Abstract
Introduction:
Narrowband ultraviolet B phototherapy (NBUVB) is safe and effective treatment for psoriasis. Vitamin D plays an important role in pathogenesis of psoriasis. It is known that psoriasis patients have low serum 25(OH)D levels, which increase after NBUVB. We assessed serum 25(OH)D levels, its correlation with Psoriasis Area and Severity Index (PASI), and the effect of NBUVB on 25(OH)D levels among Indian psoriasis patients.
Materials and Methods:
A prospective study comprising 30 adults with psoriasis with no major comorbidities (PASI > 10 and off-therapy >4 weeks) was conducted. PASI was estimated at baseline among patients and repeated after receiving 12 weeks of NBUVB therapy. Thirty age and gender-matched healthy controls were recruited to compare 25(OH)D levels at baseline and at 12 weeks. Patient demographic parameters, treatment dose, duration, side effects, and its impact on 25(OH)D levels and PASI were serially evaluated.
Results:
A total of 30 patients presenting with psoriasis and 30 healthy controls were enrolled in the study. Mean baseline PASI (M: F =19:11) among patients with mean age 36.8 (±7.7) years was 20.5 (±6.3) and all patients were either 25(OH)D deficient (n = 14) or insufficient (n = 16). Their baseline 25(OH)D levels were significantly lower than controls (25.93 nmol/L vs 47.54 nmol/L; P < 0.001). After NBUVB therapy (average cumulative dose 20.76 ± 7.1 J/cm2; average treatment sessions 32.57 ± 1.9), there was a significant improvement in PASI as well as 25(OH)D (P < 0.05). There was no correlation between the mean improvement in PASI and 25(OH)D after 12 weeks of therapy. Twelve (40%) patients had therapy-related side effects [pruritus (n = 8), erythema (n = 4)], none had major side effects.
Conclusion:
Improvement in PASI and serum 25(OH)D levels after NBUVB in psoriasis is significant but poorly correlated with each other. Vitamin D may not be the lone mediator of the therapeutic effects of NBUVB on psoriasis.
Keywords: Narrowband ultraviolet B phototherapy, psoriasis, vitamin D
INTRODUCTION
Psoriasis is a chronic inflammatory skin disease, characterized by complex alterations in epidermal growth and differentiation and multiple biochemical, immunologic, and vascular abnormalities that evolve over time due to complex interplay between genetic factors and the environment.[1] T-cell activation results in release of pro-inflammatory cytokines leading to abnormal keratinocyte proliferation and differentiation.[2] The active form of vitamin D suppresses growth and induces differentiation of keratinocytes by both genomic and nongenomic actions.[3] Various genes associated with growth and differentiations of keratinocytes that are regulated by calcitriol include involucrin, transglutaminase I, CyclinD1, and cdk4.[4] It also acts as an immunoregulatory agent. It abolishes the Thl immune response and favors Th2 immune response.[5] Calcitriol also inhibits interleukin-1-induced T-cell proliferation and inhibits the production of various cytokines involved in the pathogenesis of psoriasis.[6]
Parrish and Jaenicke discovered that psoriatic lesions responded best to narrowband ultraviolet B (NBUVB 313 nm) therapy.[7] It is also known that radiation of wavelength between 295 and 315 nm is responsible for cutaneous vitamin D3 production.[8] This suggests a link between the therapeutic effect of UVB radiation in psoriasis and the cutaneous vitamin D3 synthesis by UVB.
The exact mechanism of action of NBUVB is not known. It is proposed that it acts in the following ways: (1) Cellular DNA is converted to pyrimidine dimers which interfere with cell cycle progression,[9] (2) alteration in cytokine production,[10] (3) reduction in natural killer cell activity and induction of apoptosis of immunocompetent T cells,[11] (4) downregulation of Th17 cells,[12] and (5) synthesis of vitamin D3.[13]
A few studies have assessed the effect of NBUVB on vitamin D production and Psoriasis Area and Severity Index (PASI) in psoriasis patients.[14] The studies conducted till date have found an increase in serum levels of vitamin D3 and improvement of PASI in psoriasis patients after NBUVB therapy. This study was conducted to assess the effect of NBUVB on vitamin D levels and correlation of serum vitamin D levels with the severity of disease as assessed by PASI.
