Abstract
The prevalence of superficial mycotic infection worldwide is 20–25% of which dermatophytes are the most common agents. Recent developments in understanding the pathophysiology of dermatophytosis have confirmed the central role of cell-mediated immunity in countering these infections. Hence, a lack of delayed hypersensitivity reaction in presence of a positive immediate hypersensitivity (IH) response to trichophytin antigen points toward the chronicity of disease. Diagnosis, though essentially clinical should be confirmed by laboratory-based investigations. Several new techniques such as polymerase chain reaction (PCR) and mass spectroscopy can help to identify the different dermatophyte strains. Management involves the use of topical antifungals in limited disease, and oral therapy is usually reserved for more extensive cases. The last few years have seen a significant rise in the incidence of chronic dermatophyte infections of skin which have proven difficult to treat. However, due to the lack of updated national or international guidelines on the management of tinea corporis, cruris, and pedis, treatment with systemic antifungals is often empirical. The present review aims to revisit this important topic and will detail the recent advances in the pathophysiology and management of tinea corporis, tinea cruris, and tinea pedia while highlighting the lack of clarity of certain management issues.
Keywords: Dermatophytosis, superficial fungal infections, tinea corporis, tinea cruris, tinea pedis
INTRODUCTION
Dermatophytes are fungi that invade and multiply within keratinized tissues (skin, hair, and nails) causing infection.[1] Based upon their genera, dermatophytes can be classified into three groups: Trichophyton (which causes infections on skin, hair, and nails), epidermophyton (which causes infections on skin and nails), and Microsporum (which causes infections on skin and hair). Based upon mode of transmission, these have been classified as anthropophillic, zoophilic, and geophilic. Finally, based upon the affected site, these have been classified clinically into tinea capitis (head), tinea faciei (face), tinea barbae (beard), tinea corporis (body), tinea manus (hand), tinea cruris (groin), tinea pedis (foot), and tinea unguium (nail). Other clinical variants include tinea imbricata, tinea pseudoimbricata, and Majocchi granuloma.
Despite the increasing prevalence of cutaneous dermatophytosis across the world, and especially in tropics, research in this area has often been neglected. In fact, one has to go back nearly two decades to find guidelines on the management of tinea corporis and cruris (by the American Academy of Dermatology),[2] and these at best, appear inadequate in today's world. The more recent guidelines published by the British Association of Dermatology and in the British Medical Journal have largely focused on tinea capitis and tinea unguium with scarce reference to tinea corporis/cruris.[3,4,5] Updated Cochrane reviews on the use of topical therapy in tinea corporis, cruris, and pedis, and few on oral therapies have helped to bridge this knowledge gap but still well-designed trials, national and/or international evidence-based guidelines and recommendations on the dose and duration of the use of systemic antifungals in tinea corporis/cruris are conspicuous by their absence.[6,7,8] The present review aims to revisit this important topic and will detail the recent advances in the pathophysiology and management of tinea corporis, tinea cruris, and tinea pedis while highlighting the lack of clarity of certain management issues.
CHANGING EPIDEMIOLOGY OF DERMATOPHYTOSIS
Dermatophytes are the most common agents of superficial fungal infections worldwide and widespread in the developing countries, especially in the tropical and subtropical countries like India, where the environmental temperature and relative humidity are high. Other factors such as increased urbanization including the use of occlusive footwear and tight fashioned clothes, has been linked to higher prevalence.[9] Over the last few years, studies on epidemiology of dermatophytic infection from different part of India have shown a rising trend in the prevalence of cutaneous dermatophytosis with change in spectrum of infection and isolation of some uncommon species.[10,11,12,13] Trichophyton rubrum continues to be the most common isolate with tinea corporis and cruris the most common clinical presentation in relatively large studies from Chennai and Rajasthan. However, in studies from Lucknow and New Delhi, Trichophyton mentagrophytes[13] and Microsporum audouinii[11] were the most frequent isolate. Few studies also showed isolation of rare species like Microsporum gypseum in nonendemic part of the world.[11]
PATHOGENESIS OF DERMATOPHYTOSIS
Genetics of dermatophytosis
All people are not equally susceptible to fungal infection, even when they have similar risk factors. There is evidence of familial or genetic predispositions that could be mediated by specific defects in innate and adaptive immunity. One of the first fungal diseases thought to have a genetic predisposition was Tokelau or tinea imbricata. According to Jaradat et al., patients with low defensin beta 4 may be predisposed to all dermatophytes.[14]
The pathogenesis of dermatophyte infection involves complex interaction between the host, agent and the environment. The factors which predispose to such an infection are underlying diseases such as diabetes mellitus, lymphomas, immunocompromised status, or Cushing's syndrome, older age, which could produce severe, widespread, or recalcitrant dermatophytosis. Some areas of the body are more susceptible to the development of dermatophyte infections such as intertriginous areas (web spaces and groins) where excess sweating, maceration, and alkaline pH favor the growth of the fungus. After inoculation into the host skin, suitable condition favor the infection to progress through adherence followed by penetration mediated by proteases, serine-subtilisins, and fungolysin, which causes digestion of keratin network into oligopeptide or aminoacid and also act as a potent immunogenic stimuli.[15] In addition, the mannans produced by T. rubrum lead to inhibition of lymphocytes. Impaired function of Th17 cells leading decreased production of interleukin-17 (IL-17), IL-22 (key cytokine in clearing mucocutaneous fungal infection) results in persistence of infection.[15]
Immunology of dermatophytosis
The immune response to infection by dermatophytes ranges from a nonspecific host mechanism to a humoral and cell-mediated immune response. The currently accepted view is that a cell-mediated immune response is responsible for the control of dermatophytosis.
