Abstract
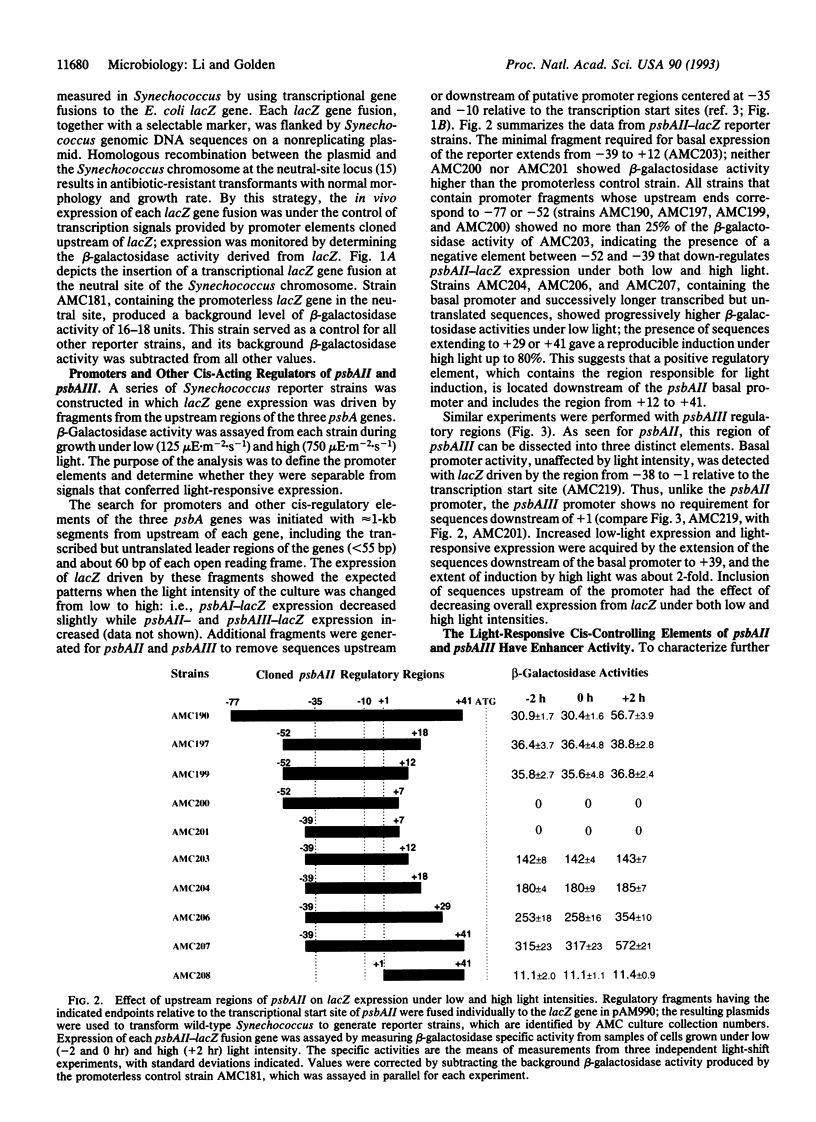
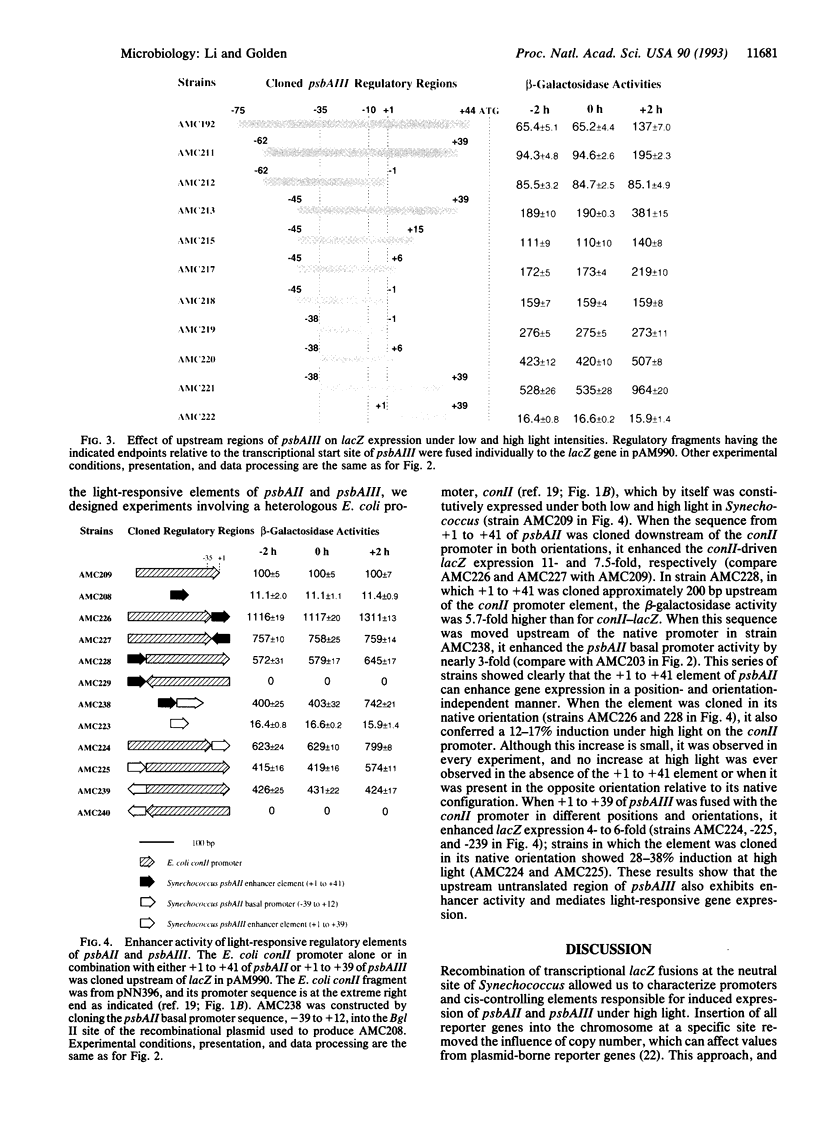
Three psbA genes encoding the D1 protein of the photosystem II reaction center are differentially expressed under different light intensities in the cyanobacterium Synechococcus sp. strain PCC 7942. Two of the three psbA genes, psbAII and psbAIII, are induced rapidly when light intensity is increased from 125 x 10(-6) mol.m-2.s-1 to 750 x 10(-6) mol.m-2.s-1. A recombinational cloning vector that carries a transcriptional lacZ reporter gene was used to characterize the controlling elements responsible for light induction. At least three distinct cis elements are present in the regulatory regions of pbsAII and psbAIII: basal promoters, comparable to Escherichia coli sigma 70 promoters in position and sequence, confer constitutive expression of the genes under both low and high light intensities; negative elements upstream of the promoters down-regulate the expression of the corresponding gene; and sequences downstream of the promoters that correspond to the untranslated leader regions of the mRNAs (+1 to +41 in psbAII and +1 to +39 in psbAIII) are responsible for increased expression under high light. When these light-responsive elements were combined with an E. coli promoter (conII) in different positions and orientations, the expression of the lacZ gene was induced 4- to 11-fold. The induction of gene expression under high light by these enhancers was position independent but orientation dependent. When the elements were combined with the conII promoter in the correct orientation, they also conferred a small but reproducible level of light-responsive expression on this E. coli promoter.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bustos S. A., Golden S. S. Expression of the psbDII gene in Synechococcus sp. strain PCC 7942 requires sequences downstream of the transcription start site. J Bacteriol. 1991 Dec;173(23):7525–7533. doi: 10.1128/jb.173.23.7525-7533.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustos S. A., Golden S. S. Light-regulated expression of the psbD gene family in Synechococcus sp. strain PCC 7942: evidence for the role of duplicated psbD genes in cyanobacteria. Mol Gen Genet. 1992 Mar;232(2):221–230. doi: 10.1007/BF00280000. [DOI] [PubMed] [Google Scholar]
