Abstract
In this study we assessed the ability of Middle East respiratory syndrome coronavirus (MERS-CoV) to replicate and induce innate immunity in human monocyte-derived macrophages and dendritic cells (MDDCs), and compared it with severe acute respiratory syndrome coronavirus (SARS-CoV). Assessments of viral protein and RNA levels in infected cells showed that both viruses were impaired in their ability to replicate in these cells. Some induction of IFN-λ1, CXCL10 and MxA mRNAs in both macrophages and MDDCs was seen in response to MERS-CoV infection, but almost no such induction was observed in response to SARS-CoV infection. ELISA and Western blot assays showed clear production of CXCL10 and MxA in MERS-CoV-infected macrophages and MDDCs. Our data suggest that SARS-CoV and MERS-CoV replicate poorly in human macrophages and MDDCs, but MERS-CoV is nonetheless capable of inducing a readily detectable host innate immune response. Our results highlight a clear difference between the viruses in activating host innate immune responses in macrophages and MDDCs, which may contribute to the pathogenesis of infection.
Introduction
The Middle East respiratory syndrome coronavirus (MERS-CoV) was first discovered in 2012 in Saudi Arabia from a man suffering from an acute respiratory distress syndrome (Zaki et al., 2012; de Groot et al., 2013). Since then, 1611 confirmed cases with 575 fatalities have been reported (as of October 2015; http://www.who.int/csr/don/29-october-2015-mers-saudi-arabia/en/). The high morbidity and novel nature of the virus have drawn comparisons with the severe acute respiratory syndrome coronavirus (SARS-CoV), which infected >8000 people causing almost 800 fatalities in 2002–2003 (Cheng et al., 2007). Whilst sharing many similarities in terms of the clinical picture (Hui et al., 2014), studies focusing on pathogenesis have identified several notable differences between the viruses, including different receptor usage (Li et al., 2003; Raj et al., 2013), differences in cell tropism (Chan et al., 2013; Zielecki et al., 2013), different susceptibility to type I IFN (Zielecki et al., 2013) and differences in host response (Josset et al., 2013). Dromedary camels are considered to be the direct source of MERS-CoV human infections as evidenced by isolation of MERS-CoV from camels (Azhar et al., 2014; Raj et al., 2014), and widespread seropositivity of camels in Africa and the Arabian Peninsula (Raj et al., 2013; Reusken et al., 2013, 2014; Haagmans et al., 2014; Meyer et al., 2014). Viruses very similar to MERS-CoV have also been isolated from bats (Ithete et al., 2013; Memish et al., 2013; Corman et al., 2014) and a recent study showed binding of bat CoV HKU4 spike protein to the MERS-CoV receptor human dipeptidyl-peptidase 4 (DPP4) (Wang et al., 2014), supporting a bat origin of MERS-CoV.
MERS-CoV causes a lower respiratory tract infection presenting as pneumonia, and common symptoms include fever, cough, sore throat and myalgia (Hui et al., 2014). A large portion of patients, especially those with severe illness, had some underlying condition such as diabetes or chronic renal disease. Gastrointestinal symptoms and renal failure were also frequently observed in patients, and MERS-CoV RNA was detected in blood, urine and rectal swabs of patients, suggesting systemic dissemination and infection of the kidneys and gastrointestinal system (Poissy et al., 2014). This view is also supported by in vitro studies on MERS-CoV host cell tropism, confirming efficient replication in human renal and intestinal cell lines (Chan et al., 2013). Macrophages and dendritic cells (DCs) are abundantly present in infected lungs and they play an important role in infection control as producers of inflammatory cytokines and as antigen-presenting cells (Kopf et al., 2015). They act as the first responders to invading pathogens and their interaction with the pathogens can be a strong determinant for the outcome of an infection. Some respiratory viruses are known to infect and replicate in macrophages and DCs, including human CoVs OC43 and 229E (Collins, 1998; Funk et al., 2012). In addition to providing a platform for propagation, infection of DCs has also been speculated to contribute to systemic spread of influenza A virus through the lymphatic system (Moltedo et al., 2011). SARS-CoV has been shown to infect macrophages and DCs, but the infection is abortive and does not result in detectable viral protein synthesis or production of progeny viral particles (Ziegler et al., 2005). In the present study we investigated the ability of MERS-CoV to replicate and induce innate antiviral genes in human lung epithelial cells and primary human monocyte-derived macrophages and DCs (MDDCs), and compared the responses with those induced by SARS-CoV.
Results
Monocyte-derived macrophages and MDDCs are non-permissive for MERS-CoV infection
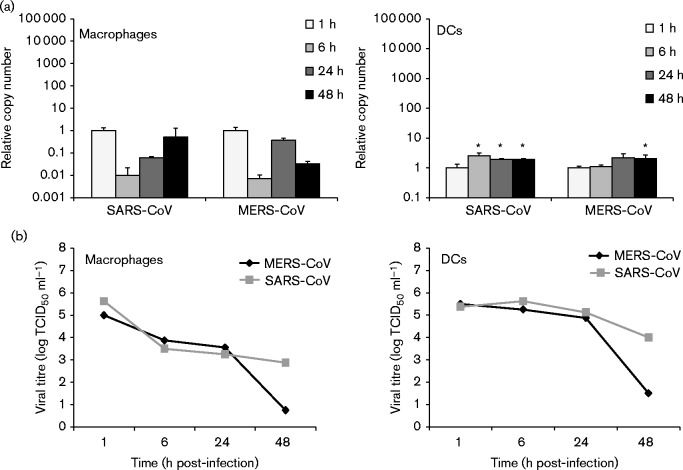
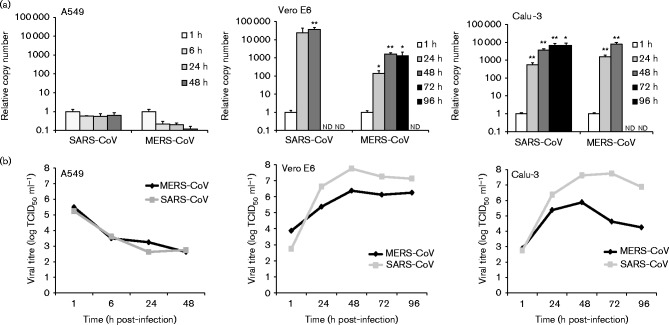
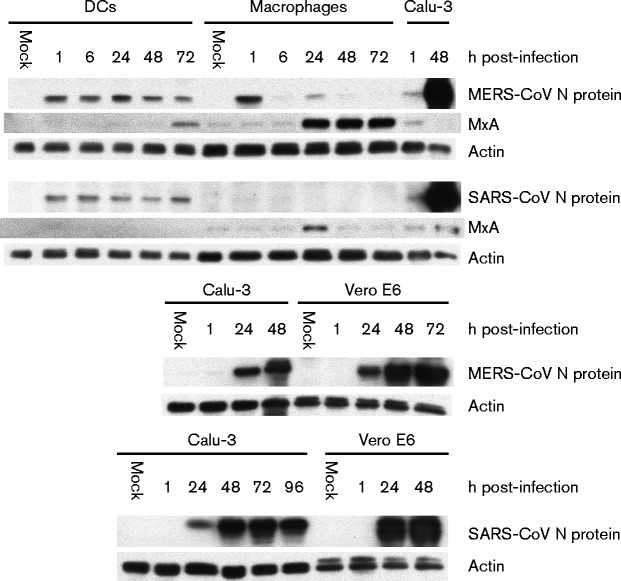
In order to determine whether primary human macrophages and MDDCs are permissive for MERS-CoV infection, we assayed supernatant and cell lysate samples from infected cells by end-point dilution and quantitative reverse transcription (qRT)-PCR to search for evidence of viral replication. SARS-CoV was included as a comparison and MDDCs were also infected with influenza A virus strain A/Beijing/89 (H3N2) as a positive control. As shown in Fig. 1(a), no evidence of efficient replication of MERS-CoV was observed in the qRT-PCR analysis in either cell type, with viral RNA levels remaining the same, decreasing or increasing only weakly throughout the infection. Similar results were obtained with SARS-CoV (Fig. 1a). End-point dilution assays performed on the supernatants confirmed the observations, showing a clear decrease in viral titre during the infection (Fig. 1b). As a control to confirm the functionality of our experimental setting, A549, Calu-3 and Vero E6 cells were infected with the viruses in a similar fashion as the leukocytes. A549 cells were found to be non-permissive for infection with either virus as evidenced by a lack of increase in viral RNA amounts and decreasing supernatant titre levels throughout the infection (Fig. 2). Both viruses showed efficient replication on Calu-3 and Vero E6 cells (Fig. 2). To further verify our observations we analysed cell lysates of infected macrophages, MDDCs, Calu-3 cells and Vero E6 cells for any increase in viral N protein amounts by Western blotting (Fig. 3). As expected, no increase was seen in macrophages or MDDCs, whereas strong expression of N protein was observed in Calu-3 and Vero E6 cells (Fig. 3).