MATERIALS AND METHODS
A prospective study was conducted in the Department of Dermatology, Biochemistry, and Pathology at PGIMER and Dr. Ram Manohar Lohia Hospital, New Delhi, during the period 2013–2014. Approval from our institute's ethical committee was taken. The study group comprised of 30 patients of psoriasis. An equal number of age and gender-matched consenting healthy volunteers who were not receiving vitamin D supplements were taken as the control group from our dermatology clinic for comparison of 25(OH)D levels.
Cases of psoriasis were diagnosed clinically on the basis of morphological appearance, positive grattage test, and Auspitz sign, and these cases were confirmed histopathologically.[15] Selection criteria for inclusion were age >18 years, patients of chronic plaque psoriasis with PASI ≥10, and had not taken any treatment for psoriasis in the last 4 weeks. Patients with more than 90% body surface area involvement, renal or hepatic disease, previous skin malignancy or other site malignancy, history of photosensitivity, previous failure or intolerance of phototherapy, or those on vitamin D supplements were excluded.
All included patients were subjected to informed written consent taken at the time of inclusion. The patients were thoroughly informed and explained about the procedure and the possible adverse events such as erythema, pruritus and so on. A detailed history and clinical examination was taken. Complete hemogram, liver function tests (LFT), kidney function tests (KFT), serum electrolytes, and blood glucose test were carried out. All the patients in the study group were biopsied to confirm the diagnosis of psoriasis. The baseline 25(OH)D levels were recorded. NBUVB phototherapy was given for 12 weeks or till achievement of complete clinical remission, whichever was earlier. 25(OH)D levels were measured after the aforementioned treatment. The severity of psoriasis was assessed by PASI scoring before and after treatment. Blood sample was taken from the controls once at approximately the same time points as the patients with psoriasis and 25(OH)D levels, complete hemogram, liver and renal function tests, serum electrolytes, and blood glucose levels were measured.
25(OH)D levels were estimated by taking a 2 mL fasting morning blood sample using Immunodiagnostic systems 25-hydroxyvitamin D Enzyme immunoassay (EIA) kit by Competitive Enzyme Linked Immunosorbent Assay (ELISA) technique. Levels of 25-OH vitamin D3 were graded as: Deficient <25 nmol/L, insufficient 25–74 nmol/L, and normal 75–250 nmol/L.
NBUVB phototherapy was given using Waldmann UV 1000 L (TL 01) machine. The irradiation dose was started at a dose of 0.3J/cm2 that was increased by 20% on each subsequent visit till just perceptible erythema appeared on the uninvolved skin. If symptomatic erythema (burning, pain) developed, phototherapy was stopped till erythema settled. On restarting therapy, the irradiation dose was decreased by 20%. Phototherapy was given thrice weekly on nonconsecutive days for 12 weeks. During treatment, genitals were shielded and eyes were protected with UV safety glasses. Side effects of therapy such as erythema, pruritus, blisters, and so on were recorded at each visit.
Statistical analysis
Data was described as proportion, mean with standard deviations, and median with range. Continuous variables were compared by using Student's t test and means were compared during pre- and postintervention analyses using paired t test. Correlation between two continuous variables was done by using Spearman's correlation. P value of 0.05 or less was considered as significant in all statistical tests.
