Abstract
Objective. This review aimed to investigate the relationship between varying levels of enteral protein intake and growth in preterm infants, regardless of feeding method. Data Sources. Electronic databases were searched for relevant studies, as were review articles, reference lists, and text books. Study Selection. Trials were included if they were randomized or quasirandomized, participants were <37 weeks gestation at birth, and protein intakes were intentionally or statistically different between study groups. Trials reporting weight, length, and head circumference gains in infants fed formula, human milk, or fortified human milk were included. Data Extraction. Studies were categorized by feeding-type and relevant data were extracted into summary tables by one reviewer and cross-checked by a second. Data Synthesis. A meta-analysis could not be conducted due to extensive variability among studies; thus, results were synthesized graphically and narratively. Twenty-four trials met the inclusion criteria and were included in a narrative synthesis and 19 in a graphical synthesis of study results. Conclusions. There was extensive variability in study design, participant characteristics, and study quality. Nonetheless, results are fairly consistent that higher protein intake results in increased growth with graphical representation indicating a potentially linear relationship. Additionally, intakes as high as 4.5 g/kg/day were shown to be safe in infants weighing >1000 g.
Keywords: infant, premature, human milk, dietary proteins, growth
The incidence of preterm births has increased in developed countries over the past decade, and due to technological advances, the survival rate of marginally viable infants has also increased.1,2 Feeding these very small infants is a challenge. Those infants born as early as 22 weeks gestation spend the entirety of the last trimester of pregnancy outside the intrauterine environment.1,2 To match intrauterine growth, very low birth weight (<1500 g) infants have high nutritional requirements.3 However, the immaturity of their organ systems can limit the safety of providing high nutrient intakes.2 Preterm infants experience postnatal growth delay, with the resulting growth deficit often not recovered during hospital admission.4 Clinical studies comparing growth curves of preterm infants with those of infants in utero show a higher proportion of preterm infants small for gestational age (weight <10th percentile) at discharge.4-6 The neonatal admission period is increasingly being shown to be the critical time for neurodevelopment.7-9 Early nutritional practices, specifically increased protein intake, and improved short-term growth outcomes during this time have been associated with beneficial long-term growth and neurodevelopment.7-9
Current opinion suggests the aim of feeding preterm infants is to replicate the growth and body composition seen in utero.3,10 Parenteral nutrition is initiated within the first hour and enteral nutrition within the first days of life, with an aim to achieve full enteral feeding as soon as is clinically possible.3 Both infant formulas and human milk (HM) are used in enteral feeding. As HM has inadequate energy, protein, and bone minerals to support optimal growth in preterm infants weighing <2000 g, the use of human milk fortifiers (HMFs) is standard clinical practice.3
The quantity of dietary protein required to enable optimal growth in preterm infants remains a contentious issue. Recommendations for protein intake vary between key bodies (Table 1) and have been revised up over the last decade. Early research with protein intakes of 6.0 to 7.0 g/kg/day resulted in metabolic acidosis, uremia, and hyperaminoacidaemia;11 however, the protein was of poor quality, and recent reviews suggest this may no longer apply to current practice.2,12
Table 1.
Current Nutrient Recommendations for Enteral Feeding Preterm Infants.
| Birth Weight | Protein Intake (g/kg/day) | Energy Intake (kcal/kg/day) | |
|---|---|---|---|
| American Academy of Pediatrics10 | 800-1200 g | 4.0 | 105-130 |
| 1200-1800 g | 3.5 | 105-130 | |
| Canadian Pediatric Society13 | <1000 g | 3.5-4.0 | 105-135 |
| >1000 g | 3.0-3.6 | 105-135 | |
| Tsang et al,14 USA, “growing”—clinically stable and gaining weight | ELBW | 3.8-4.4 | 130-150 |
| VLBW | 3.4-4.2 | 110-130 | |
| European Society for Paediatric Gastroenterology, Hepatology and Nutrition3 | <1000 g | 4.0-4.5 | 110-135 |
| 1000-1800 g | 3.5-4.0 | 110-135 |
Abbreviations: ELBW, extremely low birth weight (<1000 g); VLBW, very low birth weight (<1500 g).
Cochrane systematic reviews of growth in “high” versus “low” protein formula fed infants12 and infants fed fortified versus unfortified HM have been published.15 The former12 concluded infants receiving formula with higher protein content had improved weight gain. The review compared “high” (3.0-4.0 g/kg/day) with “low” (<3.0 g/kg/day) protein intakes and excluded trials where comparison groups fell within the same range. In a review comparing infants receiving fortified versus unfortified HM, Kuschel and Harding15 found improved weight, length, and head circumference (HC) growth. However, the review included trials comparing non-isocaloric feeds, thereby making it difficult to separate the effects of protein and energy. Additionally, neither of these Cochrane reviews included studies published since 1995; therefore, an updated review including the most recent research is required. Randomized controlled trials (RCTs) comparing the effects of HMFs with different protein concentrations on growth have shown inconsistent findings. Additionally, many neonatal units use mixed feeding and provide preterm formula to infants when the mother’s milk supply is not adequate. A comprehensive systematic review investigating increased protein and growth including all feeding methods and reflecting the mixed feeding approach in neonatal units is yet to be published.
The objective of this review is to investigate the relationship between enteral protein intake and growth in preterm infants.
Methods
Types of Studies
Randomized or quasi-randomized controlled trials were considered for inclusion in this review.
Types of Participants, Interventions, and Outcome Measures
Trials that included preterm infants with birth weight less than 2.5 kg were included in this review. Trials that compared varying protein intakes in formula, unfortified, or fortified HM fed infants were included. Trials primarily investigating parenteral nutrition and quality of enteral protein intake were beyond the scope of this review. To investigate the relationship between protein intake and growth independent of energy, only studies that held energy constant between groups were included. Similarly, only studies that provided infants with adequate energy to allow protein to be used for tissue accretion (ie, >100 kcal/kg)14 were included. Trials that reported outcomes of weight gain, length gain, or HC gain were included. The inclusion and exclusion criteria are summarized in Table 2. In trials with >2 groups, any groups not meeting the review criteria were excluded from analysis.
Table 2.
Inclusion and Exclusion Criteria for Literature Searches.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| • Gestational age at birth <37 weeks | • Protein intakes not reported |
| • Birth weight <2500 g | • Studies investigating differences in parenteral feeding solutions |
| • Protein intakes intentionally different between 2 or more groups | • Energy difference >10% relative composition or shown to be statistically significantly different |
| • Reports comparison of change between groups in any or all of the following: weight, length, head circumference | • Energy intake of any group <100 kcal/kg |
| • Protein intakes between 2 or more groups are shown to be not statistically significantly different |
Search Method and Data Extraction
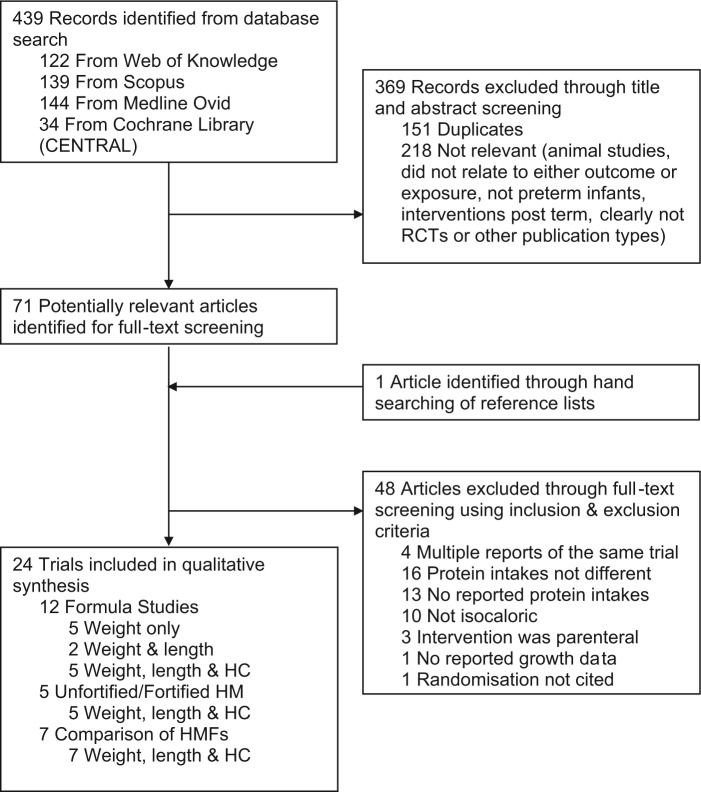
Computerized searches were conducted up to March 30, 2013. Databases, search terms, and filters used are summarized in Figure 1, and in addition the clinical trials registers, “clinicaltrials.gov,” and Australian New Zealand clinical trials registry were searched for trials in progress. A combination of MeSH terms (infant, newborn; infant, premature; infant, low birth weight; human milk; dietary proteins; infant food; growth) and keywords (preterm; neonate; breast milk; protein) were utilized in searches. English language filters were applied; however, no limits were placed on year of study. Hand-searching of reference lists was conducted and review articles and text books were used to identify further relevant studies. Studies were screened for relevance according to the selection criteria (Table 2). Studies were categorized by feed-type to facilitate comparison between studies with somewhat similar protein quality, and relevant data were extracted into summary tables by one reviewer and cross-checked by a second. A meta-analysis could not be conducted due to extensive variability among studies; thus, results have been synthesized graphically and narratively.
Figure 1.
Flow diagram of search methods.