Innate immune response
Dermatophytes contain cell wall carbohydrate molecules (β-glucan) that are recognized by innate immune mechanisms, such as Dectin-1 and Dectin-2, which activate toll-like receptor 2 and 4 (TLR-2 and TLR-4). Dectin-1 amplifies the production of tumor necrosis factor-α and IL-17, IL-6, and IL-10, all of which stimulate the adaptive immunity.[16,17] Keratinocytes in the presence of dermatophyte antigens, such as trichophytin, release IL-8, a potent neutrophillic chemo-attractant. A recent study shows the involvement of TLR-2 and TLR-4 in localized and disseminated dermatophytosis due to T. rubrum. A reduced expression of TLR-4 in the lower and upper epidermis of both localized and disseminated dermatophytosis patients was found compared to controls; TLR-2 expression was preserved in the upper and lower epidermis of all three groups.[18,19]
Adaptive immune response
Humoral immunity: Humoral immunity to dermatophytes is not protective. High levels of specific IgE and IgG4 are detected in patients with chronic dermatophytosis which is responsible for positive (IgE mediated) IH tests to Trichophyton. On the other hand, Ig levels are low in patients that present positive delayed type hypersensitivity (DTH) skin test. The IH skin test for Trichophyton is associated with the presence of serum IgE and IgG (mostly IgG4) against Trichophyton antigens, hallmarks of a Th2 response. Here, IL-4 produced by CD4 T-cells (Th2 cells) induces antibody isotype switching to IgG4 and IgE
Cell-mediated immunity: Several experiments have shown that the resolution of dermatophytosis is mediated by DTH. Immunity to pathogens could be regulated by Th1 or Th2 subsets which would ultimately determine the outcome of the infection. An acute inflammatory response correlates with a positive DTH skin test to trichophytin and clearing of the infection whereas chronic infection is associated with high IH and low DTH.[17]
Nonspecific response
Unsaturated transferrin has been found to be inhibitory to dermatophytes by binding to its hyphae. Commensal pityrosporum aids lipolysis and increases the pool of fatty acid available for inhibiting growth of fungi.
DIAGNOSIS OF DERMATOPHYTOSIS
Laboratory investigations
For a laboratory to provide optimal results, quantity and quality of material examined is critical. Scraping should be collected from active margin and transported in a presterilized black chart paper which keeps the specimen dry thus, preventing over growth of bacteria contaminants. Following are the various laboratory tests that can be used for confirming a diagnosis of dermatophytosis.
Direct microscopic examination:[20] Treatment of skin specimen with 10–20% potassium hydroxide (KOH) is a quick and inexpensive bedside tool to provide evidence of dermatophytic infection. Positive scrapings are characterized by presence of refractile, long, smooth, undulating, branching, and septate hyphal filaments with or without arthroconidiospores. False negative results are seen in 15% cases. Fluorescent staining with optical brighteners (diaminostilbene) is the most sensitive method to microscopically detect fungi in skin scales as well as in specimens from nails and hair.[21] These substances bind to chitin, the main cell wall component of fungi
Culture and antifungal sensitivity:[22] Sabouraud dextrose agar (SDA, 4% peptone, 1% glucose, agar, water) is the most commonly used isolation media for dermatophytosis and serves as the medium on which most morphologic descriptions are based. Development of colony takes 7–14 days. Modified SDA, with addition of gentamicin, chloramphenicol and cycloheximide is more selective for dermatophytes as chroramphenicol inhibits the growth of saprophytic fungus. Dermatophyte test medium is an alternative to isolation media that contain pH indicator phenol red. It is incubated at room temperature for 5–14 days. Dermatophytes utilize the protein resulting in excess ammonium ion and alkaline environment which turn the medium from yellow to bright red.
Antifungal susceptibility testing
Microdilution method: The broth microdilution assay for antifungal susceptibility testing of dermatophytes has been previously developed as a modification of the Clinical and Laboratory Standards Institute M38-A2 standard method. The final concentrations of terbinafine and itraconazole used is 0.06–32.0 μg/ml and for fluconazole, 0.13–64.0 μg/ml.[23] A standardized inoculum is prepared by counting the microconidia microscopically. Cultures are grown on SDA slants for 7 days at 35°C to produce conidia. Sterile normal saline (85%) is added to the agar slant, and the cultures are gently swabbed with a cotton-tipped applicator to dislodge the conidia from the hyphal mat. The suspension is transferred to a sterile centrifuge tube, and the volume is adjusted to 5 ml with sterile normal saline. The resulting suspension is counted on a hemacytometer and is diluted in RPMI 1640 medium to the desired concentration. Microdilution plates are set up in accordance with the reference method. The microdilution plates are incubated at 35°C and read visually after 4 days of incubation. The minimum inhibitory concentration is defined as the concentration at which the growth of the organism will be inhibited by 80% compared with the growth in the control well
Minimum fungicidal concentration (MFC) determination: For determination of the MFC, 100-μl aliquots are removed from the assay wells showing no visible growth at the end of incubation and streaked onto SDA plates. The plates are incubated at 30°C for 7 days. The MFC is defined as the lowest drug concentration at which no visible fungal growth or colonies developed
-
3.
Dermatophyte identification: This can be based on colony characteristics, microscopic morphology, and physiologic tests. Dermatophytes can be distinguished based upon their morphology of the macroconidia. Few physiological tests are available which help in confirmation of certain species. In addition, special amino acid and vitamin requirements can differentiate Trichohyton species from others. Ability to hydrolyse urea differentiates T. mentagrophytes (urease positive) from T. rubrum (urease negative).
Histopathology
Histology may be used in diagnosis of Majocchi's granuloma in which KOH examination of scale on the surface may more often be negative. When present, hyphae may be appreciated in stratum corneum on hematoxylin and eosin staining. Special stains most commonly used are periodic acid-Schiff and Gomori methanamine silver which helps to highlight hyphae.
Dermoscopy
The comma hairs, which are slightly curved, fractured hair shafts, and corkscrew hair shave been described as the dermoscopic marker of tinea capitis. Broken and dystrophic hairs are also seen. However, in tinea corporis, the involvement of vellus hair as seen on dermoscopy is an indicator of systemic therapy.[24]
Polymerase chain reaction and nucleic acid sequence based amplification
These tests not only help in the rapid and early diagnosis of infection but also help in determining drug resistance,[25] and include:
Uniplex PCR for direct dermatophyte detection in clinical samples: A PCR for the direct detection of dermatophytes in skin scales is available as in-house PCR-ELISA assay which separately identifies numerous dermatophyte species. In a pilot study, the sensitivity and specificity of the test compared to cultures was 80.1% and 80.6%
Multiplex PCR for fungal detection in dermatophytes: Commercially available multiplex PCR tests enable simultaneous amplification of 21 dermatomycotic pathogens with subsequent DNA detection by means of agarose gel electrophoresis.
New molecular methods like matrix-assisted laser desorption ionization-time of flight mass spectrometry
It is based on the detection of biochemical characteristics, proteolytic degradation product which is a result of the activity of mycological infections or noninfectious diseases. These are represented by proteolytic degradation products of native proteins. The peptide patterns of affected samples are identified by comparison with known peptide spectra from skin disorders stored in an already existing database. This procedure is immensely time saving, as it enables simultaneous identification of up to 64 dermatophyte strains, with results coming back within 24 h.[26]
Reflectance confocal microscopy
It provides in vivo imaging of the epidermis and superficial dermis at cellular level resolution and can be used to detect cutaneous fungi and parasitic infestations.[27] Branching fungal hyphae can be detected over an erythematous annular scaly patch. Advantage of the test being noninvasive and in a retrospective analysis of the test by Friedman et al. sensitivity was found to be 100%.