Fig. 1.
Replication of MERS-CoV and SARS-CoV in human monocyte-derived macrophages and MDDCs. Cells were infected at m.o.i. 1, and cell lysate and cell culture supernatant samples were collected at 1, 6, 24 and 48 h post-infection. (a) Total cellular RNA was isolated from cell lysates, and qRT-PCR analysis carried out to quantify MERS-CoV and SARS-CoV viral RNA in infected macrophages and MDDCs. Relative RNA amounts were compared with the 1 h sample. Results (mean ± sd) are representative of two independent experiments carried out in macrophages and MDDCs obtained from three different blood donors. *P < 0.05. (b) Viral titres were measured from macrophage and MDDC supernatants using the end-point dilution assay. Results were calculated using the Spearman–Karber method.
Fig. 2.
Replication of MERS-CoV and SARS-CoV in Vero E6 cells and in human A549 and Calu-3 cells. Vero E6 cells were infected at m.o.i. 0.1, and A549 and Calu-3 cells at m.o.i. 1. Cell lysate and cell culture supernatant samples were collected at the indicated time points. (a) Total cellular RNA was isolated from cell lysates and qRT-PCR analysis was carried out to quantify MERS-CoV and SARS-CoV viral RNA. Relative RNA amounts were compared with the 1 h sample. Data are presented as mean ± SD of triplicate measurements. *P < 0.05; **P < 0.01. nd, Not determined (time points where sufficient amounts of high-quality RNA could not be recovered due to extensive cytotoxicity caused by the viruses). (b) Viral titres were measured from supernatants using the end-point dilution assay. Results were calculated using the Spearman–Karber method.
Fig. 3.
Quantification of MERS-CoV and SARS-CoV N protein expression during infection. Cell lysates from MDDC, macrophage, Calu-3 and Vero E6 infection experiments collected at the indicated time points were analysed by Western blotting for the expression of actin, MxA or MERS-CoV or SARS-CoV N protein. The 1 h sample and 1/5 diluted 48 h sample from the Calu-3 experiment were included as positive controls in the MDDC/macrophage Western blots.
MERS-CoV infection results in induction of IFN-β, IFN-λ1, CXCL10 and MxA innate immune response genes
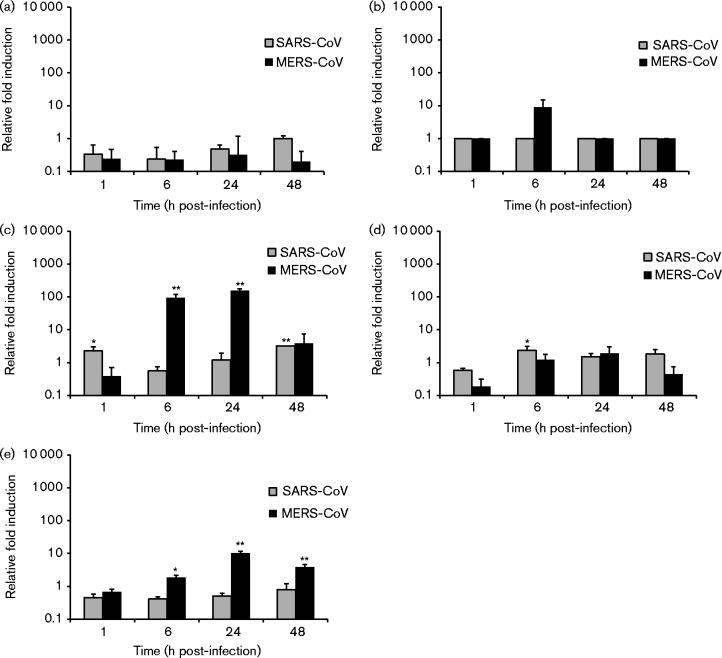
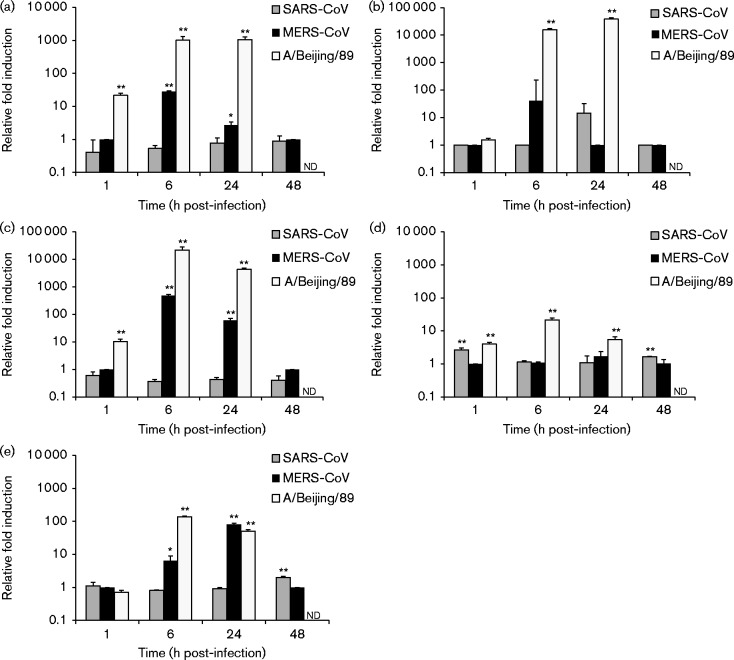
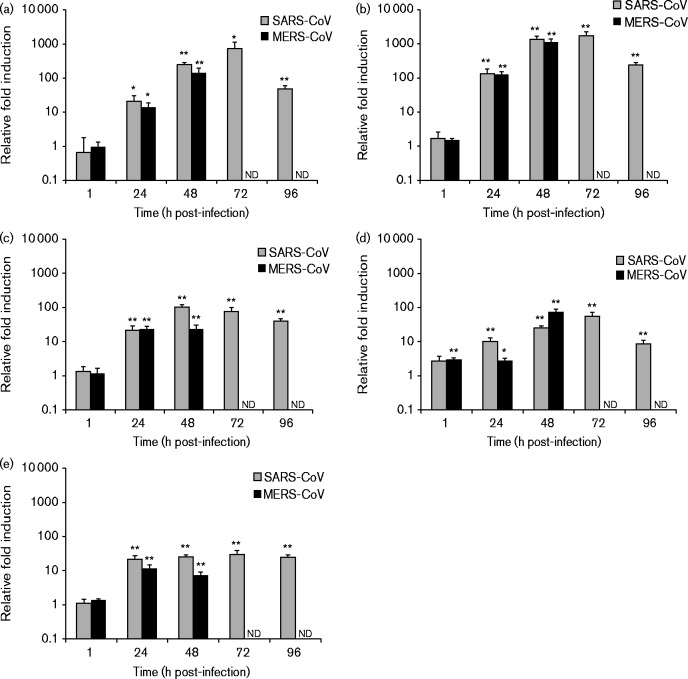
Next, we compared the ability of MERS-CoV and SARS-CoV to induce innate immune responses by analysing the expression levels of IFN-β, IFN-λ1, CXCL10, TNF-α and MxA mRNAs by qRT-PCR from cellular RNAs isolated from virus-infected cells. In macrophages, MERS-CoV infection resulted in increased levels of IFN-λ1 and CXCL10 mRNAs at the 6 h time point, and some induction of IFN-inducible MxA as well as a high induction of CXCL10 at the 24 h time point (Fig. 4). In SARS-CoV-infected macrophages, no induction of cytokine or MxA expression was observed (Fig. 4). In MERS-CoV-infected MDDCs, induction of CXCL10 and MxA was observed at the 6 and 24 h time points coupled with some induction of IFN-β and IFN-λ1 at the 6 h time point (Fig. 5). SARS-CoV infection in MDDCs led to a modest increase in the IFN-λ1 mRNA level at the 24 h time point (Fig. 5). In comparison with A/Beijing/89, the induction of innate immune response genes by MERS-CoV was weak with the exception of the MxA gene, which was induced to a similar level as seen in influenza A virus-infected cells at the 24 h time point. In Calu-3 cells, no significant difference between MERS-CoV and SARS-CoV was detected with both viruses causing a clear induction of all the tested cytokine and MxA genes (Fig. 6). In A549 cells, no induction of IFN-β, IFN-λ1, CXCL10, TNF-α or MxA mRNAs was seen in response to infection with either one of the CoVs (data not shown).