Methodological Quality
Trials were evaluated for risk of bias according to the Academy of Nutrition and Dietetics Quality Criteria Checklist for primary research.16 Briefly, this assesses trials for relevance to practice and scientific rigour.16 Individual trials were assessed against quality criteria specific for RCTs, with “Yes” or “No” being assigned to each criterion, or “Unclear” if the study report lacked adequate detail for assessment. A summary outcome of “Positive,” “Negative,” or “Neutral” is produced.
Results
The search strategy yielded 439 titles; 71 full-text articles were reviewed (Figure 1). Forty-eight of these were excluded. Characteristics of excluded studies are summarized in Figure 1. Twenty-four trials met the inclusion criteria for this review. Twelve trials compared the growth of infants fed formula with varying protein intakes; 5 compared infants fed unfortified HM with protein fortified HM, and 7 trials compared infants fed different HMFs resulting in varying protein intakes. All studies were published between May 1976 and October 2012. Trials involving formula-fed infants have been carried out throughout this entire period. Conversely, trials assessing the adequacy of unfortified HM were conducted between 1985 and 1990, after which time it was thought to be unethical to conduct these comparisons, and those comparing HMFs or fortification methods have occurred since then (1995-2012). Characteristics of included studies are summarized in Tables 3, 4 and 5.
Table 3.
Data Summary of Trials Comparing Growth in Infants Fed Isocaloric Formulas With Varying Protein Content.
| Study | Study Description |
Outcomes and Results |
Quality | |||
|---|---|---|---|---|---|---|
| Participants | Intervention | Intakes | Growth | |||
| Costa-Orvay et al17 (2011), Spain | EN n = 38 | BW < 1500 g | Alprem | Study period, average | Study end, mean (SD) | Positive |
| DO n = 4 | GA ≤ 32 wk | Group A excluded | Energy, kcal/kg/d | Weight, g; Group B: 1998 (146), Group C: 2154 (202), P = .62 | ||
| SS: on enteral nutrition, IV ceased | AGA | Not isocaloric | Group B:150, Group C: 150, P N/D | Length, cm; Group B: 44.5 (1.2), Group C: 45.6 (1.8), P N/D | ||
| SE: 28 days from study start | Healthy infants | Group B (n = 12): | Protein, g/kg/d | HC, cm; Group B: 32.3 (1.2), Group C: 32.3 (0.8), P = .74 | ||
| Promod 0.7 g/kg/d | Group B: 4.2, Group C: 4.7, P N/D | |||||
| Duocal 3.7 g/kg/d | ||||||
| Group C (n = 12): | ||||||
| Promod 1.3 g/kg/d | ||||||
| Duocal 3.3 g/kg/d | ||||||
| Cooke et al18 (2006), United Kingdom | EN n = 18 | BW ≤ 1500 g | RegPro: | Days 1-14, mean (SD) | Study period, mean (SD) | Neutral |
| DO n = 0 | GA ≤ 32 wk | Protein 3.0 g/100 kcal | Energy, kcal/kg/d; HiPro: ~120*, RegPro: ~120*, P N/D | Δ Weight, g/d; HiPro: 35 (9), RegPro: 27 (6), P = .005 | ||
| SS: N/D | Clinically stable | HiPro: | Protein, g/kg/d; HiPro: 4.6 (0.4), RegPro: 3.8 (0.2), P < .001 | Δ Weight, g/kg/d; HiPro: 23 (7), RegPro: 17 (6), P N/D | ||
| SE: 14 days after start (7 days crossover) | No steroids, diuretics, or oxygen therapy at balance study | Protein 3.6 g/100 kcal | *Calculated from target volume of 150 mL/kg/d | |||
| Titrated using CHO | ||||||
| Vit and min content same | ||||||
| Embleton and Cooke19 (2005), United Kingdom | EN n = 77 | BW ≤ 1750 g | Group A (n = 25): | Study period, mean (SD) | Enrolment–discharge, mean (SD) | Positive |
| DO n = 3 | GA ≤ 34 wk | Protein 3.3 g/100 kcal | Energy, kcal/kg/d; Group A: 131 (23), Group B: 125 (23), Group C: 129 (25), P N/D | Δ Weight, g/d; Group A: 42 (7), Group B: 37 (6), Group C: 40 (7), P > .05 | ||
| SS: full enteral feeds (150 mL/kg/d) | Intake ≥ 150 mL/kg/d for ≥48 h | Group B (n = 26): | Protein, g/kg/d; A > B by 0.5, P < .001. and B > C by 0.2, P < .05 | Δ Length, cm/wk; Group A: 1.3 (0.3), Group B: 1.2 (0.3), Group C: 1.3 (0.3), P > .05 | ||
| SE: term + 12 weeks corrected age | Healthy infants | Protein 3.0 g/100 kcal | ||||
| -Weight≥1000g | Group C (n = 26): | |||||
| Protein 2.7 g/100 kcal | ||||||
| Vit and min content same | ||||||
| Titrated using fat | ||||||
| Wauben et al20 (1995), Netherlands | EN n = 16 | BW: AGA | F1.5 (n = 8): | Days 4-7, mean (SD) | Study days 1-8, mean (SD) | Neutral |
| DO n = 0 | GA: 28-35 wk | Energy 68 kcal/100 mL | Energy, kcal/kg/d; F1.5: 118 (9), F2.0: 117 (11), P > .05 | Δ Weight, g/kg/d; F1.5: 12 (3), F2.0: 16 (4), P < .05 | ||
| SS: enteral volume 160 mL/kg/d | Healthy infants | Protein 1.5 g/100 mL | Protein, g/kg/d; F1.5: 2.7 (0.3), F2.0: 3.4 (0.3), P < .05 | |||
| SE: 8 days | F2.0 (n = 8): | |||||
| Energy 70 kcal/100 mL | ||||||
| Protein 2.0 g/100 mL | ||||||
| Vit and min content same | ||||||
| Hillman et al21 (1994), United States | EN n = 32 | BW: <1500 g | Group A (n = 9): | Study period, average | Birth to 30 days, mean (SD) | Neutral |
| DO n = 5 | GA: N/D | Protein 3.0 g/100 kcal | Energy, kcal/kg/d; Groups A, B, and C: Aim 120, P N/D | Δ Weight, g/kg/d; Group A*: 19 (4), Group B: 16 (3), Group C*: 13 (5), *P < .05 | ||
| SS: N/D | Not receiving TPN or diuretics | Group B (n = 9): | Protein, g/kg/d; Group A: 3.6, Group B: 3.2, Group C: 2.8, P N/D | Gain in HC and length not different (data N/D) | ||
| SE: 30 days | Protein 2.7 g/100 kcal | |||||
| Group C (n = 9): | ||||||
| Protein 2.2 g/100 kcal | ||||||
| Vit and min content same | ||||||
| Titrated using CHO | ||||||
| Bhatia et al22 (1991), United States | EN n = 26 | BW: <1550 g | High (n = 8): | Day 1 to study end, mean (SD) | Day 1 to study end, mean (SD) | Neutral |
| DO n = 3 | GA: N/D | Protein 3.0 g/100 kcal | Energy, kcal/kg/d; Low: 117 (4), Mid: 120 (3), High: 118 (6), P N/D | Δ Weight, g/kg/d; Low: 19 (1), Mid: 20 (3), High: 21 (2), P > .05 | ||
| SS: when enteral energy intake reached 100 kcal/kg/d | Enteral feeds by 14 days age | Mid (n = 8): | Protein, g/kg/d; Low: 2.6 (0.1), Mid: 3.1 (0.1), High: 3.8 (0.2), P N/D | Gain in HC and length not different (data N/D) | ||
| SE: 2 weeks from study day 1 | Energy intake 100 kcal/kg/d by 21 days age | Protein 2.7 g/100 kcal | ||||
| Healthy infants | Low (n = 7): | |||||
| Protein 2.2 g/100 kcal | ||||||
| Vit and min content same | ||||||
| Titrated using CHO | ||||||
| Kashyap et al23 (1988), United States | EN n = 50 | BW: 900-1750 g | Group 1 (n = 16): | Study period, mean (SD) | Study period, mean (SD) | Neutral |