Summarizing it can be safely recommended that a clinical diagnosis of cutaneous dermatophytic infection should always be supplemented by a mycologic confirmation. While traditional methods like direct demonstration of fungus by KOH offer a reasonably sensitive and inexpensive option, newer noninvasive methods such as dermoscopy have additional advantage of ease of use, ability to detect involvement of vellus hair and thus, influence the choice of treatment (topical versus systemic). Fungal culture and antifungal testing are costlier and more specialized investigations, but such infrastructure needs to build up at most centers, especially in the present scenario of rising prevalence of nonresponsive dermatophytosis. Other methods such as PCR and reflectance confocal microscopy are still used primarily for research purposes.
TREATMENT OF CUTANEOUS DERMATOPHYTOSIS
Nonpharmacologic measures
Patients should be encouraged to wear loose-fitting garments made of cotton or synthetic materials designed to wick moisture away from the surface. Socks should have similar properties. Areas likely to become infected should be dried completely before being covered with clothes. Patients should also be advised to avoid walking barefoot and sharing garments.
Medical management with antifungals
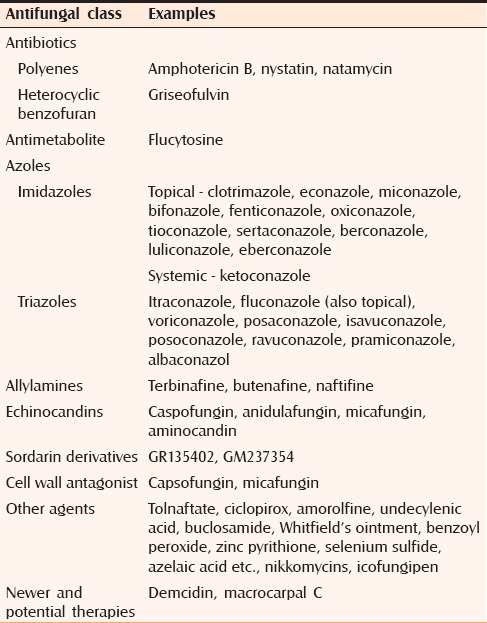
A variety of traditional agents without specific antimicrobial function are still in use, including Whitfield's ointment and Castellani's (Carbol fuchsin solution) paint. The efficacy of these preparations has not been well quantified.[28] Table 1 summarizes the classification of commonly employed antifungals.[29,30,31] Lesions covering a large body surface area fail to clear with repeated treatment using different topical agents should be considered for systemic therapy.[28] There is no definite comparative study on combination of systemic and topical versus monotherapy with systemic antifungal treatment. Topical medications have better pharmacokinetics than their systemic counterparts. Hence, combination is expected to have better mycological clearance than systemic and topical alone. Combination should be from different groups for wide coverage and also to prevent emergence of resistance. Drugs given for shorter duration with higher dose there has a less chance of development of resistance compared to lower dose for longer duration. Drug with keratophilic and lipophilic property, when given in higher doses will have reservoir effect and will lead to better mycological clearance.
Table 1.
Classification of antifungal therapy based on their structure
Indication of systemic antifungals in dermatophytosis
Tinea capitis
Tinea affecting the nails
Tinea involving more than one body region simultaneously, for example, tinea cruris and corporis, or tinea cruris and tinea pedis
Tinea corporis where the lesions are particularly extensive. However, there is no accepted definition of extensive disease
Tinea pedis when there is extensive involvement of the sole, heel, or dorsum of the foot or when there is recurring and troublesome blistering.
Topical antifungal therapy for tinea cruris, corporis, and pedis
Reviewing the evidence on the use of existing topical antifungals
Various topical antifungal agents are available for the treatment of localized tinea corporis, tineacruris, tinea faciei, and tinea pedis. It may also be used as an adjunct to oral antifungals for more extensive infection. Most of the studies in the treatment of tinea corporis and cruris have looked at the efficacy of topical antifungals with very few studies on the use of oral antifungals. A meta-analysis by Rotta et al.[32] evaluated the efficacy of antifungal treatment involving 14 different topical antifungals and included 65 randomized controlled trials (RCTs), comparing topical antifungal with one another or with placebo. Efficacy was evaluated in the form of mycological cure at the end of treatment and sustained cure. They found no statistically significant differences among the antifungals concerning the outcome of mycologic cure at the end of treatment. For sustained cure, butenafine and terbinafine each was found to be superior to clotrimazole. Pairwise comparison of topical antifungals for the outcome of fungal cure showed butenafine and terbinafine each to be superior to clotrimazole, oxiconazole, and sertaconazole; terbinafine to be superior to ciclopirox, and naftifine to be superior to oxiconazole.
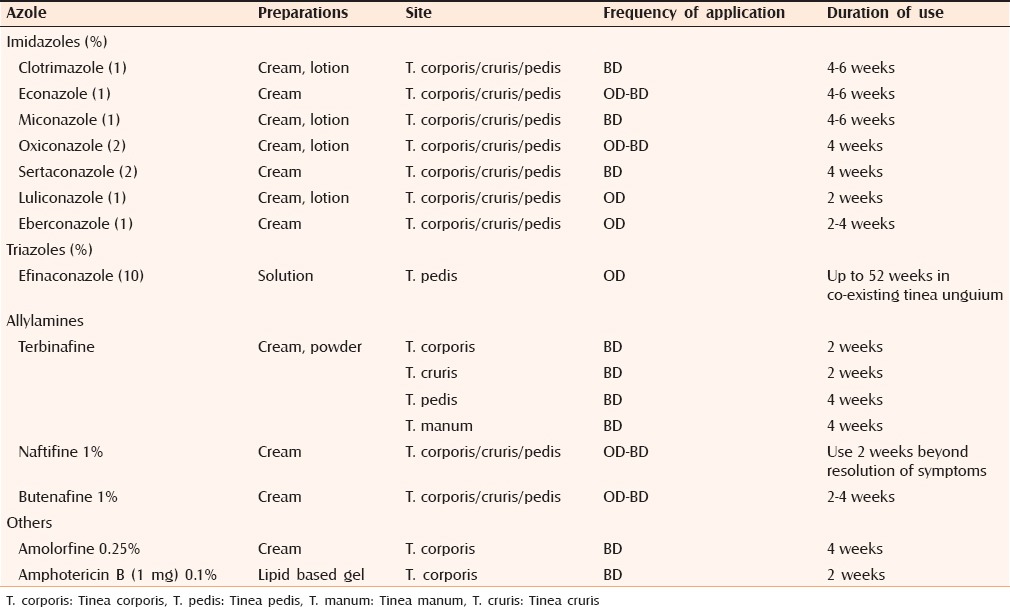
Similarly, Cochrane review[6] on the topical antifungal treatments for tinea cruris and tinea corporis suggests that the individual treatments with terbinafine and naftifine are effective with few adverse effects. Other topical antifungals like azoles treatments are also effective in terms of clinical and mycological cure rates. Regarding combinations therapy of topical steroids and antifungals though there is no standard guideline.[33,34,35] There is insufficient evidence to confidently assess relapse rates in the individual or combination treatments. Difference between the different antifungals is mostly regarding fewer application and shorter duration of treatment with some class of topical antifungals compared to others. Topical antifungal are usually given once or twice daily for 2–4 weeks as illustrated in Table 2. The end point of treatment is clinical resolution in most of the cases.