RESULTS
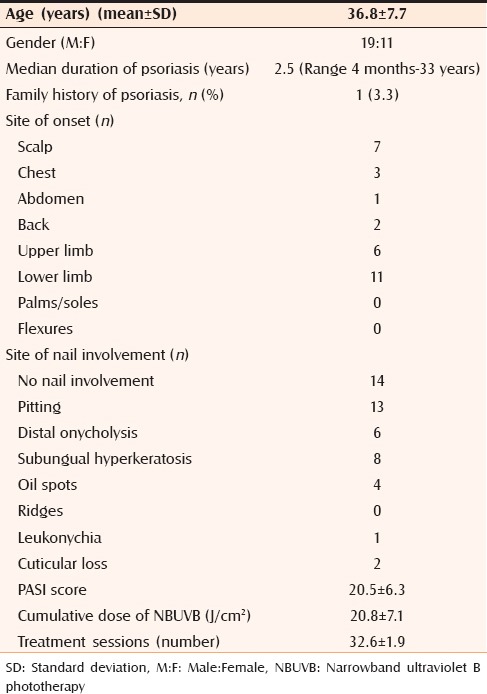
The age of presentation ranged between 18 and 65 years and the mean age at presentation was 36.9 ± 7.7 years. The maximum number of cases (30% of the total) belonged to age group 18–29 and 40–49 years each, and least number of cases (6.7%) were recorded in the age group of 50–59 years and more than 60 years. A 63.3% of our study cohort consisted of male patients. Male: female ratio was 1.7:1. Duration of disease ranged between four months and 33 years with a median of 2.5 years. Maximum number of patients presented within six years (n = 24) of onset. Only one patient of psoriasis had a positive family history of similar disease. The common site of disease onset was lower limb (36.7%) followed by scalp (23.3%). Nail involvement was found in 53.3% patients. The most frequent nail changes included nail pitting (13 patients, 43.3%), subungual hyperkeratosis (26.7% patients), and distal onycholysis in 20% of patients. Fourteen patients (46.7%) had no nail involvement. Other sites of onset in decreasing order of frequency were upper limb, chest, back, and abdomen [Table 1].
Table 1.
Baseline profile of psoriasis patients (n=30)
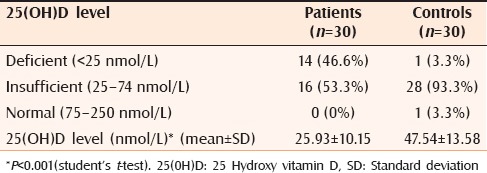
At the outset, 46.3% (n = 14) of the patients had deficient (<25 nmol/L) and 53.66% (n = 16) had insufficient serum levels of 25(OH)D (25–74 nmol/L). None had normal 25(OH)D levels (75–250 nmol/L). A 93.3% (n = 28) of the controls had insufficient levels (25–74 nmol/L), one patient had deficient 25(OH)D levels (<25 nmol/L), and one patient had normal levels (75–250 nmol/L) of 25(OH)D. Mean 25(OH)D levels at the outset in patients was 25.93 ± 10.15 nmol/L, whereas that of controls was 47.54 ± 13.58 nmol/L (P < 0.001) [Table 2]. Mean PASI at the outset was 20.49 ± 8.49. Correlation between mean PASI and mean 25(OH)D was weak (r = 0.206) and was statistically insignificant (P = 0.284).
Table 2.
Comparison of 25(OH)D level at baseline between psoriasis patients and controls
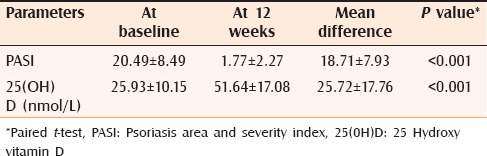
After 12 weeks, 2 (6.66%) patients achieved the normal 25(OH)D levels (75–250 nmol/L), 90% (n = 21) patients had insufficient levels (25–75 nmol/L). Only one patient still had deficient 25(OH)D level (<25 nmol/L). Patients had significant improvement in their 25(OH)D levels and PASI post 12 weeks of NBUVB phototherapy (P < 0.05) [Table 3].
Table 3.
Comparison between mean improvement in PASI and 25(OH)D before and after 12 weeks of phototherapy among patients with psoriasis (n=30)
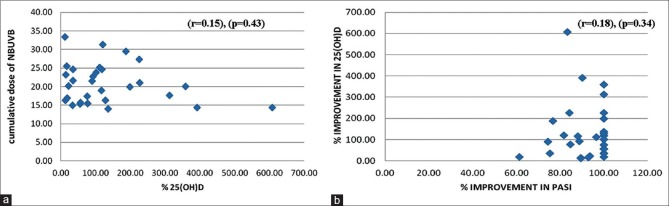
The mean improvement in PASI after 12 weeks of NBUVB phototherapy was 1.687 ± 2.21% and mean improvement in 25(OH)D levels was 51.704 ± 16.79%. The correlation between mean improvement in PASI and 25(OH)D was weak (r = 0.181) and was statistically insignificant (P = 0.339) [Figure 1]. Average cumulative dose of NBUVB phototherapy after 12 weeks was 20.76 ± 7.1 J/cm2 given in 32.57 ± 1.9 number of treatment sessions.
Figure 1.