| DO n = 6 | GA: N/D | Protein 1.6 g/100 mL | Energy, kcal/kg/d; Group 1: 119 (2), Group 2: 120 (2), P N/D | Δ Weight, g/kg/d; Group 1: 16 (2), Group 2: 19 (3), P < .05 | ||
| SS: intake 180 mLkg/d | Healthy infants | Energy 66 kcal/100 mL | Protein, g/kg/d; Group 1: 2.8 (<0.1), Group 2: 3.8 (<0.1), P N/D | Δ Length, cm/wk; Group 1: 1.0 (0.2), Group 2: 1.2 (0.3), P > .05 | ||
| SE: until infant weight 2200 g (average duration of study N/D) | Group 2 (n = 16): | Δ HC, cm/wk; Group 1: 1.0 (0.1), Group 2: 1.2 (0.3), P > .05 | ||||
| Protein 2.1 g/100 mL | ||||||
| Energy 67 kcal/100 mL | ||||||
| Minimal vit and min diff | ||||||
| Titrated with fat and CHO | ||||||
| Group 3 excluded | ||||||
| Not isocaloric | ||||||
| Bell et al24 (1986), Ireland | EN n = 75 (10 HM enrolled separately) | BW: <1800 g | Group A excluded: Increased energy intake compared with B and C (P < .05) | Study period, mean (SD) | Study period, mean (SD) | Neutral |
| DO n = 2 | GA: N/D | Group B (n = 25): | Energy, kcal/kg/d; Group B: 128 (14), Group C: 128 (15), HM: 127 (21), P NS | Δ Weight, g/kg/d; Group B*: 19 (4), Group C*: 16 (4), HM: 16 (5), *P < .05 | ||
| SS: enteral intake 150 mL/kg/day and IV ceased | Gender: both | Protein 2.4 g/100 mL | Protein, g/kg/d; Group B: 3.9 (0.4), Group C: 3.6 (0.5), HM: 2.6 (0.3), P < .001 | Δ Length, cm/wk; Group B: 1.4 (0.7), Group C: 1.5 (0.5), HM: 1.1 (0.4), P > .05 | ||
| SE: weight >2000 g (average duration of study N/D) | Healthy infants | Energy 79 kcal/100 mL | Δ OFC, cm/wk; Group B: 1.1 (0.3), Group C: 1.1 (0.3), HM: 1.1 (0.2), P > .05 | |||
| Group C (n = 25): | ||||||
| Protein 2.1 g/100 mL | ||||||
| Energy 74 kcal/100 mL | ||||||
| HM (n = 10): | ||||||
| Protein 1.5 g/100 mL | ||||||
| Energy 70 kcal/100 mL | ||||||
| Titrated with fat and CHO | ||||||
| Kashyap et al25 (1986), United States | EN n = 34 | GA: 27-37 wk | Group 1 (n = 11): | Study period, mean (SD) | Study period, mean (SD) | Neutral |
| DO n = 7 | BW: 900-1750 g | Protein 1.3 g/100 mL | Energy, kcal/kg/d; Group 1: 115 (1), Group 2: 114 (1), P N/D | Δ Weight, g/kg/d; Group 1: 14 (3), Group 2: 18 (3), P < .05 | ||
| SS: intake reached 180 mL/kg/d | Healthy infants | Energy 63 kcal/100 mL | Protein, g/kg/d; Group 1: 2.2 (0.0), Group 2: 3.6 (0.0), P N/D | Δ Length, cm/wk; Group 1: 0.9 (0.2), Group 2: 1.2 (0.3), P > .05 | ||
| SE: weight 2200 g | Group 2 (n = 11): | Δ HC, cm/wk; Group 1: 0.9 (0.2), Group 2: 1.2 (0.3), P < .05 | ||||
| Protein 2.0 g/100 mL | ||||||
| Energy 63 kcal/100 mL | ||||||
| Vit and min content same | ||||||
| Titrated with fat and CHO | ||||||
| Group 3 excluded | ||||||
| Not isocaloric | ||||||
| Darling et al26 (1985), Canada | EN n = 15 | BW: 1300-1600 g | Group 1 (n = 5): | Study period, mean (SEM) | Study period, mean (SEM) | Neutral |
| DO n = N/D | GA: N/D | Protein 1.9 g/100 mL | Energy, kcal/kg/d; Group 1: 149 (9), Group 2: 153 (6), Group 3: 147 (7), P NS | Δ Weight, g/d; Group 1*: 36 (3), Group 2*: 29 (2), Group 3: 30 (3),*P = .03 | ||
| SS: at initiation of enteral feeding | AGA | Energy 72 kcal/100 mL | Protein, g/kg/d; Group 1: 4.3 (0.2), Group 2*: 3.5 (0.1), Group 3: 4.4 (0.2), *P = .03 compared with groups 1 and 3 | Δ Length, cm/wk; Group 1*: 1.1 (0.1), Group 2*: 0.8 (0.0), Group 3: 0.9 (0.0), *P < .01 | ||
| SE: discharge at 2200 g, feeding continued on same formula until 3 months | No hemolytic disease | Whey–casein 60:40 | Δ OFC, cm/wk; Group 1: 0.8 (0.0), Group 2: 0.7 (0.0), Group 3: 0.8 (0.0), P < .05 | |||
| No hyaline membrane disease | Group 2 (n = 5): | |||||
| No notable respiratory distress | Protein 1.5 g/100 mL | |||||
| Energy 70 kcal/100 mL | ||||||
| Whey–casein 20:80 | ||||||
| Group 3 (n = 5): | ||||||
| Protein 2.0 g/100 mL | ||||||
| Energy 74 kcal/100 mL | ||||||
| Titrated with fat and CHO | ||||||
| Vit and min content same | ||||||
| Svenningsen et al27 (1982), Sweden | EN n = 48 | Mean BW: 1385 ± 343 g | HM-Group (n = 18): | 3-7 weeks age, average | 3-7 weeks age, mean (SD) | Negative |
| DO n = N/D | Mean GA: 30.8 ± 2.9 wk | Protein 1.6 g/100 kcal | Energy, kcal/kg/d; HM-Group: 116, F1-Group: 117, F2-Group: 118, P N/D | Δ Weight, g/kg/d; HM-Group: 13 (3), F1-Group: 13 (4), F2-Group: 14 (4), P NS | ||
| SS: 3rd week life | Infants with respiratory distress, septicemia were included | F1-Group (n = 14): | Protein, g/kg/d; HM-Group: 1.9, F1-Group: 2.5, F2-Group: 3.2, P N/D | Δ Length, cm/wk; HM-Group: 1.0 (N/D), F1-Group: 1.0 (N/D), F2-Group: 1.0 (N/D), P NS | ||
| SE: 7th week life | Protein 2.3 g/100 kcal | |||||
| F2-Group (n = 16): | ||||||
| Protein 3.0 g/100 kcal | ||||||
| Titration N/D | ||||||
| Raiha et al28 (1976), Finland | EN n = 106 | BW ≤ 2100 g | F1 (n = 21): | Study period, average | Regained birthweight to study end, mean (SEM) (g/wk divided by 7, Time 3 shown) | Neutral |
| DO n = 7 | GA: 28-36 wk | Protein 1.5 g/100 mL | Energy, kcal/kg/d; HM: 114, F1: 118, F2: 116, P N/D | Δ Weight, g/d; HM*: 22 (2), F1*: 27 (1), F2: 26 (2), *P NS | ||
| SS: feedings started before 24 hours life | AGA | Whey–casein 60:40 | Protein, g/kg/d; HM: 1.6, F1: 2.3, F2: 4.5, P N/D | No significant difference between any groups in mean rate of HC gain | ||
| S | SE: weight 2400 g (>28 days) | Healthy infants | F2 (n = 20): | Data N/D | ||
| Protein 3.0 g/100 mL | ||||||
| Whey–casein 60:40 | ||||||
| F3 and F4 excluded | ||||||
| Differed from F1 and F2 in protein quality only | ||||||
| Pooled HM (n = 22): | ||||||
| Pooled banked milk | ||||||
| Similar vit and mins | ||||||
| Titrated using lactose | ||||||
Abbreviations: AAs, amino acids; AGA, appropriate for gestational age; BW, birth weight; CHO, carbohydrate; DO, drop outs, that is, not included in growth outcomes; EN, enrolled; GA, gestational age; HC, head circumference; HM, human milk; LBW, low birth weight; MCT, medium-chain triglycerides; Na, sodium; NS, nonsignificant; N/D, not described; OFC, occipito-frontal circumference; PTF, preterm formula; SGA, small for gestational age; SD, standard deviation; SE, study end; SEM, standard error of the mean; SS, study start; TPN, total parenteral nutrition; VLBW, very low birth weight.
Table 4.
Data Summary of Trials Comparing Growth in Infants Fed Unsupplemented HM With Those Fed Protein-Supplemented HM.