Table 2.
Summary of the use of topical antifungals used in the treatment of tinea corporis, cruris and pedis
Moriarty et al., also emphasize upon the use of topical therapy in treating tinea corporis, cruris and pedis. They also enlist the common reasons of failure of therapy, namely; poor adherence to treatment, reinfection from close contact, drug resistance, misdiagnosis, and infection with uncommon species. Such patients should be referred to a higher center for appropriate management. They also suggest use of topical hydrocortisone for a short time in inflamed lesions. Studies have also shown that addition of topical steroid also increases the bioavailability of topical antifungals mostly imidazole groups in addition to better symptomatic relief in early inflammatory stage.[33] While it may be of benefit to patients with inflammatory lesions, such practice should be strongly discouraged in countries like India where easy over the counter availability of topical steroids render then to frequent misuse by patients who finally end up with tinea incognito. Steroids may helpful in initial improvement in symptoms but chronic use lead to a complication like atrophy, telangiectasia which is more prominent when lesions are present in flexures. Topical antifungals with potent anti-inflammatory action such as sertaconazole or luliconazole may be a better option than an antifungal-steroid combination.
Tinea pedis is usually treated with a topical antifungal cream for 4 weeks; interdigital tinea pedis may only require 1 week of therapy. Various topical antifungal effective against tinea pedis include azoles, allylamines, butenafine, ciclopirox, tolnaftate, and amorolfine as evidenced by a meta-analysis finding strong evidence of superiority of topical antifungal agents over placebo.[7] A meta-analysis of 11 randomized trials concluded that treatment with terbinafine or naftifine produces a slightly higher cure rate than treatment with an azole.[36] Nystatin is not effective for the treatment of dermatophyte infections. Naftifine hydrochloride gel was also found to be effective both for interdigital and moccasin type of tinea pedis.[37]
Newer topical antifungals
Luliconazole, an azole antifungal has fungicidal action against Trichophyton species similar to or more than that of terbinafine. Available in 1% cream formulation, it is effective as once daily application for 1–2 weeks for dematophytic infection. Approved by the US Food and Drug Administration for the treatment of interdigital tinea pedis, tinea cruris, and tinea corporis, it has a favorable safety profile.[38] Econazole nitrate foam preparation has also shown its efficacy over foam vehicle for tinea pedis.[39] However, these newer drugs are costlier which in turn may lead to issues of adherence to treatment in resource-poor settings, and may predispose to development of resistance.
Finally, use of special carrier system where parent drug attached to carriers such as micelle or use of nanostructured lipid-based carrier, microemulsions, and vesicular systems such as liposomes, niosomes, transfersomes, ethosomes, or penetration enhancer vesicles is promising as it helps in better bioavailability so as to attain better therapeutic response.[40] More recently, lipid-based amphotericin B gel has shown encouraging pharmacologic properties and clinical results in the treatment of various mucocutaneous fungal infections including dermatophytosis, with no adverse effect.[41] Amphotericin B incorporated in microemulsion shows a 100% increase in skin retention with better in vitro antifungal activity against T. rubrum.[42] One valid concern is whether use of topical amphotericin may promote its resistance in the community, thereby limiting its use for more invasive fungal infections. Microemulsion formulations of griseofulvin have shown good cure rates in dermatophytosis.[43] Adding to this is a novel formulation of terbinafine known as terbinafine film forming solution which forms a thin film forming topical application and fungicidal effect maintained for about 13 days following single application.[44] Successful treatment of tinea corporis with combination of topical isoconazole with diflucotolone (a potent topical steroid) has also been reported.[45]
Oral antifungal therapy in Tinea corporis, cruris, and pedis
Reviewing the evidence on the use of existing oral antifungals
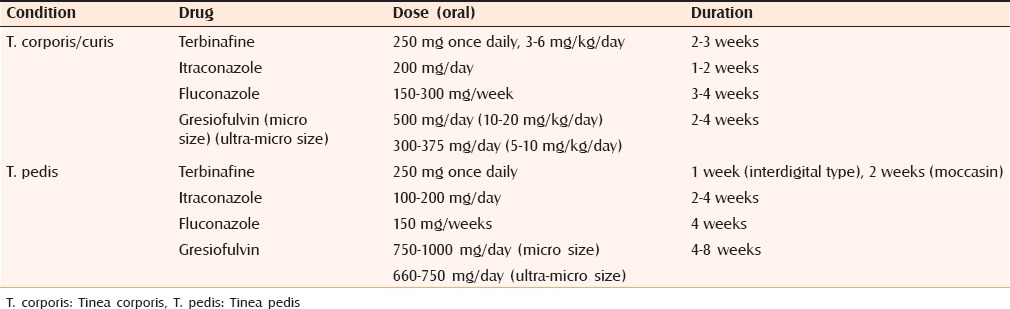
Systemic antifungals are indicated in case of extensive involvement and patients who fail topical therapy.[46] Out of the various systemic antifungals, terbinafine, and itraconazole are commonly prescribed. Griseofulvin and fluconazole are also effective but require long-term treatment. RCTs support the efficacy of systemic antifungals [Table 3].[47,48,49,50] Comparative trial between itraconazole 100 mg/day with ultramicronized griseofulvin 500 mg/day for tinea corporis or tinea cruris showed significantly better clinical and mycological outcome in favor of itraconazole after 2 weeks of therapy.[47] Similar study comparing terbinafine with griseofulvin (both 500 mg daily for 6 weeks) for tinea corporis found mycological cure rate of about 87% in former group compared to 73% in latter.[48] A double-blinded study between itraconazole (100 mg/day) and griseofulvin (500 mg/day) found itraconazole to be superior in providing mycological cure.[49]
Table 3.