- Bustos S. A., Schaefer M. R., Golden S. S. Different and rapid responses of four cyanobacterial psbA transcripts to changes in light intensity. J Bacteriol. 1990 Apr;172(4):1998–2004. doi: 10.1128/jb.172.4.1998-2004.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado-Vides J., Magasanik B., Gralla J. D. Control site location and transcriptional regulation in Escherichia coli. Microbiol Rev. 1991 Sep;55(3):371–394. doi: 10.1128/mr.55.3.371-394.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elledge S. J., Sugiono P., Guarente L., Davis R. W. Genetic selection for genes encoding sequence-specific DNA-binding proteins. Proc Natl Acad Sci U S A. 1989 May;86(10):3689–3693. doi: 10.1073/pnas.86.10.3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gober J. W., Shapiro L. A developmentally regulated Caulobacter flagellar promoter is activated by 3' enhancer and IHF binding elements. Mol Biol Cell. 1992 Aug;3(8):913–926. doi: 10.1091/mbc.3.8.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Expression of a family of psbA genes encoding a photosystem II polypeptide in the cyanobacterium Anacystis nidulans R2. EMBO J. 1986 Nov;5(11):2789–2798. doi: 10.1002/j.1460-2075.1986.tb04569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Genetic engineering of the cyanobacterial chromosome. Methods Enzymol. 1987;153:215–231. doi: 10.1016/0076-6879(87)53055-5. [DOI] [PubMed] [Google Scholar]
- Gralla J. D. Transcriptional control--lessons from an E. coli promoter data base. Cell. 1991 Aug 9;66(3):415–418. doi: 10.1016/0092-8674(81)90001-5. [DOI] [PubMed] [Google Scholar]