| Study | Study Description |
Outcomes and Results |
Quality | |||
|---|---|---|---|---|---|---|
| Participants | Intervention | Intakes | Growth | |||
| Kashyap et al29 (1990), United States | EN n = 66 | BW: 900-1750 g | Group 1 (n = 14): | Study period, mean (SD) | Study period, mean (SD) | Neutral |
| DO n = 24 | GA: N/D | Mothers HM | Energy, kcal/kg/d; Group 1: 129 (11), Group 2: 131 (12), P N/D | Δ Weight, g/kg/d; Group 1: 17 (2), Group 2: 21 (2), P < .01 | ||
| CP n = 27 | SGA and AGA infants included | Group 2 (n = 13): | Protein, g/kg/d; Group 1: 2.5 (0.5), Group 2: 3.2 (0.4), P N/D | Δ Length, cm/wk; Group 1: 0.9 (0.2), Group 2: 1.3 (0.5), P NS | ||
| SS: enteral feedings 180 mL/kg/d | Healthy infants | Mothers HM + protein (1.1 g/kg/d), Ca (3.7 mmol/kg/d), P (2.1 mmol/kg/d), Na (1.1 mmol/kg/d) | Δ HC, cm/wk; Group 1: 1.0 (0.2), Group 2: 1.2 (0.2), P NS | |||
| SE: infant weight 2200 g | Reports groups 1 and 2 isocaloric due to variation in composition of HM | |||||
| Group 3 excluded | ||||||
| Not isocaloric | ||||||
| Target volume: 180 mL/kg/d | ||||||
| HM composition: daily samples pooled for weekly analysis | ||||||
| Polberger et al30 (1989), Sweden | EN n = 34 | BW: <1500 g | HM excluded | Study period, mean (SD) | Study period, mean (SD) | Neutral |
| DO n = 6 | GA: N/D | Not isocaloric | Energy, kcal/kg/d; HMF: 121 (10) | Δ Weight, g/kg/d; HMF: 16 (2), HMP: 20 (1), P N/D | ||
| CP n= 5 (mothers milk), n = 10 (little mothers milk) | AGA | HM + HMF (n = 8): | HMP: 117 (9), P N/D | Δ Length, cm/wk; HMF: 0.9 (0.2), HMP: 1.3 (0.1), P N/D | ||
| SS: stable on 170 mL/kg/d | Tolerance of complete enteral feeding | HM with human milk fat (1 g/100 mL) | Protein, g/kg/d; HMF: 2.1 (0.3) | Δ HC, cm/wk; HMF: 1.1 (0.2), HMP: 1.2 (0.1), P N/D | ||
| SE: 2200 g or breastfeeding initiated | Healthy infants | HM + HMP (n = 9): | HMP: 3.6 (0.2), P N/D | |||
| HM with human milk protein (1 g/100 mL) | ||||||
| HM + HMPF excluded | ||||||
| Not isocaloric | ||||||
| Groups HM + HMP and HM + HMF isocaloric due to addition of fat | ||||||
| Target volume: 170 mL/kg/d | ||||||
| HM composition: daily aliquot of milk pooled for weekly analysis | ||||||
| Greer and McCormick31 (1988), United States | EN n = 38 | BW: <1600 g | HM (n = 10): Mothers HM | First 6 weeks enteral feeds, mean (SD) | First 6 weeks enteral feeds, mean (SD) | Neutral |
| DO n = N/D | GA: <32 weeks | FHM (n = 10): | Energy, kcal/kg/d; HM: 112 (10), FHM: 105 (15), P NS | Δ Weight, g/kg/d; HM: 13 (1), FHM: 17 (2), P < .01 | ||
| CP n= N/D | Healthy infants | Mothers HM + protein (0.9 g/100 mL), Ca (90 mg/100 mL), P (45 mg/100 mL) | Protein, g/kg/d; HM: 3.3 (0.6), FHM: 4.2 (0.5), P < .01 | Δ Length, cm/wk; HM: 0.8 (0.2), FHM: 1.1 (0.2), P < .01 | ||
| SS: full oral feedings achieved (120 kcal/kg/d) | AGA | HM groups similar energy intake due to higher volume feeds in HM group | Δ HC, cm/wk; HM: 0.8 (0.2), FHM: 1.1 (0.2), P < .02 | |||
| SE: 6 weeks from study start | Formula groups excluded | |||||
| Not isocaloric | ||||||
| Target volume: 120-200 mL/kg/d | ||||||
| HM composition: 5% daily aliquots of feeds pooled for weekly analysis | ||||||
| Putet et al32 (1987) | EN n = 16 | BW: <1500 g | HM (n = 8): Pooled HM | Study period (3 days), mean (SD) | 7 days overlapping balance study, mean (SD) | Neutral |
| DO n = 0 | GA: N/D | HM-Pr (n = 8): | Energy, kcal/kg/d; HM: 107 (7), HM-Pr: 106 (14), P > .05 | Δ Weight, g/kg/d; HM: 15 (3), HM-Pr: 17 (2), P > .05 | ||
| CP n = 16 | Gender: male | Pooled HM + 1 g sup/100 mL, providing (/100 g powder): | Protein*, g/kg/d; HM: 2.5 (0.4), HM-Pr: 3.8 (0.5), P < .01 | Δ Length, cm/wk; HM: 1.1 (0.3), HM-Pr: 1.2 (0.3), P > .05 | ||
| SS: N/D | Healthy infants | Nitrogen: 13.2 g, lipid: 1.4 g, Ca: 2.5 g, P: 1.1 g, Na: 8.0 mg | *Calculated as total Nitrogen × 6.25 | Δ HC, cm/wk; HM: 1.0 (0.1), HM-Pr: 1.2 (0.2), P > .05 | ||
| SE: 7 days after study start | Isocaloric due to higher volume feeds of HM group | |||||
| Target volume: N/D | ||||||
| HM composition: one aliquot taken from entire pool of milk for study | ||||||
| Ronnholm et al33 (1986) | EN n = 54 | BW: <1500 g | HM (n = 23): | 2 weeks of age, mean (SEM) | Weeks 1-6, mean (SEM) | Neutral |
| DO n = 10 | GA: ≤36 wk | Unsupplemented HM | Energy, kcal/kg/d; HM: 111 (3), HM-Pr: 110 (4), P N/D | Δ Weight, g/kg/d; HM: 10 (1), HM-Pr: 13 (1), P < .01 | ||
| CP n = 44 | SGA and AGA | HM-Pr (n = 21): | Protein, g/kg/d; HM: 1.8 (0.1), HM-Pr: 3.2 (0.2), P N/D | Δ Length, cm/wk; HM: 0.8 (0.1), HM-Pr: 1.0 (0.1), P = .04 | ||
| SS: N/D | Healthy infants | HM + HM protein (0.9 g/100 mL of milk) | 6 weeks of age, mean (SEM) | Δ HC, cm/wk; HM: 0.6 (0.0), HM-Pr: 0.7 (0.0), P = .13 | ||
| SE: N/D | All infants fed either pooled banked mature HM or mothers HM | Energy, kcal/kg/d; HM: 130 (3), HM-Pr: 133 (2), P N/D | ||||
| Reason for similarity of energy intake between groups N/D | Protein, g/kg/d; HM: 1.9 (0.0), HM-Pr: 3.7 (0.1), P N/D | |||||
| Target volume: 200 mL/kg/d | ||||||
| HM composition: 5 mL samples taken at beginning and end of each milking | ||||||
Abbreviations: AGA, appropriate for gestational age; BW, birth weight; Ca; calcium; CP, completed study protocol; DO, dropouts, that is, not included in growth outcomes; EN, enrolled; GA, gestational age; HC, head circumference; HM, human milk; LBW, low birth weight; Na, sodium; NS, nonsignificant; N/D, not described; OFC, occipito-frontal circumference; P, phosphorous; SGA, small for gestational age; SD, standard deviation; SEM, standard error of the mean; SE, study end; SS, study start; VLBW, very low birth weight.
Table 5.
Data Summary of Trials Comparing Growth in Infants Fed HM Fortified With HMFs With Varying Protein Content.
| Study | Study Description |
Outcomes and Results |
Quality | |||
|---|---|---|---|---|---|---|
| Participants | Intervention | Intakes | Growth | |||
| Miller et al34 (2012), Australia | EN n = 92 | GA: <31 wk | Higher protein (HP) (n = 43): | Study weeks 1-4, median (IQR) | Enrolment to study end, median (IQR) | Positive |
| DO n = 0 | BW: N/D | 1.4 g protein/100 mL | Energy, kcal/kg/d; HP: 137 (119-149), SP: 137 (122-150), P N/D | Δ Weight, g/d; HP: 24 (20-28), SP: 26 (24-28), P = .33 | ||
| CP n = 59 (64%) | Both healthy and unwell infants | Standard protein (SP) (Control) (n = 49): | Protein, g/kg/d; HP: 4.2 (3.6-4.7), SP: 3.6 (3.2-4.0), P N/D | Δ Length, cm/wk; HP: 1.2 (1.1-1.2), SP: 1.1 (1.1-1.1), P = .08 | ||
| SS: enteral intake ~80 mL/kg/day | SGA and AGA infants | 1.0 g protein/100 mL | Δ HC, cm/wk; HP: 0.9 (0.9-1.0), SP: 1.0 (0.9-1.0), P = .56 | |||
| SE: discharge, estimated due date | Titrated with CHO | |||||
| Minimal diff in vits and mins | ||||||
| Unfortified HM composition | ||||||
| Weekly sample | ||||||
| Brumberg et al35 (2010), United States | EN n = 23 | GA: N/D | FHM + P/E (n = 11): | Study weeks 1-4, mean (SD) | Study period, mean (SD) | Neutral |
| DO n = 3 | BW: ≤1250 g | ¼ teaspoon/30 mL fluid | Energy, kcal/kg/d; P/E: 128 (11), MCT: 124 (9), P > .05 | Δ Weight, g/kg/d; P/E: 17 (2), MCT: 12 (5), P < .01 | ||
| CP (all 4 weeks), n = 13 | Postnatal age ≥14 days | 0.3 g protein/100 mL | Protein, g/kg/d; P/E: 3.5 (0.3), MCT: 3.0 (0.5), P < .05 | Δ Length, cm/wk; P/E: 1.1 (0.4), MCT: 0.8 (0.3), P > .05 | ||
| SE: 28 days | Diet ≥75% ENT | FHM + MCT (n = 12): | Δ HC, cm/wk; P/E: 1.1 (0.3), MCT: 0.8 (0.4), P < .05 | |||
| Failure to regain BWOR weight gain <15 g/kg/d after BW regained | 2 mL/kg/d | |||||
| Otherwise healthy infants | 0 g protein | |||||
| HM and Formula fed infants randomized to P/E or MCT | ||||||