Recommended dosing of different systemic antifungals in dermatophytosis
Topical therapy is less effective than oral antifungals for the treatment of tinea pedis, and oral treatment is generally given for 4–8 weeks. In a systematic review of efficacy of oral antifungals in, terbinafine was found to be more effective than griseofulvin, whereas the efficacy of terbinafine and itraconazole were similar.[8] In addition to antifungal therapy, Burrow's (1% aluminum acetate or 5% aluminum subacetate) wet dressings, applied for 20 min 2–3 times/day, may be helpful if vesiculation or maceration is present. Of various types of tinea pedis, hyperkeratotic variety is more recalcitrant to treatment due to thick scales leading to ineffectiveness of topical antifungals and need for longer duration of systemic antifungals. Use of keratolytic agents and topical antifungals along with systemic antifungals has been found to be more useful in early achievement of clinical and mycological cure as well as decreasing the duration of oral antifungals leading to better patient compliance.[51] Secondary bacterial infection should be treated with oral antibiotics. Other adjunctive therapies include use of antifungal powder may help to prevent maceration and avoidance of occlusive footwear.
Newer oral antifungal agents
There is lack of any recent literature regarding systemic antifungals in the treatment of tinea cruris and corporis. Although few newer systemic antifungals have been approved in last two decades but most of them are reserved for more severe life-threatening invasive systemic mycoses with paucity of evidence on efficacy in superficial mycoses. Recently, posoconazole was found to be effective in a patient with extensive dermatophytic skin and nail infection with underlying CARD9 mutation.[52]
New and potential therapies
Other than the antifungals already mentioned, few plant extract (Chinese herbals) are also found to be effective against common dermatophytic infection. One of them is macrocarpal C, an active ingredient obtained from the fresh leaves of Eucalyptus globulus Labill with antifungal action against T. mentagrophytes and T. rubrum.[53] Demicidin, an antimicrobial peptide has antifungal action at a concentration normally present in sweat providing an insight to newer therapeutic target for dermatophytic infection.[54]
SPECIAL SITUATIONS
Majocchi's granuloma
It is a deep dermatophytosis that occurs when a long-standing superficial fungal infection causes progressive dissemination into the subcutaneous tissue. The most common etiological agent is T. rubrum.[55] Mechanical damage to the skin resulting from trauma may allow penetration of fungi into the reticular dermis, and the resulting cellular destruction and decreased dermal pH makes the milieu more suitable for its survival.[56] It is mostly seen in immunocompromised hosts.[57] Topical steroid application leads to local immunosuppression and development of majocchi granuloma. Systemic antifungals such as terbinafine in a dose of 250 mg/day for 4–6 weeks, itraconazole 200 mg twice daily for 1 week/month for 2 months have been successfully used.[58,59] Treatment regimens with griseofulvin and daily itraconazole have also been suggested.[60]
Tinea imbricate and pseudoimbricata
Tinea imbricata is a chronic superficial fungal infection of the glabrous skin caused by Trichophyton concentricum. Disease results from close contact with spores and filaments of T. concentricum especially between the mother and her child. It is postulated that genetic, environmental, and immunologic factors play an important part in the development of this fungal infection. The mode of inheritance is autosomal recessive pattern with a minority of autosomal dominant cases.[61] Most patients have specific antibodies to T. concentricum, thus suggesting that there is a decrease in the cellular immunity.[62] Dietary influences, iron deficiency, and malnutrition have been cited as associated factors.[63] Diagnosis is essentially clinical and isolation on culture. The disease is highly recurrent. The treatment should involve a combination of topical and systemic antifungal agents since topical therapy alone is insufficient. Griseofulvin, azole agents, such as ketoconazole and itraconazole, has been used for many years with variable success. Currently, terbinafine is the best therapeutic option, in the dose of 250 mg/day in adults.[64] Recently, there have been reports of tinea imbricate like lesions in patients abusing topical steroids. T. mentagrophytes, instead of T. concentricum is usually isolated from these lesions.[65]
Antifungal therapy in immunosuppressed and pregnancy
A special subgroup of population like with HIV infection usually present with more extensive involvement. However, characteristic morphology may be missing due to reduced inflammatory component of lesion attributed to suppressed immunity.[66] In a patient with associated comorbidities such as renal, hepatic impairment, and caution should be exercised while prescribing systemic antifungals. Terbinafine clearance significantly reduced in patient in renal impairment. So dose should be adjusted accordingly, or drug from different group should be preferred. Similarly, itraconazole should be avoided in patient with hepatic impairment. Terbinafine is a category B drug in pregnancy. However, there is no clear cut guideline available for managing dermatophytic infection and treatment should be individualized and based upon risk-benefit ratio.[67]
Chronic dermatophytosis
It has also been described in literature as T. rubrum syndrome, generalized chronically persistent rubrophytia, tinea corporis generalisata and dry-type T. rubrum infection. It is characterized by involvement of at least four body sites such as feet (plantar), hands (palmar), nails, as well as one other site with exclusion of inguinal area along with identification of T. rubrum in microscopy and culture.[68] Chronic dermatophytosis refers to persistent dermatophytosis that runs a chronic course with episodes of remission and exacerbation. Chronicity can be considered in terms of duration and recurrences of infection although there is no standard definition for chronicity. The emergence of such cases could be attributed to various pathogenic agent, host and pharmacologic factors. At present, there are no guidelines on management of chronic dermatophytosis. Although there are few studies to suggest that antifungal resistance is not common in tinea capitis, such data are lacking with respect to tinea cruris and corporis. This should also be seen with respect to the currently prevailing clinical scenario in India where there is an increasing recognition of a rising trend of nonresponsive cutaneous dermatophytosis.[69,70] The detailed recount on pathogenesis and management of chronic/recurrent dermatophytosis is beyond scope of this manuscript.