- Kulkarni R. D., Schaefer M. R., Golden S. S. Transcriptional and posttranscriptional components of psbA response to high light intensity in Synechococcus sp. strain PCC 7942. J Bacteriol. 1992 Jun;174(11):3775–3781. doi: 10.1128/jb.174.11.3775-3781.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kustu S., Santero E., Keener J., Popham D., Weiss D. Expression of sigma 54 (ntrA)-dependent genes is probably united by a common mechanism. Microbiol Rev. 1989 Sep;53(3):367–376. doi: 10.1128/mr.53.3.367-376.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang J. D., Haselkorn R. A vector for analysis of promoters in the cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol. 1991 Apr;173(8):2729–2731. doi: 10.1128/jb.173.8.2729-2731.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Schaefer M. R., Golden S. S. Differential expression of members of a cyanobacterial psbA gene family in response to light. J Bacteriol. 1989 Jul;171(7):3973–3981. doi: 10.1128/jb.171.7.3973-3981.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira S. K., Chou J., Richaud F. V., Casadaban M. J. New versatile plasmid vectors for expression of hybrid proteins coded by a cloned gene fused to lacZ gene sequences encoding an enzymatically active carboxy-terminal portion of beta-galactosidase. Gene. 1983 Nov;25(1):71–82. doi: 10.1016/0378-1119(83)90169-5. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobczyk A., Schyns G., Tandeau de Marsac N., Houmard J. Transduction of the light signal during complementary chromatic adaptation in the cyanobacterium Calothrix sp. PCC 7601: DNA-binding proteins and modulation by phosphorylation. EMBO J. 1993 Mar;12(3):997–1004. doi: 10.1002/j.1460-2075.1993.tb05740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soberon X., Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene. 1980 May;9(3-4):287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]
- Su W., Porter S., Kustu S., Echols H. DNA-looping and enhancer activity: association between DNA-bound NtrC activator and RNA polymerase at the bacterial glnA promoter. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5504–5508. doi: 10.1073/pnas.87.14.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. M., Crothers D. M. The locus of sequence-directed and protein-induced DNA bending. Nature. 1984 Apr 5;308(5959):509–513. doi: 10.1038/308509a0. [DOI] [PubMed] [Google Scholar]