| HM composition | ||||||
| Assumed values of 68 kcal and 1.0 g protein/100 mL | ||||||
| Arslanoglu et al36 (2006), Italy | EN n = 36 | BW: 600-1750 g | ADJ fortification (n = 17): | Week 2, mean (SD) | Study period, mean (SD) | Positive |
| DO n = 2 | GA: 24-34 weeks | If BUN 9-14 mg/dL, no adjustment; <9 mg/dL, increase 1 level; >14 mg/dL, decrease 1 level | Energy, kcal/kg/d; ADJ: 126 (12), STD: 127 (12), P > .05 | Δ Weight, g/kg/d; ADJ: 18 (3), STD: 14 (3), P < .01 | ||
| CP n = 36 | Enteral intake 90 mL/kg/d | Levels (g/100 mL): 0 = standard, 1 = 6.25 fortifier, 2 = 6.25 HMF + 0.4 pro | Protein, g/kg/d; ADJ: 3.2 (0.4), STD: 2.9 (0.3), P = .05 | Δ Length, cm/wk; ADJ: 1.3 (0.5), STD: 1.1 (0.4), P > .05 | ||
| SS: feed volume 150 mL/kg/day | Singletons only | STD fortification (n = 17): | Week 3, mean (SD) | Δ HC, cm/wk; ADJ: 1.4 (0.3), STD: 1.0 (0.3), P < .05 | ||
| SE: weight 2000 g | Healthy infants | 5 g HMF/100 mL | Energy, kcal/kg/d; ADJ: 128 (8), STD: 121 (8), P > .05 | |||
| Fortified HM composition: twice weekly sample | Protein, g/kg/d; ADJ: 3.4 (0.5), STD: 2.8 (0.2), P < .05 | |||||
| Same HMF (0.8 g protein/100 mL) | ||||||
| Berseth et al37 (2004), Canada, United States | EN n = 185 | GA: ≤33 wk | Trial HMF (HMF-T) (n = 96): | Study period, mean (SE) | Study period, mean (SE) | Neutral |
| DO n = 4 | BW: ≤1500 g | Protein 1.1 g/100 mL | Energy*, kcal/kg/d; HMF-T: 118 (2), HMF-C: 115 (2), P = .07 | Δ Weight, g/kg/d; HMF-T: 18 (1), HMF-C: 17 (1), P = .63 | ||
| CP n = 94 (51%) | Enteral intake >100 mL/kg/d | Control HMF (HMF-C) (n = 85): | Protein, g/kg/d; HMF-T: 3.8 (0.1), HMF-C: 3.6 (0.1), P < .01 | |||
| SS: enteral intake >100 mL/kg/d | Healthy infants | Protein 1.0 g/100 mL | *Calculated (kJ divided by 4.187) | |||
| SE: study day 28 or discharge | Had consumed no HMF | Titrated with CHO and fat | ||||
| Did not receive EPO, VD, minerals, or Fe on study day 0 | Not equivalent in vits and mins | |||||
| HM composition | ||||||
| Assumed values of 66 kcal and 1.0 g protein/100 mL | ||||||
| Reis et al38 (2000), United States | EN n = 144 | GA: ≤33 wk | Study fortifier (SF) (n = 74): | Study period, mean (SD) | Study period, mean (SD) | Neutral |
| DO n = 25 | BW: ≤1600 g | Protein 0.9 g/100 mL | Energy, kcal/kg/d; SF: 118 (13), CF: 118 (16), P > .05 | Δ Weight, g/kg/d; SF: 18 (4), CF: 15 (3), P < .01 | ||
| CP n = 89 | Healthy infants | Contains MCT oil | Protein, g/kg/d; SF: 3.5 (0.4), CF: 3.1 (0.5), P < .01 | Δ Length, cm/wk; SF: 1.1 (0.3), CF: 1.0 (0.4), P = .03 | ||
| SS: full strength fortification and enteral intake 100 mL/kg/d | Control fortifier (CF) (n = 70): | Δ HC, cm/wk; SF: 1.0 (0.2), CF: 0.9 (0.3), P = .07 | ||||
| SE: study day 29 or discharge | Protein 0.6 g/100 mL | |||||
| No MCT oil | ||||||
| Titrated with CHO and fat | ||||||
| Not equivalent in vits and mins | ||||||
| HM composition | ||||||
| Assumed values of 67 kcal and 1.4 g protein/100 mL | ||||||
| Porcelli et al39 (2000), United States | EN n = 90 | GA: 25-32 wk | New HMF (n = 47): | Study period, estimated (SD) | Study period, mean (SEM) | Neutral |
| DO n = 28 | BW: 600-1500 g | Protein 1 g/100 mL | Energy*, kcal/kg/d; New HMF: 115 (20), Std HMF: 125 (16), P N/D | Δ Weight, g/kg/d; New HMF: 20 (1), Std HMF: 17 (1), P = .04 | ||
| CP n = 64 | AGA | Energy 13 kcal/100 mL | Protein, g/kg/d; New HMF: 4.3, Std HMF: 4.2, P N/D | Δ Length, cm/wk; New HMF: 0.9 (0.1), Std HMF: 0.8 (0.1), P > .05 | ||
| SS: HMF introduced | Enteral intake >150 mL/kg/d HM | Standard HMF (n = 43): | *Calculated using energy of HM 67 kcal/100 mL and reported adjusted intakes (mL/kg/d) | Δ OFC, cm/wk; New HMF: 1.0 (0.1), Std HMF: 0.8 (0.1), P = .04 | ||
| SE: only consuming unsupplemented HM | Medically stable | Protein 0.7 g/100 mL | ||||
| Not receiving PN, formula, diuretics, or corticosteroids | Energy 14 kcal/100 mL | |||||
| Mother’s milk >14 days postpartum | Fat and protein higher in New HMF | |||||
| Not equivalent in vits and mins | ||||||
| HM composition | ||||||
| Assumed values of 67-72 kcal and 1.8 g protein/100 mL | ||||||
| Moro et al40 (1995), Italy | EN n = 42 | GA: N/D | Same HMF (0.8 g protein/100 mL HM) | Week 2, mean (SD) | Study period, mean (SD) | Neutral |
| DO n = 6 | BW: 900-1500 g | ADJ fortification (n = 17): | Energy, kcal/kg/d; ADJ: 125 (7), FIX: 119 (7), P > .05 | Δ Weight, g/kg/d; ADJ: 19 (2), FIX: 18 (2), P > .05 | ||
| CP n = 36 | Healthy infants | If CSUN 6.1-9.0 mg/100 mL, add 4.1 g fortifier/100 mL; 9.1-12.0 mg/100 mL, no adjustment; 12.1-15 mg/100 mL, add 2.9 g fortifier/100 mL | Protein, g/kg/d; ADJ: 4.0 (0.5), FIX: 3.5 (0.3), P < .01 | Δ Length, cm/wk*; ADJ: 0.9 (0.3), FIX: 1.0 (0.4), P > .05 | ||
| SS: feeding volume 160 mL/kg/d | Fixed (FIX) fortification (n = 17): | Week 3, mean (SD) | Δ HC, cm/wk*; ADJ: 0.9 (0.3), FIX: 0.9 (0.3), P > .05 | |||
| SE: discharge at ~2200 g | 3.5 g HMF/100 mL HM | Energy, kcal/kg/d; ADJ: 120 (7), FIX: 117 (7), P > .05 | *Calculated from mm/d | |||
| HMP group excluded | Protein, g/kg/d; ADJ: 3.7 (0.3), FIX: 3.4 (0.4), P > .05 | |||||
| Protein intake shown to be nonsignificantly different | ||||||
| Fortified HM composition daily sample forming weekly pools analyzed | ||||||
Abbreviations: ADJ, adjustable; AGA, appropriate for gestational age; BUN, blood urea nitrogen; BW, birth weight; CHO, carbohydrate; CP, completed study protocol; CSUN, corrected serum urea nitrogen; DO, dropouts, that is, not included in growth outcomes; EN, enrolled; ENT, enteral nutrition; EPO, erythropoietin; Fe, iron; FHM, fortified human milk; FIX, fixed; GA, gestational age; HC, head circumference; HM, human milk; HMF, human milk fortifier; IQR, interquartile range; MCT, medium-chain triglyceride; N/D, not described; OFC, occipito-frontal circumference; P/E, protein and energy; PN, parenteral nutrition; SD, standard deviation; SE, study end; SEM, standard error of the mean; SGA, small for gestational age; STD, standard; SS, study start; VD, vitamin D; VLBW, very low birth weight.
Trials Comparing Groups of Formula-Fed Infants
Summary of Studies
Twelve of the included trials compared the growth of infants fed formula with varying protein intakes (Table 3).17-28 Protein intakes ranged from 1.6 g/kg/day to 4.7 g/kg/day (Table 3). Five trials found no statistically significant differences between groups for any growth outcomes.17,19,22,27,28 Cooke et al18 and Darling et al26 found that infants with increased protein intakes (in both studies an additional 0.8 g/kg/day) had a greater rate of daily weight gain compared to controls (8 and 7 g/day greater than the control group, respectively). Five further studies showed higher protein intake groups had greater rates of fractional weight gain compared with controls (3-6 g/kg/day greater than controls).20,21,23-25 Kashyap et al25 and Darling et al26 found increased rate of HC growth in infants with higher protein intakes (0.4 and 0.1 cm/week, respectively, more than controls). Darling et al26 demonstrated increased growth in the higher protein intake group for all outcome measures (weight and HC reported above, additional 0.2 cm/week length gain; P < .01). Three trials also included a reference group of HM-fed infants and compared their growth with that of formula-fed infants.24,27,28 Bell et al24 and Svenningsen et al27 found no statistically significant difference in any outcome measures between the HM- and formula-fed groups, while Raiha et al28 found significantly increased weight gain in formula-fed infants compared with HM-fed controls (+5 g/day, P < .05).