CONCLUSIONS
Treatment of cutaneous dermatophytosis has increasingly become difficult, and dermatologists have been forced to think beyond conventional wisdom to counter this menace. Although there is sufficient evidence to demonstrate the efficacy of topical antifungals in limited disease yet, there is scarce data on the frequency of relapse once topical monotherapy is discontinued. Among various options, topical terbinafine for 4 weeks appears to be the treatment of choice for limited disease (tinea corporis/cruris/pedis). For more extensive disease, the choice is less clear. Both terbinafine (250–500 mg/day for 2–6 weeks) and itraconazole (100–200 mg/day for 2–4 weeks) appear to be effective. However, an appropriate dose and duration of administration which can produce mycologic cure and prevent recurrence remains elusive. This review also highlights the huge research gaps in the management of cutaneous dermatophytosis which need to be plugged to provide better and effective care to the patients. More stringent RCTs are the need of the hour comparing the various oral antifungal therapies to give a clear idea regarding the appropriate dose and duration of therapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Weitzman I, Summerbell RC. The dermatophytes. Clin Microbiol Rev. 1995;8:240–59. doi: 10.1128/cmr.8.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drake LA, Dinehart SM, Farmer ER, Goltz RW, Graham GF, Hardinsky MK, et al. Guidelines of care for superficial mycotic infections of the skin: Tinea corporis, tinea cruris, tinea faciei, tinea manuum, and tinea pedis. Guidelines/Outcomes Committee. American Academy of Dermatology. J Am Acad Dermatol. 1996;34(2 Pt 1):282–6. doi: 10.1016/s0190-9622(96)80135-6. [DOI] [PubMed] [Google Scholar]
- 3.Ameen M, Lear JT, Madan V, Mohd Mustapa MF, Richardson M. British Association of Dermatologists’ guidelines for the management of onychomycosis 2014. Br J Dermatol. 2014;171:937–58. doi: 10.1111/bjd.13358. [DOI] [PubMed] [Google Scholar]
- 4.Fuller LC, Barton RC, Mohd Mustapa MF, Proudfoot LE, Punjabi SP, Higgins EM. British Association of Dermatologists’ guidelines for the management of tinea capitis 2014. Br J Dermatol. 2014;171:454–63. doi: 10.1111/bjd.13196. [DOI] [PubMed] [Google Scholar]
- 5.Moriarty B, Hay R, Morris-Jones R. The diagnosis and management of tinea. BMJ. 2012;345:e4380. doi: 10.1136/bmj.e4380. [DOI] [PubMed] [Google Scholar]
- 6.El-Gohary M, van Zuuren EJ, Fedorowicz Z, Burgess H, Doney L, Stuart B, et al. Topical antifungal treatments for tinea cruris and tinea corporis. Cochrane Database Syst Rev. 2014;8:CD009992. doi: 10.1002/14651858.CD009992.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crawford F, Hollis S. Topical treatments for fungal infections of the skin and nails of the foot. Cochrane Database Syst Rev. 2007;3:CD001434. doi: 10.1002/14651858.CD001434.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bell-Syer SE, Khan SM, Torgerson DJ. Oral treatments for fungal infections of the skin of the foot. Cochrane Database Syst Rev. 2012;10:CD003584. doi: 10.1002/14651858.CD003584.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Havlickova B, Czaika VA, Friedrich M. Epidemiological trends in skin mycoses worldwide. Mycoses. 2008;51(Suppl 4):2–15. doi: 10.1111/j.1439-0507.2008.01606.x. [DOI] [PubMed] [Google Scholar]
- 10.Lakshmanan A, Ganeshkumar P, Mohan SR, Hemamalini M, Madhavan R. Epidemiological and clinical pattern of dermatomycoses in rural India. Indian J Med Microbiol. 2015;33:134–6. doi: 10.4103/0255-0857.150922. [DOI] [PubMed] [Google Scholar]
- 11.Sharma Y, Jain S, Chandra K, Khurana VK, Kudesia M. Clinico-mycological evaluation of dermatophytes and non-dermatophytes isolated from various clinical samples: A study from north India. J Res Med Sci. 2012;17:817–8. [PMC free article] [PubMed] [Google Scholar]
- 12.Agarwal US, Saran J, Agarwal P. Clinico-mycological study of dermatophytes in a tertiary care centre in Northwest India. Indian J Dermatol Venereol Leprol. 2014;80:194. doi: 10.4103/0378-6323.129434. [DOI] [PubMed] [Google Scholar]
- 13.Sahai S, Mishra D. Change in spectrum of dermatophytes isolated from superficial mycoses cases: First report from Central India. Indian J Dermatol Venereol Leprol. 2011;77:335–6. doi: 10.4103/0378-6323.79718. [DOI] [PubMed] [Google Scholar]
- 14.Jaradat SW, Cubillos S, Krieg N, Lehmann K, Issa B, Piehler S. Low DEFB4 copy number and high systemic hBD-2 and IL-22 levels are associated with dermatophytosis. J Invest Dermatol. 2015;135:750–8. doi: 10.1038/jid.2014.369. [DOI] [PubMed] [Google Scholar]
- 15.García-Romero MT, Arenas R. New insights into genes, immunity, and the occurrence of dermatophytosis. J Invest Dermatol. 2015;135:655–7. doi: 10.1038/jid.2014.498. [DOI] [PubMed] [Google Scholar]
- 16.Tainwala R, Sharma Y. Pathogenesis of dermatophytoses. Indian J Dermatol. 2011;56:259–61. doi: 10.4103/0019-5154.82476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dahl MV. Dermatophytosis and the immune response. J Am Acad Dermatol. 1994;31(3 Pt 2):S34–41. doi: 10.1016/s0190-9622(08)81265-0. [DOI] [PubMed] [Google Scholar]
- 18.Jones HE, Reinhardt JH, Rinaldi MG. Immunologic susceptibility to chronic dermatophytosis. Arch Dermatol. 1974;110:213–20. [PubMed] [Google Scholar]
- 19.Oliveira CB, Vasconcellos C, Sakai-Valente NY, Sotto MN, Luiz FG, Belda Júnior W, et al. Toll-like receptors (TLR) 2 and 4 expression of keratinocytes from patients with localized and disseminated dermatophytosis. Rev Inst Med Trop Sao Paulo. 2015;57:57–61. doi: 10.1590/S0036-46652015000100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurade SM, Amladi SA, Miskeen AK. Skin scraping and a potassium hydroxide mount. Indian J Dermatol Venereol Leprol. 2006;72:238–41. doi: 10.4103/0378-6323.25794. [DOI] [PubMed] [Google Scholar]
