Abstract
Ligand-directed signaling, biased agonism, and functional selectivity are terms that describe the propensity of a ligand to drive signaling toward one GPCR pathway over another. Most of the early examples demonstrated to date examine the divergence between GPCR signaling to G protein coupling and βarrestin2 recruitment. As biased agonists begin to become available based on cell-based screening criteria, a need arises to determine if G protein signaling biases will be maintained in the endogenous setting, wherein receptors are functioning to control relevant biological responses. This report presents our method and offers tips for evaluating G protein signaling in endogenous tissues. Predominately, brain tissues are discussed here; optimization points that can be applied to any tissues are highlighted.
Keywords: GTP gamma S, G protein coupling, GPCR, Receptor pharmacology, Opioid receptors, Endogenous receptors, GDP, Radioactivity assays, Neuropharmacology, Brain, Striatum
1 Introduction
The evaluation of ligands used to be as simple as a dose response curve in your favorite cell line expressing your favorite receptor. If the response increased, you had an agonist; if it only increased a fraction of the potential, then a “partial agonist”; and if it had no effect, you might ask if it was an antagonist. Now it is becoming increasingly clear that a ligand's actions at a receptor may not be transmitted to all downstream targets [1, 2]. In fact, a ligand may display agonism in one assay and antagonism in another. As we begin to challenge our systems and increase the array of assays and experimental endpoints, it becomes evident that ligand performance is highly context dependent.
Many factors can contribute to contextual definitions for receptor function, including but not limited to the species of the cell line or the tissue of origin, as well as localization of the receptor in proximity of signaling partners. Each of these factors defines limitations that will, in turn, affect the potential of a receptor to signal. The extent of the influence of these many variables may be more or less revealed by the nature of the agonist binding. For example, if one considers the performance of an inverse agonist, it may appear as a neutral agonist unless there is some degree of basal stimulation to displace. In this manner, the context of the receptor has determined the perception of how the ligand performs in the assay. Therefore context becomes very important in studying how a receptor responds in response to a ligand.
The fact that ligands can promote transmission of receptor signaling in a context-dependent manner is very important in considering the concept of biased agonism [1, 2]. In this paradigm, an agonist may lead to a preferential engagement of one signaling pathway over another. Most of the examples of “biased agonism” have been presented for agonists that distinguish a preference between G protein signaling and βarrestin recruitment upon ligand binding [3 – 5]; greater diversity of signaling potentials likely exists, which in turn may be more difficult to measure, particularly in an endogenous context. G protein signaling is a paramount hallmark of “G protein-coupled receptor” signaling. The heterotrimeric G protein complex consists of 3 primary subunits, the α, β, and γ subunits. Of these, there are at least 21 α subunits encoded by 16 genes, 6 β subunits encoded by 5 genes, and 12 γ subunits by 12 genes in mammals which predicts an assembly of 1512 combinations of G heterotrimeric G protein complexes, if indeed, they all had the potential to assemble as functional complexes [6, 7]. While it is unlikely that as many possibilities exist endogenously, it is still substantial to recognize the variety among Gα proteins and the recognition that most GPCRs have the capability of signaling to more than one Gα protein.
Assessment of functional selectivity in cell-based assays is generally performed by comparing the capacity of a receptor to signal through a particular G protein assay and compared to the capacity to signal to another downstream effector such as another G protein or to a βarrestin. While cell-based assays are valuable due to their high signal to noise ratios and the selectivity factors (overexpression of a particular receptor of interest and using a nontransfected cell line as a control for selectivity), they may not recapitulate the context in which the endogenous functional receptor may be found [8]. Therefore, since bias is context dependent, it becomes necessary to examine signaling parameters in a more biologically relevant system. While there are currently no good assays for evaluating βarrestin interaction in the endogenous system, there are means to assess G protein signaling potentials in primary cells and in tissues from animals [9 – 25]. In this methods report, we discuss G protein signaling assays usually performed in cell-based assays and discuss our adaptations for optimization in tissues and primary cultures.
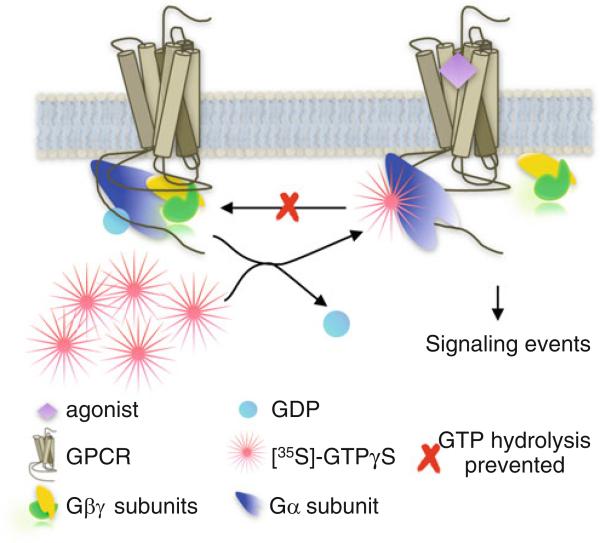
Overall, the [35S]-GTPγS binding assay is based on the principle of GDP to GTP exchange that occurs upon activation of the Gα subunit of the heterotrimeric G protein (Fig. 1). G protein signaling can be assessed in animal or human tissue samples if the samples are immediately frozen in liquid nitrogen using a traditional radioisotope labeled GTPγS binding assay. The limitation of this assay is that it is only suitable for detection of G protein signaling that is the most robust in the system. In most tissue, as well as in cell lines, Gαi family of G α proteins is the most robust. However, in brain, other G proteins such as Gαz are also highly expressed [26, 27]. Upon agonist stimulation of membranes, G protein signaling can be detected in a dose responsive manner by the accumulation of radioactivity remaining associated with proteins stuck to the filters after aspiration of the samples. Using this method, it is not possible to distinguish between the different Gα proteins and this remains a major limitation. However, the assay should capture the propensity of the receptor to signal the available G proteins in the relevant endogenous system. Other means to resolve the identity of G proteins involve using antibodies specific to different G protein subunits in an immunoprecipitation assay as opposed to a membrane filtration assay [28 – 33]. In this approach, only the immunoprecipitated Gα proteins will be counted. Below, we include some specific details that our lab has used to evaluate G proteins signaling in brain regions isolated from mouse using the membrane filtration assessment of global G protein signaling.