Scatter plots showing correlation between Vitamin D with narrow band ultraviolet B (NBUVB) photo-therapy and psoriasis area and severity index (PASI) (a) Correlation of % 25(OH)D increase with mean cumulative dose of Narrowband ultraviolet B phototherapy (NBUVB). (b) Correlation between mean improvement in Psoriasis Area and Severity Index (PASI) and 25(OH)D after 12 weeks of phototherapy
Correlation of mean percentage improvement of 25(OH)D levels with an average cumulative dose of NBUVB was weak (r = 0.150) and statistically insignificant (P = 0.43) [Figure 1]. Correlation of mean baseline serum 25(OH)D level and mean improvement in serum 25(OH)D vitamin D3 level was strong and statistically significant.
Sixty percent (n = 18) of the patients had no side effects. Most common side effect observed was marked pruritus in 26.66% (n = 8) of the patients. The incidence of marked erythema was 13.33% (n = 4) [Table 4]. None of the patients developed burning, acneiform eruptions, or herpes labialis.
Table 4.
Side effects of NBUVB phototherapy among patients with psoriasis

DISCUSSION
Psoriasis is a chronic inflammatory disease, which affects about 2% of the population.[16] Among the various treatment modalities used to treat psoriasis, vitamin D analogues occupy a prime position in the therapeutic armamentarium. Low levels of 25(OH)D were found in psoriasis patients. Various studies have found that psoriasis patients who are treated with NBUVB (310–315 nm) show increase in serum 25(OH)D levels as well as improvement in PASI. This has led to the speculation that there may be some correlation between serum 25(OH)D, NBUVB phototherapy, and improvement in PASI. But the exact correlation between these factorshas not been clearly explored. The few studies done till date have given conflicting results. This study was designed to estimate serum 25(OH)D levels in patients of psoriasis before and after narrowband UVB phototherapy and to find whether correlation of serum 25(OH)D levels with PASI exists.
Vitamin D deficiency prevails in epidemic proportions all over the Indian subcontinent, with a prevalence of 70%–100% in the general population and as high as 85%–100% in Delhi region.[17] We found that baseline serum level of 25(OH)D was low in both patients and the controls. Only one of the controls had normal 25(OH)D level. Low levels in the controls may be due to darker skin color of the Indians, poor dietary intake of vitamin D, lack of food fortification, and inadequate sun exposure especially in females probably due to the traditional clothing. 25(OH)D level in patients was significantly lower than that in controls (P < 0.001). Statistically significant difference was similarly found in 25(OH)D serum levels among patients and controls in previous studies conducted in Italy and Spain.[18,19]
Various studies conducted on healthy individuals have found that NBUVB increases serum 25(OH)D levels in healthy adults. But very few studies have been done on psoriasis patients to assess the effect of NBUVB on 25(OH)D serum levels and severity of psoriasis. The findings of these studies corroborated with our study showing that NBUVB increases 25(OH)D levels in psoriasis patients and decreases severity of psoriasis. In a recent study by Ala-Houhala et al., 12 NB-UVB exposures given during 4 weeks increase serum 25(OH)D concentration significantly more than 20 μg of oral cholecalciferol daily.[20] This along with the findings that lower levels of 25(OH)D are found in psoriasis patients than the healthy controls has raised the question that does vitamin D play an important role in the pathogenesis of psoriasis and whether the major mechanism of action of NBUVB in treatment of psoriasis is by increasing synthesis of 25(OH)D.
In our study, mean improvement in PASI as well as increase in 25(OH)D levels after the completion of treatment were individually significant. Similarly, in a previous study by Ala-Houhala et al., 9th exposure to NB-UVB 25(OH)D had increased by 13.2 nmol/L and at the 18th exposure by 49.4 nmol/L above baseline. Psoriasis Area Severity Index score improved from 8.7±3.5 to 4.5±2.0 (P<0.001).[21]
However, on comparing the mean improvement in PASI and mean improvement in 25(OH)D level after 12 weeks of NBUVB phototherapy, their correlation was weak and statistically insignificant.
These findings show that the major mechanism of action of NBUVB in treatment of psoriasis is not through vitamin D. Clinical improvement of psoriasis by NB-UVB may be linked to suppression of Th17 and type I and type II IFN signaling pathways, which are critical in the pathogenesis of the disease.[22] Effect of NBUVB on psoriasis mediated through cytokines and immune cells may be more significant than its effect through vitamin D.