Fig. 4.
MERS-CoV- and SARS-CoV-induced cytokine and MxA gene expression in human macrophages. Human monocyte-derived macrophages were left uninfected or infected with MERS-CoV and SARS-CoV at m.o.i. 1. Cells were collected at 1, 6, 24 and 48 h after infection. Total cellular RNA was isolated, and the levels of (a) IFN-β, (b) IFN-λ1, (c) CXCL10, (d) TNF-α and (e) MxA mRNAs were determined by qRT-PCR. Values were normalized to 18S rRNA levels. Gene expression data are presented as relative fold induction of gene expression in relation to uninfected samples. *P < 0.05; **P < 0.01. Results (mean ± sd) are representative of two independent experiments carried out in cells obtained from three different blood donors.
Fig. 5.
MERS-CoV-, SARS-CoV- and influenza A virus-induced cytokine and MxA gene expression in human MDDCs. Human MDDCs were left uninfected or infected with MERS-CoV and SARS-CoV or influenza A virus strain A/Beijing/89 (H3N2) at m.o.i. 1. Cells were collected at 1, 6, 24 and 48 h after infection. Total cellular RNA was isolated, and the levels of (a) IFN-β, (b) IFN-λ1, (c) CXCL10, (d) TNF-α and (e) MxA mRNAs were determined by qRT-PCR. Values were normalized to 18S rRNA levels. Gene expression data are presented as relative fold induction of gene expression in relation to uninfected samples. *P < 0.05; **P < 0.01. nd, Not determined (time points where sufficient amounts of high-quality RNA could not be recovered due to extensive cytotoxicity caused by the viruses). Results (mean ± sd) are representative of two independent experiments carried out in cells obtained from three different blood donors.
Fig. 6.
MERS-CoV- and SARS-CoV-induced cytokine and MxA gene expression in human Calu-3 cells. Calu-3 cells were left uninfected or infected with MERS-CoV and SARS-CoV at m.o.i. 1. Cells were collected at 1, 24, 48, 72 and 96 h after infection. Total cellular RNA was isolated, and the levels of (a) IFN-β, (b) IFN-λ1, (c) CXCL10, (d) TNF-α and (e) MxA mRNAs were determined by qRT-PCR. Values were normalized to 18S rRNA levels. Gene expression data (mean ± SD of triplicate measurements) are presented as relative fold induction of gene expression in relation to uninfected samples *P < 0.05; **P < 0.01. nd, Not determined (time points where sufficient amounts of high-quality RNA could not be recovered due to extensive cytotoxicity caused by the viruses).
In support of the qRT-PCR data we performed ELISAs on supernatants from the macrophage, MDDC and Calu-3 infection experiments to determine whether increased cytokine gene expression could be seen at the protein level. Despite some expression at the mRNA level, analysis of IFN-λ1 and TNF-α showed no production of either cytokine in any cell type in response to MERS-CoV or SARS-CoV infection (data not shown). However, strong induction of CXCL10 mRNA by MERS-CoV in macrophages and MDDCs did correlate with high amounts of CXCL10 produced in some of the donors (Fig. 7). There was a large variation between different donors, as three of the six tested donors produced high levels of CXCL10 in both macrophages and MDDCs, whilst the other three showed no detectable CXCL10 production (Fig. 7). In Calu-3 cells, only modest CXCL10 production at the 48 h time point was observed in response to MERS-CoV (Fig. 7). SARS-CoV induced strong CXCL10 production in Calu-3 cells, but no production was detected in either macrophages or MDDCs (Fig. 7). Western blot analysis of MxA from cell lysates revealed high expression in MERS-CoV-infected macrophages and a more modest expression in MDDCs (Fig. 3). SARS-CoV infection resulted in MxA expression in macrophages at the 24 h time point, but no expression in MDDCs was detected (Fig. 3). In order to estimate the biological significance of the observed cytokine production, UV-inactivated supernatants from the infection experiments were used to prime A549 cells for 24 h followed by a 6 h infection with A/Beijing/89 virus (Fig. S1, available in the online Supplementary Material). Cells primed with macrophage and MDDC supernatants from MERS-CoV and A/Beijing/89 infection experiments were the only cells to show a statistically significant reduction in influenza A virus M1 RNA expression levels (Fig. S1).
Fig. 7.
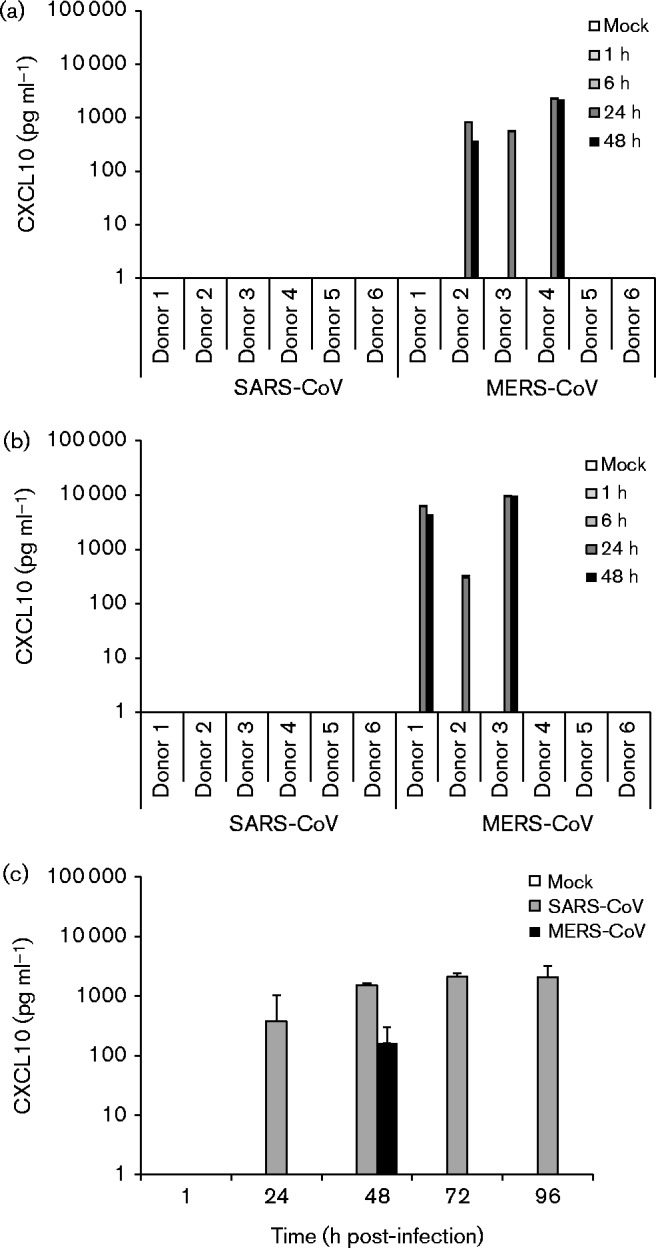
MERS-CoV- and SARS-CoV-induced CXCL10 production during infection. Supernatants collected from virus-infected (a) macrophages, (b) MDDCs and (c) Calu-3 cells at the indicated time points were analysed for CXCL10 concentration by ELISA. Six different donors were analysed for macrophages and MDDCs. Representative data (mean ± sd) from two independent experiments are shown for Calu-3 cells.
