Case Report
A 15-year-old male with severe cognitive impairment and history of speech delay presented to his primary physician with fungal infection of his toe nail. Review of systems revealed that he had been complaining of diffuse dull, right-sided abdominal discomfort of few months duration without any complaints of blood in stools. He denied tiredness, shortness of breath, palpitations, or any syncope. Dietary history was not indicative of iron deficiency. His family history revealed that a maternal uncle had died at 23 years of age due to metastatic rectal cancer. On physical examination, his height was in the 10th percentile and weight between the 10th and 25th percentile, with a body mass index of 18 kg/m2. Positive physical findings included mild dysmorphic facial features including light hair, broad forehead with a receding hairline, and pectus excavatum. He had no other bony abnormalities. The rest of his physical examination was normal except for significant pallor and mild tachycardia.
He was born at 36 weeks to a 17-year-old G1P1 mother via spontaneous vaginal delivery, which was complicated by a tight nuchal cord and meconium aspiration. Respiratory distress at birth required supplemental nasal oxygen for 24 hours. Gross motor, fine motor, and personal social development was normal, but he had difficulty with speech and required speech therapy. He had severe cognitive impairment and required special education classes since kindergarten.
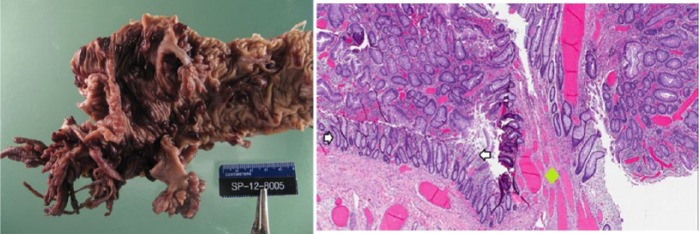
Prior to starting antifungal medication, a baseline complete blood count (CBC) was obtained that revealed very low hemoglobin (Hb) of 5.6 g/dL, and hence he was referred to our institution for further workup of anemia. CBC repeated at our institution showed a Hb value of 4.8 g/dL and mean corpuscular volume of 57 fL; Hb electrophoresis, Von Willebrand panel, direct and indirect Coomb’s testing, antinuclear antibody, haptoglobin, and serum uric acid were within normal limits. Microcytic, hypochromic red cells seen in the peripheral smear supported iron deficiency, which was confirmed by the very low serum ferritin level of 2 ng/mL and an elevated total iron binding capacity of 464 µg/dL. Initial stool guaiac testing was negative in the clinic. Given his right-sided abdominal discomfort and concern of possible gastrointestinal cause for severe anemia, Meckel’s scan was done, which showed mildly increased uptake. A pediatric surgeon was consulted and he underwent diagnostic laparoscopy, and Meckel’s diverticulum was ruled out. Colonoscopy was done as the next step to rule out other gastrointestinal causes of anemia, which revealed numerous polyps (>100) extending from ileum to rectum (see right image in figure 1). Biopsy of these polyps was pathologically identified as classic tubular adenomas, which confirmed the diagnosis of familial adenomatous polyposis (FAP) (see left image in figure 1).
Figure 1.
Left image: Proctocolectomy specimen showing multiple polyps. Right image: Histopathology specimen of colon.
The area between the white arrows is normal colonic mucosa. The entire area above the arrows is tubular adenoma with hyperchromatic and elongated pseudostratified nuclei. The green diamond marks the stalk of the tubular adenoma.
Final Diagnosis
FAP in a cognitively impaired teenage male with a rare genetic abnormality was our final diagnosis.
Hospital Course
Metastatic workup including computed tomography of the chest, abdomen, and pelvis and ultrasound of thyroid was negative. Targeted testing for 2 common MUTYH (formerly MYH) gene mutations was also negative. Adenomatous polyposis coli (APC) gene analysis in chromosome 5 detected a sequence alteration at exon 15 c.5465T>A, p.Val1822Asp. Total proctocolectomy was done followed by ileal pouch-anal anastamosis. Postoperative course was uneventful. His anemia resolved with continued oral iron supplements. Histopathology of the specimen identified tubular adenomas without any signs of malignancy, and the surgical margins were free of dysplasia. He has been disease free since surgery with no further manifestations of FAP for over a year. We plan to screen his entire family for FAP.