Correlation between cumulative dose of NBUVB phototherapy and change in 25(OH)D level was not significant. This might be explained by autoregulation of the synthesis, storage, and slow and steady release of vitamin D3 from the skin into the circulation. Photoequilibrium is set up as a result of continued exposure to ultraviolet radiation, which explains the nonlinear vitamin D synthesis.[23] Vitamin D synthesis also depends on other factors such as dietary intake.
In our study, the magnitude of increase in 25(OH)D response was greater in the cases who had low initial level of 25(OH)D (t0).
Since very few patients developed side effects such as itching and erythema and PASI decreased significantly, our study also supports the well-known fact that NBUVB is a safe and effective therapy for psoriasis.
Our study had few limitations. This study was time bound and thus a small sample size was achieved, which has its scientific limitations. It was a prospective study with an interventional component in single arm to document efficacy of NBUVB in treatment of psoriasis. No control arm for comparison was another limitation to this study. Factors influencing 25(OH)D levels such as occupation, exposure to sunlight, seasonal exposure, and so on were not recorded or adjusted during the analysis.
CONCLUSION
The levels of 25(OH)D in psoriasis patients were significantly lower than that of the normal controls. NBUVB resulted in a significant rise in the levels of 25(OH)D. All patients improved after NBUVB phototherapy with significant improvement in PASI. But correlation between the mean improvement in PASI and the mean improvement in 25(OH)D level after 12 weeks of NBUVB phototherapy was weak and was statistically insignificant. Hence our study does not support vitamin D as the most important factor in NBUVB-induced improvement in psoriasis. Further studies are needed in this regard.
What's already known?
NBUVB is a safe and effective therapy for psoriasis
NBUVB increases vitamin D levels in psoriasis patients.
What does this study add?
Correlation between mean improvement in PASI and mean improvement in 25(OH)D level after NBUVB is weak and hence vitamin D is not the major mediator of the therapeutic effects of NBUVB on psoriasis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Gudjonsson JE, Elder JT. Psoriasis. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick's Dermatology In General Medicine. 7th ed. Vol. 1. USA: McGraw-Hill; 2008. p. 169. [Google Scholar]
- 2.Krueger JG. The immunologic basis for the treatment of psoriasis with new biologic agents. J Am Acad Dermatol. 2002;46:1–26. doi: 10.1067/mjd.2002.120568. [DOI] [PubMed] [Google Scholar]
- 3.Kira M, Kobayashi T, Yoshikawa K. Vitamin D and the skin. J Dermatol. 2003;30:429–37. doi: 10.1111/j.1346-8138.2003.tb00412.x. [DOI] [PubMed] [Google Scholar]
- 4.Lehmann B. The vitamin D3 pathway in human skin and its role for regulation of biological processes. Photochem Photobiol. 2005;81:1246–51. doi: 10.1562/2005-02-02-IR-430. [DOI] [PubMed] [Google Scholar]
- 5.Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O’Garra A. 1alpha, 25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J. Immunol. 2001;167:4974–80. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 6.van de Kerkhof PC. Biological activity of vitamin D analogues in the skin, with special reference to antipsoriatic mechanisms. Br J Dermatol. 1995;132:675–82. doi: 10.1111/j.1365-2133.1995.tb00710.x. [DOI] [PubMed] [Google Scholar]
- 7.Parrish JA, Jaenicke KF. Action spectrum for phototherapy of psoriasis. J Invest Dermatol. 1981;76:359–62. doi: 10.1111/1523-1747.ep12520022. [DOI] [PubMed] [Google Scholar]