DPP4 is expressed in macrophages and MDDCs
Finally, a quantitative assay for MERS-CoV receptor DPP4 mRNA expression was set up to investigate whether differences in DPP4 expression would explain the observed differences in CoV replication. DPP4 was expressed at similar levels in macrophages and MDDCs, whereas approximately six- to eightfold higher expression levels (copy numbers) were seen in Calu-3 cells compared with macrophages and MDDCs (Fig. 8a). This observation is an unlikely explanation for differences in replication though, as the DPP4 copy number was two to three times lower in Vero E6 cells than in macrophages or MDDCs (Fig. 8a). Interestingly, almost no DPP4 mRNA expression was detected in A549 cells (Fig. 8a). To study the DDP4 protein expression in different cell types we carried out Western blot analysis for DPP4 protein expression using highly specific antibodies (Fig. 8b). As shown in Fig. 8(b), DPP4 was detected in all tested cell types, with A549 cells showing the lowest DPP4 expression levels.
Fig. 8.
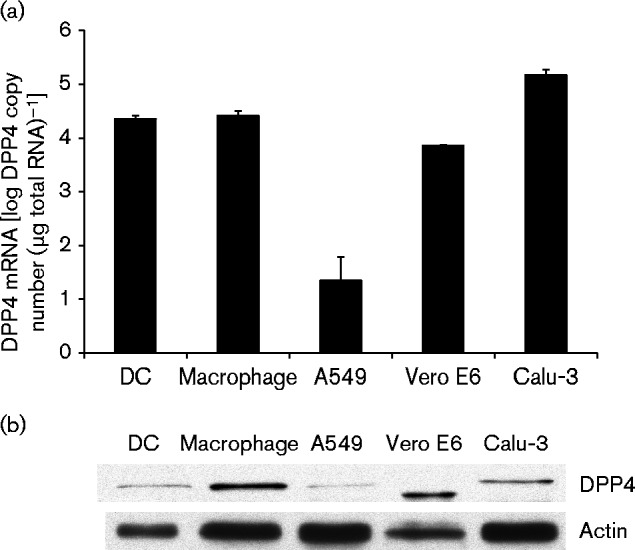
Quantification of DPP4 mRNA expression in different cell types. (a) Equal amounts of total cell RNA isolated from uninfected control samples from the infection experiments were used to quantify the level of DPP4 expression in our experimental settings by qRT-PCR. A standard curve was produced by serial dilution of plasmid pcDNA3.1(+)-human DPP4 and used to estimate DPP4 mRNA copy numbers for each sample. Data are presented as mean ± sd of triplicate measurements. (b) Cell lysates from uninfected control samples were analysed by Western blotting for the expression of DPP4 and actin.
Discussion
In the present study we investigated the characteristics of MERS-CoV infection in human monocyte-derived macrophages and MDDCs. Our results show that like SARS-CoV, MERS-CoV is unable to establish a productive infection in human macrophages and MDDCs in vitro, as evidenced by no significant increase in viral titres, RNA levels or expression of viral N protein during the infection. We did, however, observe an increase in IFN-β, IFN-λ1, CXCL10 and MxA mRNA expression, and in CXCL10 protein expression, in response to MERS-CoV infection. This increase was not seen with SARS-CoV, revealing a clear difference between the viruses. Calu-3 cells supported efficient growth of both viruses, and exhibited a strong expression of IFN-β, IFN-λ1, CXCL10 and MxA mRNAs, but only SARS-CoV induced strong CXCL10 protein expression.
An obvious explanation for the lack of MERS-CoV replication in macrophages and MDDCs would be the lack of MERS-CoV receptor DPP4 on the cell surface. DPP4 is ubiquitously expressed in many different tissues (Lambeir et al., 2003) and has been reported to be expressed on the cell surface of most monocyte-derived macrophages and MDDCs (Zhong et al., 2013). In order to verify the expression of DPP4 in our cell systems, we quantified DPP4 mRNA copy numbers in our samples by qRT-PCR and also analysed DPP4 protein levels in cell lysates (Fig. 8). We found that both macrophages and MDDCs readily expressed DPP4, with macrophages showing even higher DPP4 protein levels than Calu-3 cells (Fig. 8). However, low DPP4 expression correlated with the lack of MERS-CoV replication on A549 cells. Even though receptor availability is evidently not an issue, a block at a very early stage of macrophage/MDDC infection is suggested as no production of MERS-CoV or SARS-CoV virus RNA or proteins is seen. Another possible explanation for impaired internalization besides receptor availability is the deficiency in spike protein cleavage. CoV S protein is classified as a class I viral fusion protein and it needs to be cleaved for efficient internalization into a host cell (Bosch et al., 2003). This cleavage can occur either during the infection before budding or upon the entry of the virus into a new host cell. Several different host cell proteases have been reported to be involved in MERS-CoV S protein cleavage, including TMPRSS2 (Gierer et al., 2013), cathepsin L (Shirato et al., 2013) and furin (Burkard et al., 2014; Millet & Whittaker, 2014). Of these, overexpression of TMPRSS2 or furin has been shown to increase host cell susceptibility to MERS-CoV infection (Shirato et al., 2013; Millet & Whittaker, 2014). Shirato et al. (2013) reported that MERS-CoV can utilize different proteases for S protein cleavage in different cell types. Thus, the range of proteases expressed by a cell appears to be one of the determinants of host cell tropism, and could also be a factor in the lack of replication in macrophages and MDDCs. However, our stock viruses are expected to be highly infectious, as both Vero E6 and Calu-3 cells were effectively infected.
Our findings support the recent results of Scheuplein et al. (2015), but conflict with the observations of Zhou et al. (2014) and Chu et al. (2014) who showed that MERS-CoV can replicate weakly in monocyte-derived macrophages and MDDCs. This apparent discrepancy with our study may be related to differences in experimental conditions or the differentiation protocol used in different laboratories. Variability between studies involving macrophages and DCs is not uncommon. For instance, influenza A infection of macrophages and DCs can have varying results depending on virus strain, maturation state of the immune cells and cell subset being studied (Short et al., 2012). Individual variability may also play a role, as macrophages from different donors differ in their permissibility to human immunodeficiency virus infection (Bergamaschi & Pancino, 2010). This variability also became apparent in our own study, as different donors showed significant differences in their CXCL10 responses (Fig. 7). Therefore, some variability is to be expected when studying primary cells and the exact reason for certain discrepancies between different studies is difficult to identify.
