar]
- 4.Ho HT, Lipman LJ, Gaastra W. Arcobacter, what is known and unknown about a potential foodborne zoonotic agent. Vet Microbiol. 2006;115:1–13. doi: 10.1016/j.vetmic.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Bogantes EV, Fallas-Padilla KL, Rodríguez-Rodríguez CE, Jaramillo HF, Echandi ML. Zoonotic species of the genus Arcobacter in poultry from different regions of Costa Rica. J Food Prot. 2015;78:808–811. doi: 10.4315/0362-028X.JFP-14-494. [DOI] [PubMed] [Google Scholar]
- 6.Calvo G, Arias ML, Fernández H. Arcobacter: un patógeno de origen alimentario. Arch Latinoam Nutr. 2013;63:164–172. [PubMed] [Google Scholar]
- 7.De Boer E, Tilburg J, Woodward D, Lior H, Johnson M. A selective medium for the isolation of Arcobacter from meats. Lett Appl Microbiol. 1996;23:64–66. doi: 10.1111/j.1472-765x.1996.tb00030.x. [DOI] [PubMed] [Google Scholar]
- 8.Aydin F, Gumussoy KS, Atabay HI, Iça T, Abay S. Prevalence and distribution of Arcobacter species in various sources in Turkey and molecular analysis of isolated strains by ERIC-PCR. J Appl Microbiol. 2007;103:27–35. doi: 10.1111/j.1365-2672.2006.03240.x. [DOI] [PubMed] [Google Scholar]
- 9.Collado L, Jara R, Vásquez N, Telsaint C. Antimicrobial resistance and virulence genes of Arcobacter isolates recovered from edible bivalve molluscs. Food Control. 2014;46:508–512. [Google Scholar]
- 10.Van Driessche E, Houf K. Survival capacity in water of Arcobacter species under different temperature conditions. J Appl Microbiol. 2008;105:443–451. doi: 10.1111/j.1365-2672.2008.03762.x. [DOI] [PubMed] [Google Scholar]
- 11.Arias ML, Cid A, Fernández H. Arcobacter butzleri: first isolation report from chicken carcasses in Costa Rica. Braz J Microbiol. 2011;42:703–706. doi: 10.1590/S1517-838220110002000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fallas-Padilla KL, Rodríguez-Rodríguez C, Fernández H, Arias ML. Arcobacter: comparison of isolation methods, diversity, and potential pathogenic factors in commercially retailed chicken breast meat from Costa Rica. J Food Prot. 2014;77:880–884. doi: 10.4315/0362-028X.JFP-13-368. [DOI] [PubMed] [Google Scholar]
- 13.Kjeldgaard J, Jørgensen K, Ingmer H. Growth and survival at chiller temperatures of Arcobacter butzleri. Int J Food Microbiol. 2009;131:256–259. doi: 10.1016/j.ijfoodmicro.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 14.Fernández H, Flores S, Inzunza F. Arcobacter butzleri strains isolated from different sources display adhesive capacity to epithelial cell in vitro. Acta Sci Vet. 2010;38:287–291. [Google Scholar]
- 15.Levican A, Aleskas A, Günter C, Forsythe SJ, Figueras MJ. Adherence to and invasion of human intestinal cells by Arcobacter species and their virulence genotypes. Appl Environ Microbiol. 2013;79:4951–4957. doi: 10.1128/AEM.01073-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Öngör H, Çentinkaya B, Açik MN, Atabay HI. Investigation of arcobacters in meat and faecal samples of clinically healthy cattle in Turkey. Lett Appl Microbiol. 2004;38:339–344. doi: 10.1111/j.1472-765x.2004.01494.x. [DOI] [PubMed] [Google Scholar]
- 17.Villaruel-Lopez A, Márquez-González M, Garay-Martínez L, Zepeda H, Castillo A, Mota de la Garza L. Isolation of Arcobacter spp: from retail meats and cytotoxic effects of isolates against vero cells. J Food Prot. 2003;66:1374–1378. doi: 10.4315/0362-028x-66.8.1374. [DOI] [PubMed] [Google Scholar]
- 18.Hilton CL, Mackey BM, Hargreaves AJ, Forsythe SJ. The recovery of Arcobacter butzleri NCTC 12481 from various temperature treatments. J Appl Microbiol. 2001;91:929–932. doi: 10.1046/j.1365-2672.2001.01457.x. [DOI] [PubMed] [Google Scholar]
- 19.Brown HL, Reder M, Salt LJ, Cross KL, Betts RP, van Vliet AH. Chicken juice enhances surface attachment and biofilm formatin of Campylobacter jejuni. Appl Environ Microbiol. 2014;80:7053–7060. doi: 10.1128/AEM.02614-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rasmussen LH, Kjeldgaard J, Chistensen JP, Ingmer H. Multilocus sequence typing and biocide tolerance of Arcobacter butzleri from Danish broiler carcasses. BMC Res Notes. 2013;6:322–322. doi: 10.1186/1756-0500-6-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah AH, Saleha AA, Zunita Z, Murugaiyah M. Arcobacter: an emerging threat to animals and animal origin food products? Trends Food Sci Technol. 2011;22:225–236. [Google Scholar]