- 21.Lasseter G, Palmer M, Morgan J, Watts J, Yoxall H, Kibbler C, et al. Developing best practice for fungal specimen management: Audit of UK microbiology laboratories. Br J Biomed Sci. 2011;68:197–202. doi: 10.1080/09674845.2011.11730350. [DOI] [PubMed] [Google Scholar]
- 22.Singh J, Zaman M, Gupta AK. Evaluation of microdilution and disk diffusion methods for antifungal susceptibility testing of dermatophytes. Med Mycol. 2007;45:595–602. doi: 10.1080/13693780701549364. [DOI] [PubMed] [Google Scholar]
- 23.Fernández-Torres B, Cabañes FJ, Carrillo-Muñoz AJ, Esteban A, Inza I, Abarca L, et al. Collaborative evaluation of optimal antifungal susceptibility testing conditions for dermatophytes. J Clin Microbiol. 2002;40:3999–4003. doi: 10.1128/JCM.40.11.3999-4003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gómez-Moyano E, Crespo-Erchiga V. Tinea of vellus hair: An indication for systemic antifungal therapy. Br J Dermatol. 2010;163:603–6. doi: 10.1111/j.1365-2133.2010.09811.x. [DOI] [PubMed] [Google Scholar]
- 25.Yang CY, Lin TL, Tzung TY, Cheng LC, Wang JT, Jee SH. Direct identification of dermatophyte DNA from clinical specimens by a nested polymerase chain reaction assay. Arch Dermatol. 2007;143:799–800. doi: 10.1001/archderm.143.6.799. [DOI] [PubMed] [Google Scholar]
- 26.Theel ES, Hall L, Mandrekar J, Wengenack NL. Dermatophyte identification using matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2011;49:4067–71. doi: 10.1128/JCM.01280-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hui D, Xue-cheng S, Ai-e X. Evaluation of reflectance confocal microscopy in dermatophytosis. Mycoses. 2013;56:130–3. doi: 10.1111/j.1439-0507.2012.02222.x. [DOI] [PubMed] [Google Scholar]
- 28.Weinstein A, Berman B. Topical treatment of common superficial tinea infections. Am Fam Physician. 2002;65:2095–102. [PubMed] [Google Scholar]
- 29.Gupta AK, Tomas E. New antifungal agents. Dermatol Clin. 2003;21:565–76. doi: 10.1016/s0733-8635(03)00024-x. [DOI] [PubMed] [Google Scholar]
- 30.Grillot R, Lebeau B. Antimicrobial Agents. Washington, DC: ASM Press; 2005. Systemic antifungal agents; pp. 1260–87. [Google Scholar]
- 31.High WA, Fitzpatrick JE. Fitzpatrick's Dermatology in General Medicine. 8th ed. New Delhi: Tata McGraw Hill; 2012. Topical antifungal agents; pp. 2116–21. [Google Scholar]
- 32.Rotta I, Ziegelmann PK, Otuki MF, Riveros BS, Bernardo NL, Correr CJ. Efficacy of topical antifungals in the treatment of dermatophytosis: A mixed-treatment comparison meta-analysis involving 14 treatments. JAMA Dermatol. 2013;149:341–9. doi: 10.1001/jamadermatol.2013.1721. [DOI] [PubMed] [Google Scholar]
- 33.Havlickova B, Friedrich M. The advantages of topical combination therapy in the treatment of inflammatory dermatomycoses. Mycoses. 2008;51(Suppl 4):16–26. doi: 10.1111/j.1439-0507.2008.01615.x. [DOI] [PubMed] [Google Scholar]
- 34.Nadalo D, Montoya C, Hunter-Smith D. What is the best way to treat tinea cruris? J Fam Pract. 2006;55:256–8. [PubMed] [Google Scholar]
- 35.Alston SJ, Cohen BA, Braun M. Persistent and recurrent tinea corporis in children treated with combination antifungal/corticosteroid agents. Pediatrics. 2003;111:201–3. doi: 10.1542/peds.111.1.201. [DOI] [PubMed] [Google Scholar]
- 36.Haedersdal M, Svejgaard EL. Systematic treatment of tinea pedis – Evidence for treatment?. A result of a Cochrane review. Ugeskr Laeger. 2003;165:1436–8. [PubMed] [Google Scholar]
- 37.Stein Gold LF, Vlahovic T, Verma A, Olayinka B, Fleischer AB., Jr Naftifine hydrochloride gel 2%: An effective topical treatment for moccasin-type tinea pedis. J Drugs Dermatol. 2015;14:1138–44. [PubMed] [Google Scholar]
- 38.Khanna D, Bharti S. Luliconazole for the treatment of fungal infections: An evidence-based review. Core Evid. 2014;9:113–24. doi: 10.2147/CE.S49629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elewski BE, Vlahovic TC. Econazole nitrate foam 1% for the treatment of tinea pedis: Results from two double-blind, vehicle-controlled, phase 3 clinical trials. J Drugs Dermatol. 2014;13:803–8. [PubMed] [Google Scholar]
- 40.Bseiso EA, Nasr M, Sammour O, Abd El Gawad NA. Recent advances in topical formulation carriers of antifungal agents. Indian J Dermatol Venereol Leprol. 2015;81:457–63. doi: 10.4103/0378-6323.162328. [DOI] [PubMed] [Google Scholar]
- 41.Sheikh S, Ahmad A, Ali SM, Paithankar M, Barkate H, Raval RC, et al. Topical delivery of lipid based amphotericin B gel in the treatment of fungal infection: A clinical efficacy, safety and tolerability study in patients. J Clin Exp Dermatol Res. 2014;5:248. [Google Scholar]
- 42.Butani D, Yewale C, Misra A. Amphotericin B topical microemulsion: Formulation, characterization and evaluation. Colloids Surf B Biointerfaces. 2014;116:351–8. doi: 10.1016/j.colsurfb.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 43.Aggarwal N, Goindi S, Khurana R. Formulation, characterization and evaluation of an optimized microemulsion formulation of griseofulvin for topical application. Colloids Surf B Biointerfaces. 2013;105:158–66. doi: 10.1016/j.colsurfb.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 44.Li RY, Wang AP, Xu JH, Xi LY, Fu MH, Zhu M, et al. Efficacy and safety of 1% terbinafine film-forming solution in Chinese patients with tinea pedis: A randomized, double-blind, placebo-controlled, multicenter, parallel-group study. Clin Drug Investig. 2014;34:223–30. doi: 10.1007/s40261-014-0171-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Czaika VA. Effective treatment of tinea corporis due to Trichophyton mentagrophytes with combined isoconazole nitrate and diflucortolone valerate therapy. Mycoses. 2013;56(Suppl 1):30–2. doi: 10.1111/myc.12068. [DOI] [PubMed] [Google Scholar]
- 46.Lesher JL., Jr Oral therapy of common superficial fungal infections of the skin. J Am Acad Dermatol. 1999;40(6 Pt 2):S31–4. doi: 10.1016/s0190-9622(99)70395-6. [DOI] [PubMed] [Google Scholar]