Critique of Studies
Random sequence generation and allocation concealment were typically poorly reported in trials finding an effect compared with those showing no effect. Conversely, 5 of these trials used a standard operating procedure for anthropometric measurements, thus ensuring consistency and accuracy,20,23-26 compared with no clear description of measurement methods in all trials showing no effect.18,19,21,22,27,28 Furthermore, only 2 trials conducted an intention-to-treat analysis.18,19 The study duration (>28 days) was a strength of 6 trials.17,19,21,26-28 Longer trial duration limits the effect of daily fluid fluctuations on weight gain, enabling meaningful changes in length and HC to be observed. The difference in sodium content of the formula between comparison groups is a limitation of the trials by Cooke et al18 and Bell et al.24 The change in weight seen in these trials may have been due to the influence of sodium on fluid balance rather than tissue growth. Supporting this, neither trial showed a significant difference in length or HC gain (Table 3). The small sample sizes of the trials by Costa-Orvay et al17 and Bhatia et al22 may have limited their ability to show a significant difference between groups, as both trials showed a trend toward increased growth in infants with higher protein intakes. The trials by Costa-Orvay et al17 and Embleton and Cooke19 did not reach the required sample size, thus making them vulnerable to Type II error (see Supplementary Table 1, available online at http://gph.sagepub.com/supplemental).
Few trials showed significant improvements in multiple outcome measures, limiting the consistency of this evidence. Many of the trials showing significantly increased weight gain in higher-protein intake groups did show a trend for increased rates of growth in length and HC but failed to reach significance.23-25 It may be that these trials were underpowered to detect statistically significant differences in these growth measures as they are more variable than weight. Nine studies did not report a power calculation, and all trials that did based their sample size on expected effect size of other outcomes such as nitrogen or fat-free mass accretion.
Given the clinical heterogeneity among the trials, it is difficult to draw robust conclusions from this evidence. The maturity and size of the infants studied varied between trials. Reasonably mature infants were studied overall (range = 1130-1958 g). This limits the generalizability of this evidence to very immature infants (<1000 g). The selection criteria varied widely between trials also, with some including infants with intrauterine growth failure or those small for gestational age, while others excluded these infants. However, the clinical stability of infants was relatively uniform. Almost all studies described their sample as “healthy” or “clinically stable” (Table 3). Only one trial21 did not exclude infants with respiratory distress or on oxygen/ventilator support. Again, this limits the generalizability of this evidence to infants who experience multiple medical issues associated with premature birth.41 A further difficulty encountered when comparing these studies is the variation in method for calculating rate of growth gain. Different calculation methods have been shown to produce varying results, with some more accurate than others.42 However, many studies simply did not report their method for calculating growth rate.
The variance in effect size seen may reflect other key differences between the trials. The difference in protein intake between comparison groups ranged from 0.2 g/kg/day19 to 2.3 g/kg/day.28 Nine trials compared groups with less than 1 g/kg difference in intake (Table 3). Thus, differences in protein intake between comparison groups may have been too small to show the possible effect of increased protein intake in some trials. Differences in the composition of trial formulas and quality of protein may further contribute to statistical heterogeneity. Additionally, the medical management of infants also likely varied between trials, as these trials were conducted steadily over a period of 35 years and standards of care in neonatal intensive care units continue to improve.
These trials provide some evidence that increased enteral protein intake (intakes between 3.5 and 4.5 g/kg/day) results in increased weight gain of 3 to 6 g/kg/day in formula-fed infants, but little evidence suggesting increased length or HC growth.
Trials Comparing Infants Fed Unfortified HM With Those Fed Protein-Fortified HM
Summary of Studies
Five trials compared infants fed unfortified HM with those fed protein-fortified HM.29-33 These trials achieved similarity in energy intake between groups through increased volume31,32 or fat30 of unfortified HM feeds, or natural variation in composition of HM.29 All trials showed a trend toward increased weight, length, and HC in infants fed protein-fortified HM compared with unfortified HM (Table 4). A statistically significantly greater increment of weight gain in infants fed higher protein intakes was shown in 3 trials (range of 3-4 g/kg/day greater than controls).29,31,33 Two of these also showed significantly increased length growth in infants with higher protein intakes (0.2 and 0.4 cm/week more than controls)31,33 and one significantly increased HC growth (0.3 cm/week greater than controls, P < .02).31
Critique of Studies
The quality of these pre-1991 trials is difficult to assess due to lack of adequate reporting of trial methods. None reported using random sequence generation, and only one adequately concealed group allocation,30 introducing the possibility of allocation bias. Furthermore, personnel and outcome blinding were only described in one trial;30 thus, bias may be introduced during unblinded measurement of outcomes. However, it is difficult to blind a trial of this type without changing the caloric density of the control feed as nonnutritive substances should not be added to preterm infant feeds. Three trials limited measurement error through the use of one outcome assessor, standardized techniques, and repeated measures 29.31,33 (see Supplementary Table 2, available online at http://gph.sagepub.com/supplemental).
The 4 trials showing increased growth with increased protein intake measured protein intakes through analysis of pooled daily samples of each infant’s milk, strengthening their findings. The only study showing no effect measured milk only once, at the beginning of the trial.32 The sample size used in this trial was also small (16 infants) compared to the other trials (34-66 infants; Table 4), increasing vulnerability to Type II error. Furthermore, the short study duration (7 days) may be limiting the ability of the study to show a significant effect. The generalizability of this study is also questionable, as it investigated male infants only. All studies were strengthened by their achievement of a substantially different protein intake between groups (range = 0.7 g/kg/day to 1.8 g/kg/day) ensuring any potential effect of increased protein intake was likely to be seen. However, the results of 3 trials may be confounded by the inclusion of bone minerals in the HMF.29,31,32 Polberger et al30 did not report P values for any group comparisons, limiting interpretation of these results
This evidence is strongly consistent, with all trials showing a trend to increased growth in all outcomes measured, with multiple outcomes reaching statistical significance in 3 trials. This may in part be due to the clinical homogeneity between studies. All trials investigated healthy infants of similar size (mean birth weights = 1090-1435 g) and maturity at study start (Table 4). The effect size is also remarkably consistent between trials showing significantly increased growth (weight = +3.8 to +4.1 g/kg/day; length = +0.35 to +0.36 cm/week) with only one study deviating from this.33 This trial was conducted earlier than the others, with feed and fortifier quality likely to have improved since.
There are quality issues with this evidence, primarily due to the age of the trials. However, it is highly consistent; all trials show a trend to increased growth in all outcomes measures with none showing the opposite trend. Thus, this evidence suggests increased protein intake (addition of 0.9-1.0 g/100 mL milk) in HM-fed infants does result in increased weight, length, and HC growth.
Trials Comparing HMFs Resulting in Different Protein Intakes
Summary of Studies
Seven trials compared the growth of HM-fed infants fed HMFs or supplements resulting in different protein intakes.34-40 All trials used multicomponent HMFs including protein, energy, bone minerals, and a variable selection of micronutrients. Berseth and Moro were the only 2 trials that showed no trend toward better growth in the higher protein intake groups.37,40 Four trials showed significantly increased rates of fractional weight gain in infants with higher protein intakes (range = 3-6 g/kg/day greater than controls).35,36,38,39 Three of these trials also showed significantly increased gains in HC with higher protein intakes (0.2-0.4 cm/week greater than controls).35,36,39 Two trials showed a trend toward better length growth;34,38 however, in the study by Miller et al34 this did not reach statistical significance (0.1 cm/week greater than controls; P = .08).
Critique of Studies
The trials are of varying quality. Miller et al34 alone reported random sequence generation, while 3 trials reported adequate concealment of group allocation.34,36,40 For some of these studies,37-39 study quality was primarily limited by inadequate reporting of random sequence generation and allocation concealment. Only 3 trials were satisfactorily blinded, possibly introducing bias during outcome assessment. However, all but one study reported groups to be similar at baseline (Miller et al34 had uneven multiple births between groups). Furthermore, 4 trials conducted statistical analysis on an intention-to-treat basis. This ensured groups remained balanced and thus similar at baseline, strengthening their results. Three of these trials found a significant increase in growth in the higher protein intake group (see Supplementary Table 3, available online at http://gph.sagepub.com/supple-mental).
All trials are strengthened by adequate study duration (range = 21-74 days). The trial by Miller et al34 was the most generalizable as it included healthy, sick, and small for gestational age infants. All other trials investigated “healthy” infants only. Furthermore, 3 trials34,36,40 reported accurate protein intakes through analysis of HM samples. As it has been shown that assumed intakes can deviate from actual intakes significantly,43 the use of assumed HM composition values limits the accuracy of the protein intakes reported by the other trials, and thus the results. The differences in protein intake between groups were small (range = 0.2-0.6 g/kg/day), and may not have been large enough to show a significant effect, despite satisfying the selection criteria to be included in this review. However, as many of these trials reported protein intakes that meet current recommendations (Table 1), assessing the effect of smaller increases in protein intake is clinically relevant.
There is some clinical heterogeneity among these trials. The birth weight of infants varied widely (range = 862-1407 g), as did clinical condition and maturity at study initiation (13-25 days postmenstrual age). Furthermore, compliance to feeding protocol within and between trials was wide-ranging, some infants receiving none of the assigned intervention38 while others fully completed feeding protocols.35 This may partly explain the variance in effect size seen between trials (Table 5). Variations in fortifier composition, different fortification methods, and diverse standards of care may also contribute. Overall, however, this evidence is reasonably consistent, as all trials showing significantly improved growth rate in one outcome variable also show a trend to improved growth in all outcome measures (Table 5). Thus, it is unlikely to be simply changes in fluid and fat mass confounding the results.
These trials provide evidence that increased protein intake (additional 0.2-0.6 g/kg/day) results in small weight, length, and HC gains in infants fed fortified HM.