- 8.Young AR, Walker SL. Acute and chronic effects of ultraviolet radiation on the skin. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick's Dermatology in General Medicine. USA: McGraw-Hill; 2008. p. 814. [Google Scholar]
- 9.Dogra S, De D. Narrowband ultraviolet B in the treatment of psoriasis: The journey so far! Indian J Dermatol Venereol Leprol. 2010;76:652–61. doi: 10.4103/0378-6323.72461. [DOI] [PubMed] [Google Scholar]
- 10.Piskin G, Tursen U, Sylva- Steenland RM, Bos JD, Teunissen MB. Clinical improvement in chronic plaque-type psoriasis lesions after narrow-band UVB therapy is accompanied by a decrease in the expression of IFN-gamma inducers—IL-12, IL-18 and IL-23. Exp Dermatol. 2004;13:764–72. doi: 10.1111/j.0906-6705.2004.00246.x. [DOI] [PubMed] [Google Scholar]
- 11.Ozawa M, Ferenczi K, Kikuchi T, Cardinale I, Austin LM, Coven TR, et al. 312-nanometer ultraviolet B light (narrow- band UVB) induces apoptosis of T cells within psoriatic lesions. J Exp Med. 1999;189:711–8. doi: 10.1084/jem.189.4.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nickoloff BJ. Cracking the cytokine code in psoriasis. Nat Med. 2007;13:242–4. doi: 10.1038/nm0307-242. [DOI] [PubMed] [Google Scholar]
- 13.Ryan C, Moran B, McKenna MJ, Murray BF, Brady J, Collins P, et al. The effect of narrowband UV-B treatment for psoriasis on vitamin D status during wintertime in Ireland. Arch Dermatol. 2010;146:836–42. doi: 10.1001/archdermatol.2010.195. [DOI] [PubMed] [Google Scholar]
- 14.Fredriksson T, Pettersson U. Severe psoriasis—Oral therapy with a new retinoid. Dermatologica. 1978;157:238–44. doi: 10.1159/000250839. [DOI] [PubMed] [Google Scholar]
- 15.Murphy M, Kerr P, Grant-Kels JM. The histopathologic spectrum of psoriasis. Clin Dermatol. 2007;25:524–8. doi: 10.1016/j.clindermatol.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Cristophers E. Psoriasis—Epidemiology and clinical spectrum. Clin Exp Dermatol. 2001;26:314–20. doi: 10.1046/j.1365-2230.2001.00832.x. [DOI] [PubMed] [Google Scholar]
- 17.Ritu G, Gupta A. Vitamin D deficiency in India: Prevalence, causalities and interventions. Nutrients. 2014;6:729–75. doi: 10.3390/nu6020729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gisondi P, Rossini M, Di Cesare A, Idolazzi L, Farina S, Beltrami G, et al. Vitamin D status in patients with chronic plaque psoriasis. Br J Dermatol. 2012;166:505–10. doi: 10.1111/j.1365-2133.2011.10699.x. [DOI] [PubMed] [Google Scholar]
- 19.Orgaz-Molina J, Buendía-Eisman A, Arrabal-Polo MA, Ruiz JC, Arias-Santiago S. Deficiency of serum concentration of 25-hydroxyvitamin D in psoriatic patients: A case-control study. J Am Acad Dermatol. 2012;67:931–8. doi: 10.1016/j.jaad.2012.01.040. [DOI] [PubMed] [Google Scholar]
- 20.Ala-Houhala MJ, Vähävihu K, Hasan T, Kautiainen H, Ylianttila L, Viljakainen HT, et al. Comparison of narrowband ultraviolet B exposure and oral vitamin D substitution on serum 25-hydroxyvitamin D concentration. Br J Dermatol. 2012;167:160–4. doi: 10.1111/j.1365-2133.2012.10990.x. [DOI] [PubMed] [Google Scholar]
- 21.Ala-Houhala MJ, Karppinen T, Vähävihu K, Kautiainen H, Dombrowski Y, Snellman E, et al. Narrow-band ultraviolet B treatment boosts serum 25-hydroxyvitamin D in patients with psoriasis on oral vitamin D supplementation. Acta Derm Venereol. 2014;94:146–51. doi: 10.2340/00015555-1685. [DOI] [PubMed] [Google Scholar]
- 22.Rácz E, Prens EP, Kurek D, Kant M, de Ridder D, Mourits S, et al. Effective treatment of psoriasis with narrow-band UVB phototherapy is linked to suppression of the IFN and Th17 pathways. J Invest Dermatol. 2011;131:1547–58. doi: 10.1038/jid.2011.53. [DOI] [PubMed] [Google Scholar]
- 23.Holick MF, MacLaughlin JA, Doppelt SH. Regulation of cutaneous previtamin D3 photosynthesis in man: Skin pigment is not an essential regulator. Science. 1981;211:590–3. doi: 10.1126/science.6256855. [DOI] [PubMed] [Google Scholar]