Discussion
We report an interesting case of a cognitively impaired teenage male, who was incidentally found to have profound microcytic anemia and, on further workup, was diagnosed with FAP. Genetic testing revealed an uncommon sequence alteration of APC gene.
Microcytic anemia is typically an incidental finding in asymptomatic patients when CBC was done for other reasons. The causes of microcytic anemia are iron deficiency anemia, thalassemia trait, anemia of chronic disease, lead toxicity, and sideroblastic anemia. Red blood cell distribution width using the CBC, serum iron levels, serum ferritin levels, total iron binding capacity, transferrin saturation, Hb electrophoresis, reticulocyte blood count, and peripheral blood smears help in differentiating the causes of microcytic anemia. Low ferritin level is indicative of iron deficiency. Once a presumptive diagnosis of iron deficiency anemia has been made, an underlying source for the deficiency should be determined. Iron deficiency anemia in adolescents is always presumed to be caused by overt or occult blood loss, most often due to bleeding from malignancy or other lesions in the gastrointestinal tract.1 It is critical to exclude gastrointestinal malignancy in men and non–menstruating women.2,3
FAP is a well-known genetic disease characterized by the development of hundreds to thousands of adenomatous colorectal polyps, which, if not treated, eventually become cancerous by 50 years. Mean age of polyp emergence is 16 years. Most patients are asymptomatic for years until the polyps enlarge and increase in number, causing rectal bleeding or anemia, or until cancer develops. Nonspecific symptoms may include constipation or diarrhea, abdominal pain, palpable abdominal masses, and weight loss. Of patients with FAP, 75% to 80% have a family history of polyps and/or colorectal cancer at age 40 years or younger. Extracolonic manifestations of FAP include polyps of gastric fundus and small bowel, periampullary carcinoma, desmoid tumors, epithelial inclusion cysts, fibromas, lipomas, osteoid osteomas, supernumerary teeth, and congenital hypertrophy of the retinal pigmented epithelium. Other less common manifestations reported in FAP include hepatoblastoma, medulloblastoma, pancreatobiliary carcinoma, papillary thyroid carcinoma, and adrenal cortical tumors. Diagnosis of FAP is based on clinical findings, colonoscopy, and a suggestive family history. Clinical diagnosis should be confirmed by genetic testing. Classic FAP is an autosomal dominant syndrome that results from a germline mutation in the APC tumor suppressor gene located on chromosome 5q band 21, which has a penetrance close to 100%. In a subset of individuals, an MUTYH mutation causes a recessively inherited polyposis condition, which is characterized by a slightly increased risk of developing colorectal cancer and adenomatous polyps in both the upper and lower gastrointestinal tract.
APC gene mutation has been identified in 80% of classic FAP patients. The most recent genetic database reveals 858 germline mutations of APC gene at chromosome 5q21 in patients with FAP.4 Majority of these are frameshift and nonsense mutations leading to a nonfunctional APC protein. Mental retardation has been reported in only a few patients with FAP. Heald et al5 summarized 14 reported cases of FAP associated with mental retardation. These patients had varying degrees of mental retardation but with the majority falling in mild to moderate range. All of them were found to have interstitial deletions of chromosome 5q.6 Deletions involving the proximal portion of the long arm of chromosome 5 extending from q15 to q22 were associated with mild phenotype with regard to both mental retardation and physical features, whereas larger deletions including the distal portion, extending from q22 to q31, were associated with rather distinct phenotype.7 However, our index case with mild dysmorphic features and severe mental retardation was found to have a sequence alteration (c.5465T>A, p.Val1822Asp) in exon 15 of APC gene, which is a base substitution of asparagine to valine at codon 1822. Thus far in the literature, there have been 3 cases reported with this homozygous single base substitution.8,9 One of them had FAP, but the other 2 cases were non-FAP patients. Of the non-FAP patients, one patient had isolated colorectal cancer and the other patient had 30 to 40 adenomatous colonic polyps with rectal sparing and hepatic metastases. None of them had mental retardation or dysmorphic features. Our index case did not have any metastatic involvement or other extracolonic manifestations of FAP. More virulent forms of FAP have been associated with a mutation in exon 15 between codons 1250 and 1464, the middle portion of APC gene.10
Conclusion
Microcytic anemia in a male should always raise the suspicion of any causes for chronic blood loss such as gastrointestinal bleeding. Recognizing at-risk patients with vague abdominal symptoms, obtaining thorough history, and performing complete workup will lead to early identification of the disease and prevent progression to cancer. As our index case, patients with FAP, cognitive deficits, and mild dysmorphic features may be associated with small sequence alterations of APC gene rather than large deletions or typical point mutations.