- 47.Bourlond A, Lachapelle JM, Aussems J, Boyden B, Campaert H, Conincx S, et al. Double-blind comparison of itraconazole with griseofulvin in the treatment of tinea corporis and tinea cruris. Int J Dermatol. 1989;28:410–2. doi: 10.1111/j.1365-4362.1989.tb02491.x. [DOI] [PubMed] [Google Scholar]
- 48.Cole GW, Stricklin G. A comparison of a new oral antifungal, terbinafine, with griseofulvin as therapy for tinea corporis. Arch Dermatol. 1989;125:1537–9. [PubMed] [Google Scholar]
- 49.Panagiotidou D, Kousidou T, Chaidemenos G, Karakatsanis G, Kalogeropoulou A, Teknetzis A, et al. A comparison of itraconazole and griseofulvin in the treatment of tinea corporis and tinea cruris: A double-blind study. J Int Med Res. 1992;20:392–400. doi: 10.1177/030006059202000504. [DOI] [PubMed] [Google Scholar]
- 50.Faergemann J, Mörk NJ, Haglund A, Odegård T. A multicentre (double-blind) comparative study to assess the safety and efficacy of fluconazole and griseofulvin in the treatment of tinea corporis and tinea cruris. Br J Dermatol. 1997;136:575–7. [PubMed] [Google Scholar]
- 51.Shi TW, Zhang JA, Zhang XW, Yu HX, Tang YB, Yu JB. Combination treatment of oral terbinafine with topical terbinafine and 10% urea ointment in hyperkeratotic type tinea pedis. Mycoses. 2014;57:560–4. doi: 10.1111/myc.12198. [DOI] [PubMed] [Google Scholar]
- 52.Jachiet M, Lanternier F, Rybojad M, Bagot M, Ibrahim L, Casanova JL, et al. Posaconazole treatment of extensive skin and nail dermatophytosis due to autosomal recessive deficiency of CARD9. JAMA Dermatol. 2015;151:192–4. doi: 10.1001/jamadermatol.2014.2154. [DOI] [PubMed] [Google Scholar]
- 53.Wong JH, Lau KM, Wu YO, Cheng L, Wong CW, Yew DT, et al. Antifungal mode of action of macrocarpal C extracted from Eucalyptus globulus Labill (Lan An) towards the dermatophyte Trichophyton mentagrophytes. Chin Med. 2015;10:34. doi: 10.1186/s13020-015-0068-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arai S, Yoshino T, Fujimura T, Maruyama S, Nakano T, Mukuno A, et al. Mycostatic effect of recombinant dermcidin against Trichophyton rubrum and reduced dermcidin expression in the sweat of tinea pedis patients. J Dermatol. 2015;42:70–6. doi: 10.1111/1346-8138.12664. [DOI] [PubMed] [Google Scholar]
- 55.Smith KJ, Neafie RC, Skelton HG, 3rd, Barrett TL, Graham JH, Lupton GP. Majocchi's granuloma. J Cutan Pathol. 1991;18:28–35. doi: 10.1111/j.1600-0560.1991.tb00598.x. [DOI] [PubMed] [Google Scholar]
- 56.Gill M, Sachdeva B, Gill PS, Arora B, Deep A, Karan J. Majocchi's granuloma of the face in an immunocompetent patient. J Dermatol. 2007;34:702–4. doi: 10.1111/j.1346-8138.2007.00363.x. [DOI] [PubMed] [Google Scholar]
- 57.Akiba H, Motoki Y, Satoh M, Iwatsuki K, Kaneko F. Recalcitrant trichophytic granuloma associated with NK-cell deficiency in a SLE patient treated with corticosteroid. Eur J Dermatol. 2001;11:58–62. [PubMed] [Google Scholar]
- 58.Gupta AK, Prussick R, Sibbald RG, Knowles SR. Terbinafine in the treatment of Majocchi's granuloma. Int J Dermatol. 1995;34:489. doi: 10.1111/j.1365-4362.1995.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 59.Gupta AK, Groen K, Woestenborghs R, De Doncker P. Itraconazole pulse therapy is effective in the treatment of Majocchi's granuloma: A clinical and pharmacokinetic evaluation and implications for possible effectiveness in tinea capitis. Clin Exp Dermatol. 1998;23:103–8. doi: 10.1046/j.1365-2230.1998.00319.x. [DOI] [PubMed] [Google Scholar]
- 60.Feng WW, Chen HC, Chen HC. Majocchi's granuloma in a 3-year-old boy. Pediatr Infect Dis J. 2006;25:658–9. doi: 10.1097/01.inf.0000224312.87417.fc. [DOI] [PubMed] [Google Scholar]
- 61.Ravine D, Tuner KJ, Alpers MP. Genetic inheritance of susceptibility to tinea imbricata. J Med Genet. 1980;17:342–8. doi: 10.1136/jmg.17.5.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jones HE. Immune response and host resistance of humans to dermatophyte infection. J Am Acad Dermatol. 1993;28(5 Pt 1):S12–8. doi: 10.1016/s0190-9622(09)80302-2. [DOI] [PubMed] [Google Scholar]
- 63.Hay RJ, Reid S, Talwat E, Macnamara K. Immune responses of patients with tinea imbricata. Br J Dermatol. 1983;108:581–6. doi: 10.1111/j.1365-2133.1983.tb01060.x. [DOI] [PubMed] [Google Scholar]
- 64.Budimulja U, Kuswadji K, Bramono S, Basuki J, Judanarso LS, Untung S, et al. A double-blind, randomized, stratified controlled study of the treatment of tinea imbricata with oral terbinafine or itraconazole. Br J Dermatol. 1994;130(Suppl 43):29–31. doi: 10.1111/j.1365-2133.1994.tb06091.x. [DOI] [PubMed] [Google Scholar]
- 65.Verma S, Hay RJ. Topical steroid-induced tinea pseudoimbricata: A striking form of tinea incognito. Int J Dermatol. 2015;54:e192–3. doi: 10.1111/ijd.12734. [DOI] [PubMed] [Google Scholar]
- 66.Millikan LE. Role of oral antifungal agents for the treatment of superficial fungal infections in immunocompromised patients. Cutis. 2001;68(1 Suppl):6–14. [PubMed] [Google Scholar]
- 67.Elston CA, Elston DM. Treatment of common skin infections and infestations during pregnancy. Dermatol Ther. 2013;26:312–20. doi: 10.1111/dth.12075. [DOI] [PubMed] [Google Scholar]
- 68.Piñeiro L, Larruskain J, Idigoras P, Pérez-Trallero E. Trichophyton rubrum syndrome: The tip of the iceberg and a preventable outcome. Mycoses. 2010;53:186. doi: 10.1111/j.1439-0507.2008.01685.x. [DOI] [PubMed] [Google Scholar]
- 69.Ghannoum M, Isham N, Sheehan D. Voriconazole susceptibilities of dermatophyte isolates obtained from a worldwide tinea capitis clinical trial. J Clin Microbiol. 2006;44:2579–80. doi: 10.1128/JCM.00818-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ghannoum MA, Wraith LA, Cai B, Nyirady J, Isham N. Susceptibility of dermatophyte isolates obtained from a large worldwide terbinafine tinea capitis clinical trial. Br J Dermatol. 2008;159:711–3. doi: 10.1111/j.1365-2133.2008.08648.x. [DOI] [PubMed] [Google Scholar]