Despite a lack of efficient replication, MERS-CoV infection was associated with some induction of IFN-β, IFN-λ1, CXCL10 and MxA mRNAs in macrophages (Fig. 4) and MDDCs (Fig. 5). Although the induction seems modest, as might be expected considering the lack of replication, this induction may still be significant. In our previous study we showed that even a modest increase in MxA expression induced by low amounts (1 IU ml− 1) of IFN-α/β was sufficient to trigger antiviral responses against influenza viruses (Osterlund et al., 2010). MERS-CoV-induced IFN production also proved to be modest, as we failed to detect any IFNs in infected cell supernatants, but it was still enough to cause strong expression of MxA, especially in macrophages (Fig. 3). The induction of CXCL10 mRNA correlated with abundant CXCL10 protein expression, but intriguingly striking differences between the donors were observed (Fig. 7). Significant individual variation in response to MERS-CoV has been previously reported in patients (Faure et al., 2014), but our data show that such variation can be evident in any blood cell donor. In accordance with strong CXCL10 and MxA expression, priming of A549 cells with supernatants from MERS-CoV-infected macrophages and MDDCs caused a clear reduction in A/Beijing/89 replication, indicating significant antiviral cytokine production (Fig. S1). Our results are mostly in accordance with studies by Zhou et al. (2014) and Chu et al. (2014) who also reported CXCL10 induction in MERS-CoV-infected macrophages and MDDCs, albeit to a somewhat lesser degree. Neither study analysed MxA gene expression and this is, to the best of our knowledge, the first study to report on MERS-CoV-induced MxA expression in macrophages and MDDCs. In Calu-3 cells, MERS-CoV infection resulted in only modest CXCL10 expression (Fig. 7) despite a strong induction of CXCL10, IFN-λ1 and TNF-α mRNAs (Fig. 6). SARS-CoV infection of Calu-3 cells resulted in significant CXCL10 production. However, no IFN-λ1 and TNF-α production was seen despite strong mRNA induction. This highlights the importance of verifying gene expression data at the protein level. Our results in Calu-3 cells are similar to previous studies where SARS-CoV was found to be a better inducer of innate immune responses than MERS-CoV (Lau et al., 2013). In addition to a small increase in IFN-λ1 mRNA levels at a later time point of infection in MDDCs, no mRNA induction or protein expression of any antiviral or proinflammatory mediators at any point of SARS-CoV infection was seen in macrophages or MDDCs (Figs. 4, 5 and 7). Also, no inhibitory effect on A/Beijing/89 replication was observed in the priming experiment (Fig. S1). These results highlight a difference between MERS-CoV and SARS-CoV, which might be explained by the different composition of accessory proteins exhibited by the viruses.
Both SARS-CoV and MERS-CoV encode several accessory proteins with proven IFN antagonistic properties. SARS-CoV ORF3b protein is a direct inhibitor of IFN-β induction (Spiegel et al., 2005), ORF3a protein suppresses IFN signalling through the activation of the PERK pathway (Minakshi et al., 2009) and ORF6 protein inhibits IFN signalling by interfering with STAT1 activation (Frieman et al., 2007; Kopecky-Bromberg et al., 2007). The structural M and N proteins and several non-structural proteins of SARS-CoV are also involved in blocking type I IFN production (Kopecky-Bromberg et al., 2007; Siu et al., 2009; DeDiego et al., 2014). MERS-CoV ORF4b protein has been shown to inhibit IFN signalling by an unknown mechanism (Yang et al., 2013; Matthews et al., 2014) and the ORF4a protein blocks IFN induction by interaction with dsRNA (Niemeyer et al., 2013; Siu et al., 2014). In addition, MERS-CoV NSP3, M and ORF5 proteins have been shown to have IFN antagonistic functions (Yang et al., 2013, 2014). This differing array of antagonistic proteins is a plausible explanation for the variation in host response, but other factors are also likely involved. The SARS-CoV receptor ACE2 is reportedly not expressed in MDDCs (Law et al., 2005) and our Western blot analysis failed to detect any SARS-CoV N protein at any point of infection in macrophages (Fig. 3), possibly indicating a failure in SARS-CoV virion adherence. Thus, inefficient entry of SARS-CoV virions into the leukocytes could also play a role in the observed difference in the host response in macrophages and MDDCs.
Our data indicate that even if the replication capacity of MERS-CoV is restricted in human leukocytes, it can induce a clearly detectable IFN-mediated antiviral response. This antiviral state might partly explain the clinical outcome of the MERS-CoV infection, where the severe infections are more restricted to patients with other underlying clinical conditions as compared with SARS-CoV infections. More detailed analysis of the possible capacity of MERS-CoV genetic material or proteins to activate or interfere with host innate immune system is clearly warranted.
Methods
Ethical standards
Our study complied with the current laws of Finland. Adult human blood used in the experiments was obtained from anonymous healthy blood donors through the Finnish Red Cross Blood Transfusion Service (permission no. 29/2014, renewed once a year).
Cells and viruses
MERS-CoV (GenBank accession number JX869059) and SARS-CoV (HKU-39849) were provided by the Erasmus Medical Center (Rotterdam, The Netherlands), and propagated in Vero E6 cells for two passages to obtain virus stocks for the experiments. The titres of MERS-CoV and SARS-CoV stocks were determined to be 1.5 × 106 and 2.5 × 107 TCID50 ml− 1, respectively, by end-point dilution assay. The propagation of CoVs and all experiments with them were carried out under strict Biosafety Level 3 conditions. Influenza A virus strain A/Beijing/89 (H3N2) was propagated in 11-day-old embryonated chicken eggs at 34 °C for 3 days. Macrophages and DCs were differentiated from monocytes derived from voluntary blood donors as previously described (Pirhonen et al., 1999; Osterlund et al., 2005) and identified as macrophages or DCs by their typical morphology. The cell populations generated with these methods have been characterized previously in our laboratory (Lehtonen et al., 2007). Macrophages were maintained in macrophage serum-free substitution medium (Gibco Invitrogen) supplemented with 0.6 μg penicillin ml− 1, 60 μg streptomycin ml− 1 and 10 ng human granulocyte–macrophage colony-stimulating factor (GM-CSF) ml− 1 (Biosource), and used at 7 days after cultivation. MDDCs were maintained in RPMI medium (Sigma-Aldrich) supplemented with 0.6 μg penicillin ml− 1, 60 μg streptomycin ml− 1, 2 mM l-glutamine, 20 mM HEPES, 10 % FCS (Sigma-Aldrich), 10 ng GM-CSF ml− 1 and 20 ng IL-4 ml− 1 (R&D Systems), and used at 6 days after cultivation. A549 (ATCC CCL-185), Vero (ATCC CCL-81), Vero E6 (ATCC CRL-1586) and Calu-3 (ATCC HTB-55) cells were maintained in Eagle's minimum essential medium (Invitrogen) supplemented with 0.6 μg penicillin ml− 1, 60 μg streptomycin ml− 1, 2 mM l-glutamine, 20 mM HEPES and 10 % FCS at 37 °C in 5 % CO2.
Infection experiments
Macrophages and A549 cells on 24-well plates were infected with MERS-CoV or SARS-CoV at m.o.i. 1 for up to 48 h with fresh medium changed at 1 h post-infection. MDDCs on 24-well plates were infected with MERS-CoV, SARS-CoV or A/Beijing/89 at m.o.i. 1 for up to 48 h. Three different donors were used for macrophages and MDDCs. Supernatant samples were collected at 1, 6, 24 and 48 h post-infection followed by PBS wash, lysing of the cells into either RLT-buffer or passive lysis buffer (Promega) and pooling of the lysates from different donors. Calu-3 and Vero E6 cells were infected similarly to A549 using m.o.i. 1 for Calu-3 and m.o.i. 0.1 for Vero E6 cells. Supernatant samples were taken at 1, 24, 48, 72 and 96 h post-infection followed by PBS wash, lysing of the cells into either RLT-buffer or passive lysis buffer and pooling of lysates from three wells.