Acknowledgments
We acknowledge Dr Matthew Dacso, Director of Centre for Global Health Education, University of Texas Medical Branch, and Dr Keith Bly, program director of pediatric residency, University of Texas Medical Branch, for their support. We acknowledge the help of Dr Sean Fitzgerald for providing pathology images.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Annibale B, Capurso G, Chistolini A, et al. Gastrointestinal causes of refractory iron deficiency anemia in patients without gastrointestinal symptoms. Am J Med. 2001;111:439-445. [DOI] [PubMed] [Google Scholar]
- 2. Moreno Chulilla JA, Romero Colas MS, Gutierrez Martin M. Classification of anemia for gastroenterologists. World J Gastroenterol. 2009;15:4627-4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Killip S, Bennett JM, Chambers MD. Iron deficiency anemia. Am Fam Physician. 2007;75:671-678. [PubMed] [Google Scholar]
- 4. Kerr SE, Thomas CB, Thibodeau SN, Ferber MJ, Halling KC. APC germline mutations in individuals being evaluated for familial adenomatous polyposis: a review of the Mayo Clinic experience with 1591 consecutive tests. J Mol Diagn. 2013;15:31-43. [DOI] [PubMed] [Google Scholar]
- 5. Heald B, Moran R, Milas M, Burke C, Eng C. Familial adenomatous polyposis in a patient with unexplained mental retardation. Nat Clin Pract Neurol. 2007;3:694-700. [DOI] [PubMed] [Google Scholar]
- 6. Raedle J, Friedl W, Engels H, Koenig R, Trojan J, Zeuzem S. A de novo deletion of chromosome 5q causing familial adenomatous polyposis, dysmorphic features, and mild mental retardation. Am J Gastroenterol. 2001;96:3016-3020. [DOI] [PubMed] [Google Scholar]
- 7. Lindgren V, Bryke CR, Ozcelik T, Yang-Feng TL, Francke U. Phenotypic, cytogenetic, and molecular studies of three patients with constitutional deletions of chromosome 5 in the region of the gene for familial adenomatous polyposis. Am J Hum Genet. 1992;50:988-997. [PMC free article] [PubMed] [Google Scholar]
- 8. Plawski A, Slomski R. APC gene mutations causing familial adenomatous polyposis in Polish patients. J Appl Genet. 2008;49:407-414. [DOI] [PubMed] [Google Scholar]
- 9. Wallis YL, Morton DG, McKeown CM, Macdonald F. Molecular analysis of the APC gene in 205 families: extended genotype-phenotype correlations in FAP and evidence for the role of APC amino acid changes in colorectal cancer predisposition. J Med Genet. 1999;36:14-20. [PMC free article] [PubMed] [Google Scholar]
- 10. Wachsmannova-Matelova L, Stevurkova V, Adamcikova Z, Holec V, Zajac V. Different phenotype manifestation of familial adenomatous polyposis in families with APC mutation at codon 1309. Neoplasma. 2009;56:486-489. [DOI] [PubMed] [Google Scholar]