Discussion
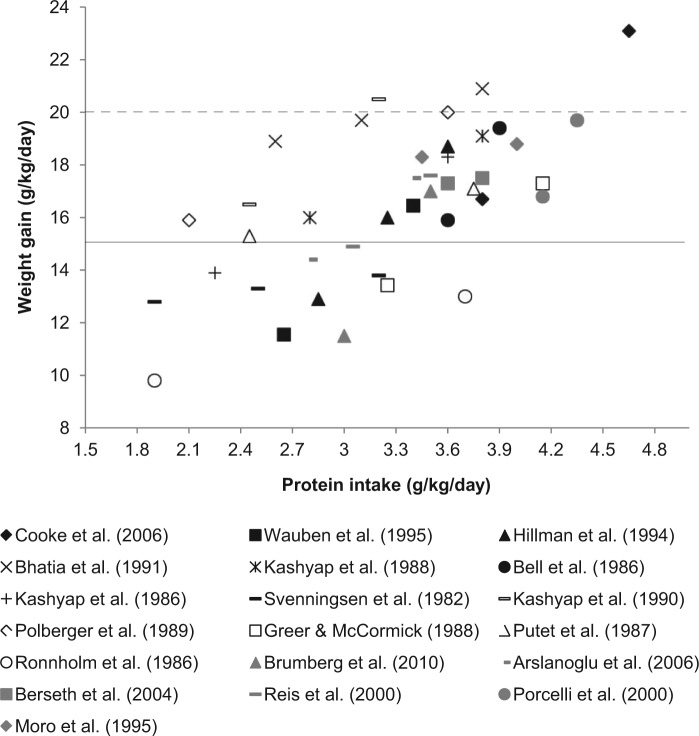
All 3 study categories show increased weight gain in infants fed higher protein intakes. When considered together and represented graphically, a somewhat linear dose–response relationship can be seen (Figure 2). However, weight gain increases from below intrauterine rates to above are larger in the trials comparing infants fed unfortified with fortified HM (Figure 2). This likely indicates protein intakes of unfortified HM-fed infants are inadequate for growth. This is consistent with the Cochrane review of the area, which also concluded unfortified HM is inadequate for infants <1500 g.15 Conversely, in infants fed formula or fortified HM, the growth of most comparison groups fell between 15 g/kg/day and 20 g/kg/day (Figure 2). This may indicate that generally protein intakes were adequate; thus, overall these studies compare adequate intakes with intakes supporting optimal growth. The findings of the Cochrane review investigating this in formula-fed infants are consistent with those of the present review: increased weight gain with higher protein intake, but little evidence for increased length or HC growth.12 Overall, statistically significant improvement in length or HC growth was shown in only 10 of the 18 studies investigating these outcomes. This may be due to the duration of the trials, as changes in these outcomes take longer to observe compared with weight gain.44
Figure 2.
Relationship between protein intake and weight gain.
Five studies did not report weight gain in g/kg/day and thus were excluded.17,19,26,28,34 Formula studies are indicated with black, unfortified versus fortified HM studies with white, and studies comparing different HMFs with grey markers. The solid black line represents the clinically used weight gain target of 15 g/kg/day.45 The dashed line represents the recently updated weight gain target that accommodates catch-up growth, 20 g/kg/day.46
Comparing these trials is limited by variation in protein quality, micronutrient composition, and nonnutritive effects on growth of different feed types. This variation, along with differing medical management,27 energy intakes, race, clinical stability,22,27,29 and size for gestational age of infants studied30,33 may explain the spread of results seen in Figure 2. This comparison is very clinically relevant however, as mixed feeding is a reality in clinical practice. The growth achieved in many trials met the clinical growth target of 15 g/kg/day (Figure 2).45 However, only 4 trials achieved the growth target46 required for adequate catch-up growth, to prevent the disparity seen in the number of infants small for gestational age at discharge (Figure 2). This suggests that many of the protein intakes studied remain inadequate for truly optimal growth. However, the impact of the substantial discrepancies between studies in the calculation of rate of weight gain should not be underestimated. Methods used ranged from the simplest average of weight over time18 to complex statistical modelling.34 Patel et al42 showed large differences in the growth estimates produced using different calculation methods; thus, this undoubtedly contributes to the spread of results seen in Figure 2.
Any benefits of increased protein intake need to be balanced with potential adverse effects due to the immature organ systems of these infants. Two formula trials withdrew participants due to perceived adverse effects of higher protein intake. Svenningsen et al27 reported late-onset metabolic acidosis in 5 infants (4 in higher protein intake group), and Raiha et al28 reported 2 infants (both higher protein intake group) developed progressive nitrogen retention and metabolic acidosis. This may be plausibly explained by the age of these trials and therefore likely poorer protein quality of feeds. This effect was not shown in the more recent trials with even higher protein intakes. Additionally, medical management of preterm infants has advanced such that greater clinical and metabolic stability can be achieved during feeding.2 Seven other trials reported either higher serum urea or elevated plasma amino acid concentration in infants with higher protein intakes.17,19,24,25,31,32,34 These authors report, however, that although higher than in control infants, elevated biochemical parameters were not clinically affecting the health of the infant, or resolved without intervention. No studies reported increased incidence of necrotizing enterocolitis, patent ductus arteriosus, or sepsis in higher protein intake groups. The present evidence suggests, therefore, that in very low birth weight infants protein intakes up to 4.5 g/kg/day are well tolerated and do not result in adverse outcome. However, this evidence does not assess the safety of such intakes in the smallest and sickest infants.
The evidence base presented in this review is satisfactory, as RCTs with moderate risk of bias are included. The consistency and generalizability of the evidence is good as the included trials represent a number of geographical regions and thus are highly applicable to health care internationally. The outcomes measured represent increments of growth. Therefore, the small improvements shown accumulate over the hospital admission to have substantial implications for the infant’s overall growth. These results satisfactorily47 show that infants fed higher protein intakes achieve small improvements in weight in the order of 3 to 6 g/kg/day, length of 0.2 to 0.4 cm/week, and HC of 0.1 to 0.4 cm/week over infants receiving lower protein. Thus, preterm infants with birth weight <1750 g fed HM should have it fortified with a multicomponent fortifier including protein. It may also be beneficial to increase the protein content of HMFs to 1.4 g/100 mL milk, and of formulas to 2.4 to 2.9 g/100 mL as standard, as no adverse effects of these protein intakes were shown.
The evidence presented here is of less than high quality, as many of these trials were conducted before clear guidelines for reporting of RCTs were established. Thus, any future research needs to be done using adequately randomized and blinded trials, with large sample sizes. The smallest and sickest infants should be included, as currently very little research includes this group of preterm infants. Furthermore, trials involving HM-fed infants must accurately measure protein intakes through HM composition analysis. Importantly, a standardized method for calculating rate of weight gain needs to be adopted by all researchers in the field to facilitate comparison of growth velocity between studies. This evidence suggests increased enteral protein intake results in increased growth in preterm infants. Thus, future research should aim to determine the protein intakes that provide not only adequate but also truly optimal growth, with a focus on safety.
Footnotes
Authors’ Note: There was no commercial/corporate financial support provided for this contribution. Jacqueline Miller and Carmel Collins, but not Emma Tonkin, were authors on the following paper which is however cited in this contribution. The study fortifiers reported on in the paper below were supplied by Nestle. Miller J, Makrides M, Gibson RA, et al. Effect of increasing protein content of human milk fortifier on growth in preterm infants born at <31 wk gestation: a randomized controlled trial. Am J Clin Nutr. 2012;95:648-655. Nestle Nutrition had no role in the study design or conduct, data collection, management, analysis, or interpretation; or in the writing, review or approval of the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Emma Tonkin received no financial support to complete this work. Carmel Collins is supported by a Postdoctoral Research Fellowship from the MS McLeod Research Fund of the Women’s and Children’s Hospital Foundation. Jacqueline Miller received no financial support to complete this work.