RNA isolation and qRT-PCR
Total cellular RNA was isolated from the cell lysates using a RNeasy Mini kit (Qiagen) according to the manufacturer's instructions. RNase-Free DNase Set (Qiagen) was used for DNA digestion during isolation. A TaqMan Reverse Transcriptase kit (Life Technologies) with random hexamer primers was used to transcribe 1 μg RNA into cDNA. TaqMan Universal PCR Mastermix and Gene Expression Assays (Life Technologies) were used for the real-time PCR amplification of the cDNA with the Stratagene Mx3005P instrument (Stratagene). Previously described primers (Drosten et al., 2003; Corman et al., 2012) targeting the ORF1b region of SARS-CoV and MERS-CoV genomic RNA were used for assaying viral copy number, and commercially available primers (Life Technologies) were used for analysing the expression levels of IFN-β, IFN-λ1, CXCL10, TNF-α and MxA mRNAs. Fold inductions were calculated by normalizing C t values of each sample to their 18S rRNA levels (primers from Life Technologies) and then comparing them either to the 1 h sample (viral copy number) or to uninfected control samples collected at each time point (cytokines and MxA).
End-point dilution assay
For determining viral titres in MERS-CoV and SARS-CoV samples, Vero or Vero E6 cells, respectively, were used. A dilution series up to 10− 8 was made for each sample and used to infect cells on 96-well plates with eight infected wells for each dilution. After 3 days of incubation the wells were observed for cytopathic effect under a light microscope and scored positive or negative for virus infection. Results were calculated using the Spearman–Karber method and presented as TCID50 ml− 1.
Antibodies
Guinea pig antibody against SARS-CoV N protein has been described previously (Ziegler et al., 2005) and was used at a 1 : 2000 dilution. Polyclonal rabbit antibody against MERS-CoV N protein (1 : 1000 dilution) has been described previously (Adney et al., 2014). Commercial rabbit antibodies against MERS-CoV N protein (1 : 1000 dilution; Sino Biological), human DPP4 (1 : 1000 dilution; AbCam) and actin (1 : 500 dilution; Santa Cruz Biotechnology) were used according to the manufacturer's instructions. Rabbit antibody against MxA has been described previously (Ronni et al., 1993) and was used at a 1 : 500 dilution. As secondary antibodies, HRP-conjugated rabbit anti-guinea pig (1 : 1000 dilution; Dako) and goat anti-rabbit (1 : 2000 dilution; Dako) immunoglobulins were used.
Western blot analysis
Protein concentrations of cell lysates in passive lysis buffer were measured by the Bradford method, and equal amounts of proteins (10 μg of total protein per lane) were loaded and separated on 12 % SDS-PAGE. Proteins were transferred onto Immobilon P PVDF membranes, blocked with PBS+5 % milk powder for 40 min at room temperature, stained for 1 h at room temperature with antibodies against MERS-CoV N, SARS-CoV N and actin followed by staining with HRP-conjugated secondary antibodies for 1 h at room temperature. PBS+0.05 % Tween was used for washes between treatments and PBS+5 % milk was used as diluent for antibodies. Protein bands were visualized on HyperMax films using an ECL Plus System (GE Healthcare).
Quantification of DPP4 mRNA expression
For quantification of human DPP4 mRNA expression, we generated a dilution series of plasmid pcDNA3.1(+)-human DPP4 (van Doremalen et al., 2014). Samples containing 300 000, 30 000, 3000, 300 and 30 copies of the DPP4 gene were analysed together with samples from the infection experiments in qRT-PCR. Copy numbers for each sample were calculated from a standard curve produced by the dilution series. Commercially available primers were used in the detection of DPP4 (Bio-Rad).
ELISA
IFN-λ1 levels from cell supernatants were analysed using a VeriKine-DIY Human Interferon Lambda ELISA kit (PBL Interferon Source) according to the manufacturer's instructions. TNF-α and CXCL10 levels were analysed using antibodies provided by BD Pharmingen according to the manufacturer's instructions.
Statistical analysis
Statistical significances were calculated by using Student's t-test.
Acknowledgements
This work was supported by the Medical Research Council of the Academy of Finland (project numbers 252252 and 255780), the Sigrid Jusélius Foundation, Finnish Cultural Foundation, and Jenny and Antti Wihuri Foundation. The authors wish to thank Hanna Valtonen for expert technical assistance, Sinikka Latvala for help in preparing macrophages and MDDCs, and Susanna Sissonen for training and guidance in Biosafety Level 3 practices. We are indebted to Dr Ron A. M. Fouchier (Erasmus Medical Center, Rotterdam, the Netherlands) for providing us with the MERS-CoV and SARS-CoV viruses. V. J. M. is supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Supplementary Data
Supplementary Data
References
- Adney D. R., van Doremalen N., Brown V. R., Bushmaker T., Scott D., de Wit E., Bowen R. A., Munster V. J. (2014). Replication and shedding of MERS-CoV in upper respiratory tract of inoculated dromedary camels Emerg Infect Dis 20 1999–2005 10.3201/eid2012.141280 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azhar E. I., El-Kafrawy S. A., Farraj S. A., Hassan A. M., Al-Saeed M. S., Hashem A. M., Madani T. A. (2014). Evidence for camel-to-human transmission of MERS coronavirus N Engl J Med 370 2499–2505 10.1056/NEJMoa1401505 . [DOI] [PubMed] [Google Scholar]
- Bergamaschi A., Pancino G. (2010). Host hindrance to HIV-1 replication in monocytes and macrophages Retrovirology 7 31 10.1186/1742-4690-7-31 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch B. J., van der Zee R., de Haan C. A., Rottier P. J. (2003). The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex J Virol 77 8801–8811 10.1128/JVI.77.16.8801-8811.2003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkard C., Verheije M. H., Wicht O., van Kasteren S. I., van Kuppeveld F. J., Haagmans B. L., Pelkmans L., Rottier P. J., Bosch B. J., de Haan C. A. (2014). Coronavirus cell entry occurs through the endo-/lysosomal pathway in a proteolysis-dependent manner PLoS Pathog 10 e1004502 10.1371/journal.ppat.1004502 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J. F., Chan K. H., Choi G. K., To K. K., Tse H., Cai J. P., Yeung M. L., Cheng V. C., Chen H., other authors (2013). Differential cell line susceptibility to the emerging novel human betacoronavirus 2c EMC/2012: implications for disease pathogenesis and clinical manifestation J Infect Dis 207 1743–1752 10.1093/infdis/jit123 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng V. C. C., Lau S. K. P., Woo P. C. Y., Yuen K. Y. (2007). Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection Clin Microbiol Rev 20 660–694 10.1128/CMR.00023-07 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H., Zhou J., Wong B. H., Li C., Cheng Z. S., Lin X., Poon V. K., Sun T., Lau C. C., other authors (2014). Productive replication of Middle East respiratory syndrome coronavirus in monocyte-derived dendritic cells modulates innate immune response Virology 454–455 197–205 10.1016/j.virol.2014.02.018 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins A. R. (1998). Human macrophages are susceptible to coronavirus OC43 Adv Exp Med Biol 440 635–639 10.1007/978-1-4615-5331-1_82 . [DOI] [PubMed] [Google Scholar]
- Corman V. M., Eckerle I., Bleicker T., Zaki A., Landt O., Eschbach-Bludau M., van Boheemen S., Gopal R., Ballhause M. (2012). Detection of a novel human coronavirus by real-time reverse-transcription polymerase chain reaction Euro Surveill 17 3–8 . [DOI] [PubMed] [Google Scholar]