References
- 1. De Curtis M, Rigo J. The nutrition of preterm infants. Early Hum Dev. 2012;88:S5-S7. doi: 10.1016/j.earlhumdev.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 2. Hay WW, Thureen P. Protein for preterm infants: how much is needed? How much is enough? How much is too much? Pediatr Neonatol. 2010;51:198-207. [DOI] [PubMed] [Google Scholar]
- 3. Agostoni C, Buonocore G, Carnielli VP, et al. Enteral nutrient supply for preterm infants: commentary from the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2010;50:85-91. doi: 10.1097/MPG.0b013e3181adaee0. [DOI] [PubMed] [Google Scholar]
- 4. Ehrenkranz RA. Growth outcomes of very low-birth weight infants in the newborn intensive care unit. Clin Perinatol. 2000;27:325-345. doi: 10.1016/s0095-5108(05)70024-5. [DOI] [PubMed] [Google Scholar]
- 5. Gill A, Yu VYH, Bajuk B, Astbury J. Postnatal-growth in infants born before 30 weeks gestation. Arch Dis Child. 1986;61:549-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Berry MA, Conrod H, Usher RH. Growth of very premature infants fed intravenous hyperalimentation and calcium-supplemented formula. Pediatrics. 1997;100:647-653. doi: 10.1542/peds.100.4.647. [DOI] [PubMed] [Google Scholar]
- 7. Belfort MB, Rifas-Shiman SL, Sullivan T, et al. Infant growth before and after term: effects on neurodevelopment in preterm infants. Pediatrics. 2011;128:E899-E906. doi: 10.1542/peds.2011-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ehrenkranz RA, Dusick AM, Poole WK, Vohr BR, Wrage LA, Wright LL. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. 2006;117:1253-1261. [DOI] [PubMed] [Google Scholar]
- 9. Stephens BE, Walden RV, Gargus RA, et al. First-week protein and energy intakes are associated with 18-month developmental outcomes in extremely low birth weight infants. Pediatrics. 2009;123:1337-1343. doi: 10.1542/peds.2008-0211. [DOI] [PubMed] [Google Scholar]
- 10. American Academy of Pediatrics. American Academy of Pediatrics Committee on Nutrition: nutritional needs of low-birth-weight infants. Pediatrics. 1985;75:976-986. [PubMed] [Google Scholar]
- 11. Goldman H, Freudenthal R, Holland B, Karelitz S. Clinical effects of two different levels of protein intake on low-birth-weight infants. J Pediatr. 1969;74:881-889. [DOI] [PubMed] [Google Scholar]
- 12. Premji SS, Fenton TR, Sauve RS. Higher versus lower protein intake in formula-fed low birth weight infants. Cochrane Database Syst Rev. 2006;(1):CD003959. [DOI] [PubMed] [Google Scholar]
- 13. Malhotra TR, Zlotkin SH, Boland MP, Issenman RM, Rousseauharsany E, Vanaerde EE. Nutrient needs and feeding of premature-infants. Can Med Assoc J. 1995;152:1765-1785.7773894 [Google Scholar]
- 14. Tsang R, Uauy R, Koletzko B, Zlotkin S, eds. Nutrition of the Preterm Infant. Scientific Basis and Practical Guidelines. 2nd ed. Cincinnati, OH: Digital Education Publishing; 2005. [Google Scholar]
- 15. Kuschel CA, Harding JE. Protein supplementation of human milk for promoting growth in preterm infants. Cochrane Database Syst Rev. 2000;(2):CD000433. [DOI] [PubMed] [Google Scholar]
- 16. Academy of Nutrition and Dietetics. Evidence Analysis Manual: Steps in the Academy Evidence Analysis Process. Chicago, IL: Academy of Nutrition and Dietetics; 2012. [Google Scholar]
- 17. Costa-Orvay J, Figueras-Aloy J, Romera G, Closa-Monasterolo R, Carbonell-Estrany X. The effects of varying protein and energy intakes on the growth and body composition of very low birth weight infants. Nutr J. 2011;10:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cooke R, Embleton N, Rigo J, Carrie A, Haschke F, Ziegler E. High protein pre-term infant formula: effect on nutrient balance, metabolic status and growth. Pediatr Res. 2006;59:265-270. [DOI] [PubMed] [Google Scholar]
- 19. Embleton ND, Cooke RJ. Protein requirements in preterm infants: effect of different levels of protein intake on growth and body composition. Pediatr Res. 2005;58:855-860. doi: 10.1203/01.pdr.0000182586.46532.7c. [DOI] [PubMed] [Google Scholar]
- 20. Wauben I, Westerterp K, Gerver WJ, Blanco C. Effect of varying protein intake on energy balance, protein balance and estimated weight gain composition in premature infants. Eur J Clin Nutr. 1995;49:11-16. [PubMed] [Google Scholar]
- 21. Hillman LS, Salmons SS, Erickson MM, Hansen JW, Hillman RE, Chesney R. Calciuria and aminoaciduria in very low birth weight infants fed a high-mineral premature formula with varying levels of protein. J Pediatr. 1994;125:288-294. [DOI] [PubMed] [Google Scholar]
- 22. Bhatia J, Rassin DK, Cerreto MC, Bee DE. Effect of protein/energy ratio on growth and behavior of premature infants: preliminary findings. J Pediatr. 1991;119:103-110. [DOI] [PubMed] [Google Scholar]
- 23. Kashyap S, Schulze KF, Forsyth M, et al. Growth, nutrient retention, and metabolic response in low birth-weight infants fed varying intakes of protein and energy. J Pediatr. 1988;113:713-721. doi: 10.1016/s0022-3476(88)80388-3. [DOI] [PubMed] [Google Scholar]
- 24. Bell A, Halliday H, McClure G, Reid M. Controlled trial of new formulae for feeding low birth weight infants. Early Hum Dev. 1986;13:97-105. [DOI] [PubMed] [Google Scholar]
- 25. Kashyap S, Forsyth M, Zucker C. Effects of varying protein and energy intakes on growth and metabolic response in low birth weight infants. J Pediatr. 1986;108:955-963. [DOI] [PubMed] [Google Scholar]
- 26. Darling P, Lepage G, Tremblay P. Protein quality and quantity in preterm infants receiving the same energy intake. Am J Dis Child. 1985;139:186-190. [DOI] [PubMed] [Google Scholar]
- 27. Svenningsen NW, Lindroth M, Lindquist B. A comparative study of varying protein intake in low birthweight infant feeding. Acta Paediatr Scand Suppl. 1982;296:28-31. [DOI] [PubMed] [Google Scholar]
- 28. Raiha NC, Heinonen K, Rassin DK, Gaull GE. Milk protein quantity and quality in low-birthweight infants: I. Metabolic responses and effects on growth. Pediatrics. 1976;57:659-684. [PubMed] [Google Scholar]
- 29. Kashyap S, Schulze KF, Forsyth M, Dell RB, Ramakrishnan R, Heird WC. Growth, nutrient retention, and metabolic response of low-birth-weight infants fed supplemented and unsupplemented preterm human milk. Am J Clin Nutr. 1990;52:254-262. [DOI] [PubMed] [Google Scholar]
- 30. Polberger SKT, Axelsson IA, Raiha NCE. Growth of very low birth weight infants on varying amounts of human milk protein. Pediatr Res. 1989;25:414-419. [DOI] [PubMed] [Google Scholar]
- 31. Greer FG, McCormick A. Improved bone mineralization and growth in premature infants fed fortified own mother’s milk. J Pediatr. 1988;112:961-969. [DOI] [PubMed] [Google Scholar]
- 32. Putet G, Rigo J, Salle B, Senterre J. Supplementation of pooled human milk with casein hydrolysate: energy and nitrogen balance and weight gain composition in very low birth weight infants. Pediatr Res. 1987;21:458-461. [DOI] [PubMed] [Google Scholar]
- 33. Ronnholm KA, Perheentupa J, Siimes MA. Supplementation with human milk protein improves growth of small premature infants fed human milk. Pediatrics. 1986;77:649-653. [PubMed] [Google Scholar]
- 34. Miller J, Makrides M, Gibson RA, et al. Effect of increasing protein content of human milk fortifier on growth in preterm infants born at <31 wk gestation: a randomized controlled trial. Am J Clin Nutr. 2012;95:648-655. [DOI] [PubMed] [Google Scholar]
- 35. Brumberg HL, Kowalski L, Troxell-Dorgan A, et al. Randomized trial of enteral protein and energy supplementation in infants less than or equal to 1250 g at birth. J Perinatol. 2010;30:517-521. [DOI] [PubMed] [Google Scholar]
- 36. Arslanoglu S, Moro GE, Ziegler EE. Adjustable fortification of human milk fed to preterm infants: does it make a difference? J Perinatol. 2006;26:614-621. [DOI] [PubMed] [Google Scholar]
- 37. Berseth CL, Van Aerde JE, Gross S, Stolz SI, Harris CL, Hansen JW. Growth, efficacy, and safety of feeding an iron-fortified human milk fortifier. Pediatrics. 2004;114:e699-e706. [DOI] [PubMed] [Google Scholar]
- 38. Reis BB, Hall RT, Schanler RJ, et al. Enhanced growth of preterm infants fed a new powdered human milk fortifier: a randomized, controlled trial. Pediatrics. 2000;106:581-588. doi: 10.1542/peds.106.3.581. [DOI] [PubMed] [Google Scholar]
- 39. Porcelli P, Schanler R, Greer F, et al. Growth in human milk-Fed very low birth weight infants receiving a new human milk fortifier. Ann Nutr Metab. 2000;44:2-10. [DOI] [PubMed] [Google Scholar]
- 40. Moro GE, Minoli I, Ostrom M, et al. Fortification of human milk: evaluation of a novel fortification scheme and of a new fortifier. J Pediatr Gastroenterol Nutr. 1995;20:162-172. [DOI] [PubMed] [Google Scholar]
- 41. Higgins RD, Devaskar S, Hay WW, Jr, et al., et al. Executive summary of the workshop “Nutritional Challenges in the High Risk Infant.” J Pediatr. 2012;160:511-516. doi: 10.1016/j.jpeds.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Patel AL, Engstrom JL, Meier PP, Kimura RE. Accuracy of methods for calculating postnatal growth velocity for extremely low birth weight infants. Pediatrics. 2005;116:1466-1473. doi: 10.1542/peds.2004-1699. [DOI] [PubMed] [Google Scholar]
- 43. Arslanoglu S, Moro GE, Ziegler EE. Preterm infants fed fortified human milk receive less protein than they need. J Perinatol. 2009;29:489-492. [DOI] [PubMed] [Google Scholar]
- 44. Griffin IJ. Nutritional assessment in preterm infants. In: Cooke RJ, Vandenplas Y, Wahn U, eds. Nutrition Support for Infants and Children at Risk. Vevey, Switzerland: Nestle Nutrition Services; 2007:177-192. [DOI] [PubMed] [Google Scholar]
- 45. Sparks JW. Human intrauterine growth and nutrient accretion. Semin Perinatol. 1984;8:74-93. [PubMed] [Google Scholar]
- 46. Adamkin D. Feeding the preterm infant. In: Bhatia J, ed. Perinatal Nutrition: Optimizing Infant Health and Development. New York, NY: Marcel Dekker; 2005. [Google Scholar]
- 47. National Health and Medical Research Council. NHMRC Additional Levels of Evidence and Grades for Recommen-dations for Developers of Guidelines. Canberra, ACT: Australian Government; 2010. [Google Scholar]