- Corman V. M., Ithete N. L., Richards L. R., Schoeman M. C., Preiser W., Drosten C., Drexler J. F. (2014). Rooting the phylogenetic tree of Middle East respiratory syndrome coronavirus by characterization of a conspecific virus from an African bat J Virol 88 11297–11303 10.1128/JVI.01498-14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot R. J., Baker S. C., Baric R. S., Brown C. S., Drosten C., Enjuanes L., Fouchier R. A. M., Galiano M., Gorbalenya A. E., other authors (2013). Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the Coronavirus Study Group J Virol 87 7790–7792 10.1128/JVI.01244-13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeDiego M. L., Nieto-Torres J. L., Jimenez-Guardeño J. M., Regla-Nava J. A., Castaño-Rodriguez C., Fernandez-Delgado R., Usera F., Enjuanes L. (2014). Coronavirus virulence genes with main focus on SARS-CoV envelope gene Virus Res 194 124–137 10.1016/j.virusres.2014.07.024 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C., Günther S., Preiser W., van der Werf S., Brodt H. R., Becker S., Rabenau H., Panning M., Kolesnikova L., other authors (2003). Identification of a novel coronavirus in patients with severe acute respiratory syndrome N Engl J Med 348 1967–1976 10.1056/NEJMoa030747 . [DOI] [PubMed] [Google Scholar]
- Faure E., Poissy J., Goffard A., Fournier C., Kipnis E., Titecat M., Bortolotti P., Martinez L., Dubucquoi S., other authors (2014). Distinct immune response in two MERS-CoV-infected patients: can we go from bench to bedside? PLoS One 9 e88716 10.1371/journal.pone.0088716 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieman M., Yount B., Heise M., Kopecky-Bromberg S. A., Palese P., Baric R. S. (2007). Severe acute respiratory syndrome coronavirus ORF6 antagonizes STAT1 function by sequestering nuclear import factors on the rough endoplasmic reticulum/Golgi membrane J Virol 81 9812–9824 10.1128/JVI.01012-07 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk C. J., Wang J., Ito Y., Travanty E. A., Voelker D. R., Holmes K. V., Mason R. J. (2012). Infection of human alveolar macrophages by human coronavirus strain 229E J Gen Virol 93 494–503 10.1099/vir.0.038414-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierer S., Bertram S., Kaup F., Wrensch F., Heurich A., Krämer-Kühl A., Welsch K., Winkler M., Meyer B., other authors (2013). The spike protein of the emerging betacoronavirus EMC uses a novel coronavirus receptor for entry, can be activated by TMPRSS2, and is targeted by neutralizing antibodies J Virol 87 5502–5511 10.1128/JVI.00128-13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haagmans B. L., Al Dhahiry S. H., Reusken C. B., Raj V. S., Galiano M., Myers R., Godeke G. J., Jonges M., Farag E., other authors (2014). Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation Lancet Infect Dis 14 140–145 10.1016/S1473-3099(13)70690-X . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui D. S., Memish Z. A., Zumla A. (2014). Severe acute respiratory syndrome vs. the Middle East respiratory syndrome Curr Opin Pulm Med 20 233–241 10.1097/MCP.0000000000000046 . [DOI] [PubMed] [Google Scholar]
- Ithete N. L., Stoffberg S., Corman V. M., Cottontail V. M., Richards L. R., Schoeman M. C., Drosten C., Drexler J. F., Preiser W. (2013). Close relative of human Middle East respiratory syndrome coronavirus in bat, South Africa Emerg Infect Dis 19 1697–1699 10.3201/eid1910.130946 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josset L., Menachery V. D., Gralinski L. E., Agnihothram S., Sova P., Carter V. S., Yount B. L., Graham R. L., Baric R. S., Katze M. G. (2013). Cell host response to infection with novel human coronavirus EMC predicts potential antivirals and important differences with SARS coronavirus MBio 4 e00165–15 10.1128/mBio.00165-13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecky-Bromberg S. A., Martínez-Sobrido L., Frieman M., Baric R. A., Palese P. (2007). Severe acute respiratory syndrome coronavirus open reading frame (ORF) 3b, ORF 6, and nucleocapsid proteins function as interferon antagonists J Virol 81 548–557 10.1128/JVI.01782-06 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf M., Schneider C., Nobs S. P. (2015). The development and function of lung-resident macrophages and dendritic cells Nat Immunol 16 36–44 10.1038/ni.3052 . [DOI] [PubMed] [Google Scholar]
- Lambeir A. M., Durinx C., Scharpé S., De Meester I. (2003). Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV Crit Rev Clin Lab Sci 40 209–294 10.1080/713609354 . [DOI] [PubMed] [Google Scholar]
- Lau S. K., Lau C. C., Chan K. H., Li C. P., Chen H., Jin D. Y., Chan J. F., Woo P. C., Yuen K. Y. (2013). Delayed induction of proinflammatory cytokines and suppression of innate antiviral response by the novel Middle East respiratory syndrome coronavirus: implications for pathogenesis and treatment J Gen Virol 94 2679–2690 10.1099/vir.0.055533-0 . [DOI] [PubMed] [Google Scholar]
- Law H. K., Cheung C. Y., Ng H. Y., Sia S. F., Chan Y. O., Luk W., Nicholls J. M., Peiris J. S., Lau Y. L. (2005). Chemokine up-regulation in SARS-coronavirus-infected, monocyte-derived human dendritic cells Blood 106 2366–2374 10.1182/blood-2004-10-4166 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtonen A., Ahlfors H., Veckman V., Miettinen M., Lahesmaa R., Julkunen I. (2007). Gene expression profiling during differentiation of human monocytes to macrophages or dendritic cells J Leukoc Biol 82 710–720 10.1189/jlb.0307194 . [DOI] [PubMed] [Google Scholar]
- Li W., Moore M. J., Vasilieva N., Sui J., Wong S. K., Berne M. A., Somasundaran M., Sullivan J. L., Luzuriaga K., other authors (2003). Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus Nature 426 450–454 10.1038/nature02145 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews K. L., Coleman C. M., van der Meer Y., Snijder E. J., Frieman M. B. (2014). The ORF4b-encoded accessory proteins of Middle East respiratory syndrome coronavirus and two related bat coronaviruses localize to the nucleus and inhibit innate immune signalling J Gen Virol 95 874–882 10.1099/vir.0.062059-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memish Z. A., Mishra N., Olival K. J., Fagbo S. F., Kapoor V., Epstein J. H., Alhakeem R., Durosinloun A., Al Asmari M., other authors (2013). Middle East respiratory syndrome coronavirus in bats, Saudi Arabia Emerg Infect Dis 19 1819–1823 10.3201/eid1911.131172 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B., Müller M. A., Corman V. M., Reusken C. B., Ritz D., Godeke G. J., Lattwein E., Kallies S., Siemens A., other authors (2014). Antibodies against MERS coronavirus in dromedary camels, United Arab Emirates, 2003 and 2013 Emerg Infect Dis 20 552–559 10.3201/eid2004.131746 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet J. K., Whittaker G. R. (2014). Host cell entry of Middle East respiratory syndrome coronavirus after two-step, furin-mediated activation of the spike protein Proc Natl Acad Sci U S A 111 15214–15219 10.1073/pnas.1407087111 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minakshi R., Padhan K., Rani M., Khan N., Ahmad F., Jameel S. (2009). The SARS Coronavirus 3a protein causes endoplasmic reticulum stress and induces ligand-independent downregulation of the type 1 interferon receptor PLoS One 4 e8342 10.1371/journal.pone.0008342 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moltedo B., Li W., Yount J. S., Moran T. M. (2011). Unique type I interferon responses determine the functional fate of migratory lung dendritic cells during influenza virus infection PLoS Pathog 7 e1002345 10.1371/journal.ppat.1002345 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeyer D., Zillinger T., Muth D., Zielecki F., Horvath G., Suliman T., Barchet W., Weber F., Drosten C., Müller M. A. (2013). Middle East respiratory syndrome coronavirus accessory protein 4a is a type I interferon antagonist J Virol 87 12489–12495 10.1128/JVI.01845-13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund P., Veckman V., Sirén J., Klucher K. M., Hiscott J., Matikainen S., Julkunen I. (2005). Gene expression and antiviral activity of alpha/beta interferons and interleukin-29 in virus-infected human myeloid dendritic cells J Virol 79 9608–9617 10.1128/JVI.79.15.9608-9617.2005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund P., Pirhonen J., Ikonen N., Rönkkö E., Strengell M., Mäkelä S. M., Broman M., Hamming O. J., Hartmann R., other authors (2010). Pandemic H1N1 2009 influenza A virus induces weak cytokine responses in human macrophages and dendritic cells and is highly sensitive to the antiviral actions of interferons J Virol 84 1414–1422 10.1128/JVI.01619-09 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirhonen J., Sareneva T., Kurimoto M., Julkunen I., Matikainen S. (1999). Virus infection activates IL-1 beta and IL-18 production in human macrophages by a caspase-1-dependent pathway J Immunol 162 7322–7329 . [PubMed] [Google Scholar]
- Poissy J., Goffard A., Parmentier-Decrucq E., Favory R., Kauv M., Kipnis E., Mathieu D., Guery B., MERS-CoV Biology Group (2014). Kinetics and pattern of viral excretion in biological specimens of two MERS-CoV cases J Clin Virol 61 275–278 10.1016/j.jcv.2014.07.002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj V. S., Mou H., Smits S. L., Dekkers D. H. W., Müller M. A., Dijkman R., Muth D., Demmers J. A. A., Zaki A., other authors (2013). Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC Nature 495 251–254 10.1038/nature12005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj V. S., Farag E. A., Reusken C. B., Lamers M. M., Pas S. D., Voermans J., Smits S. L., Osterhaus A. D., Al-Mawlawi N., other authors (2014). Isolation of MERS coronavirus from a dromedary camel, Qatar, 2014 Emerg Infect Dis 20 1339–1342 10.3201/eid2008.140663 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusken C. B., Haagmans B. L., Müller M. A., Gutierrez C., Godeke G. J., Meyer B., Muth D., Raj V. S., Smits-De Vries L., other authors (2013). Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: a comparative serological study Lancet Infect Dis 13 859–866 10.1016/S1473-3099(13)70164-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusken C.B.E.M., Messadi L., Feyisa A., Ularamu H., Godeke G. J., Danmarwa A., Dawo F., Jemli M., Melaku S., other authors (2014). Geographic distribution of MERS coronavirus among dromedary camels, Africa Emerg Infect Dis 20 1370–1374 10.3201/eid2008.140590 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronni T., Melén K., Malygin A., Julkunen I. (1993). Control of IFN-inducible MxA gene expression in human cells J Immunol 150 1715–1726 . [PubMed] [Google Scholar]
- Scheuplein V. A., Seifried J., Malczyk A. H., Miller L., Höcker L., Vergara-Alert J., Dolnik O., Zielecki F., Becker B., other authors (2015). High secretion of interferons by human plasmacytoid dendritic cells upon recognition of Middle East respiratory syndrome coronavirus J Virol 89 3859–3869 10.1128/JVI.03607-14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirato K., Kawase M., Matsuyama S. (2013). Middle East respiratory syndrome coronavirus infection mediated by the transmembrane serine protease TMPRSS2 J Virol 87 12552–12561 10.1128/JVI.01890-13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short K. R., Brooks A. G., Reading P. C., Londrigan S. L. (2012). The fate of influenza A virus after infection of human macrophages and dendritic cells J Gen Virol 93 2315–2325 10.1099/vir.0.045021-0 . [DOI] [PubMed] [Google Scholar]
- Siu K. L., Kok K. H., Ng M. H., Poon V. K., Yuen K. Y., Zheng B. J., Jin D. Y. (2009). Severe acute respiratory syndrome coronavirus M protein inhibits type I interferon production by impeding the formation of TRAF3·TANK·TBK1/IKKepsilon complex J Biol Chem 284 16202–16209 10.1074/jbc.M109.008227 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu K. L., Yeung M. L., Kok K. H., Yuen K. S., Kew C., Lui P. Y., Chan C. P., Tse H., Woo P. C., other authors (2014). Middle East respiratory syndrome coronavirus 4a protein is a double-stranded RNA-binding protein that suppresses PACT-induced activation of RIG-I and MDA5 in the innate antiviral response J Virol 88 4866–4876 10.1128/JVI.03649-13 . [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Spiegel M., Pichlmair A., Martínez-Sobrido L., Cros J., García-Sastre A., Haller O., Weber F. (2005). Inhibition of beta interferon induction by severe acute respiratory syndrome coronavirus suggests a two-step model for activation of interferon regulatory factor 3 J Virol 79 2079–2086 10.1128/JVI.79.4.2079-2086.2005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Miazgowicz K. L., Milne-Price S., Bushmaker T., Robertson S., Scott D., Kinne J., McLellan J. S., Zhu J., Munster V. J. (2014). Host species restriction of Middle East respiratory syndrome coronavirus through its receptor, dipeptidyl peptidase 4 J Virol 88 9220–9232 10.1128/JVI.00676-14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Qi J., Yuan Y., Xuan Y., Han P., Wan Y., Ji W., Li Y., Wu Y., other authors (2014). Bat origins of MERS-CoV supported by bat coronavirus HKU4 usage of human receptor CD26 Cell Host Microbe 16 328–337 10.1016/j.chom.2014.08.009 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Zhang L., Geng H., Deng Y., Huang B., Guo Y., Zhao Z., Tan W. (2013). The structural and accessory proteins M, ORF 4a, ORF 4b, and ORF 5 of Middle East respiratory syndrome coronavirus (MERS-CoV) are potent interferon antagonists Protein Cell 4 951–961 10.1007/s13238-013-3096-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Chen X., Bian G., Tu J., Xing Y., Wang Y., Chen Z. (2014). Proteolytic processing, deubiquitinase and interferon antagonist activities of Middle East respiratory syndrome coronavirus papain-like protease J Gen Virol 95 614–626 10.1099/vir.0.059014-0 . [DOI] [PubMed] [Google Scholar]
- Zaki A. M., van Boheemen S., Bestebroer T. M., Osterhaus A.D.M.E., Fouchier R. A. M. (2012). Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia N Engl J Med 367 1814–1820 10.1056/NEJMoa1211721 . [DOI] [PubMed] [Google Scholar]
- Zhong J., Rao X., Deiuliis J., Braunstein Z., Narula V., Hazey J., Mikami D., Needleman B., Satoskar A. R., Rajagopalan S. (2013). A potential role for dendritic cell/macrophage-expressing DPP4 in obesity-induced visceral inflammation Diabetes 62 149–157 10.2337/db12-0230 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Chu H., Li C., Wong B. H., Cheng Z. S., Poon V. K., Sun T., Lau C. C., Wong K. K., other authors (2014). Active replication of Middle East respiratory syndrome coronavirus and aberrant induction of inflammatory cytokines and chemokines in human macrophages: implications for pathogenesis J Infect Dis 209 1331–1342 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler T., Matikainen S., Rönkkö E., Osterlund P., Sillanpää M., Sirén J., Fagerlund R., Immonen M., Melén K., Julkunen I. (2005). Severe acute respiratory syndrome coronavirus fails to activate cytokine-mediated innate immune responses in cultured human monocyte-derived dendritic cells J Virol 79 13800–13805 10.1128/JVI.79.21.13800-13805.2005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielecki F., Weber M., Eickmann M., Spiegelberg L., Zaki A. M., Matrosovich M., Becker S., Weber F. (2013). Human cell tropism and innate immune system interactions of human respiratory coronavirus EMC compared to those of severe acute respiratory syndrome coronavirus J Virol 87 5300–5304 10.1128/JVI.03496-12 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data