Fig. 1.
G protein signaling. First, the isolated membranes are incubated with GDP in order to prime the available Gα protein in the active state. Upon activation of the G protein by the GPCR, GDP is exchanged for GTP. By providing a nonhydrolyzable (or hydrolysis resistant) radiolabeled form of GTP, we are able to quantify the stimulated accumulation of GTP-bound G protein as a function of agonist stimulation in a set amount of membrane protein
2 Materials
2.1 Buffers, Reagents
Homogenization Buffer: 10 mM Tris–HCl pH 7.4, 100 mM NaCl, 1 mM EDTA, and 1 mM DTT (add fresh to the needed amount for the study just prior to use).
Assay Buffer: 10 mM Tris–HCl pH 7.4, 100 mM NaCl, 1 mM EDTA, 5 mM MgCl2, and 20 μM GDP (add GDP just prior to use).
Wash: deionized H2O (ice cold)-recommended storage in a large carboy in the cold room prior to use.
DMSO as drug solvent.
[35S]-GTPγS: 1250 Ci/mmol, 12.5 mCi/mL stock from Perkin Elmer, aliquot and freeze at −20 °C.
Liquid Nitrogen.
Scintillation fluid.
2.2 Tools and Instruments
-
8.
Rotating Blade homogenizer with tip appropriate for tissue size (like a “Polytron” or “Tissue Tearor®” device).
-
9.
Glass-on-glass dounce homogenizer (1 mL unless large tissue sections are used, such as whole mouse brain).
-
10.
26 Gauge needle and 10 mL syringe
-
11.
Refrigerated centrifuge capable of 20,000 × g (4 °C).
-
12.
96-Well plates with glass fiber B (GF/B) filters.
-
13.
Microplate sealing film to cover plate.
-
14.
96-Well plate harvester (Brandel Inc., Gaithersburg, MD).
-
15.
Scintillation counter (Beckman Coulter LS6500).
-
16.
TopCount NXT HTS microplate scintillation and luminescence counter (PerkinElmer).
3 Method
3.1 Tissue Procurement and Storage
Acquire tissues as soon after euthanasia as possible and snap frozen in liquid nitrogen. The means of euthanasia should be performed in a manner that will have the least likelihood of affecting the receptor function of interest.
If dissections are to occur, they should be performed prior to freezing the samples. Following dissection, tissues are then snap frozen in liquid nitrogen in a sealed plastic tube.
It is our observation that brain tissues can be stored for several years at −80 °C.
3.2 Membrane Preparations
Tissues should be stored on ice prior to thawing, subjected to blade on blade rotating homogenization (see Note 1).
Homogenization Buffer is composed of 10 mM Tris–HCl pH 7.4, 100 mM NaCl, 1 mM EDTA, and 1 mM DTT (add fresh prior to use to the needed amount for the study).
It is absolutely critical that the rotating blade device be thoroughly cleaned prior to use. Performing the homogenization step while the tissue is still frozen can facilitate a smooth and homogenous disruption of the tissue. For analogy, consider making a smoothie from pineapple. If it has thawed, it has the tendency to become stringy; if frozen, the disruption of the fruit becomes smooth. For some tissues, such as highly muscular tissue, such as heart or colon, it may become necessary to use a more extreme approach for homogenization. In this case, a French press or a hammer to steel approach may be utilized. We have used the latter with success in preparing colon membranes in solution. Keeping tissues frozen as well as keeping samples and buffers on ice will help to offset the damaging effects of heat generated in the homogenization process. Thorough homogenization is critical for reproducibility in performing G protein signaling assays. It is recommended that the homogenizer be rinsed with a 70 % methanol solution followed by rinsing with water, then homogenization buffer.
Homogenization is performed in a buffer that may vary in composition. It is recommended that a thorough literature search is performed to determine what is most commonly used for detection of GTPγS binding for the receptor of interest. This will provide a good starting point; however, buffer optimization will be necessary to elucidate the highest signal to noise ratio for a particular receptor of interest. The simplest homogenization buffer that we have utilized is a 10 mM Tris buffer at pH 7.4 with NaCl and EDTA (see Notes 2–4).
Generally, the less buffer that can be used, the better as a more dilute sample will have less contact with the blades of the homogenizer. However, too little buffer will also present problems. As a general rule, for small tissue samples, such as a mouse striatum, 200 μL is used with a small homogenizer size head (~5–10 mm diameter). A small rounded bottom tube (~10–15 mm diameter) should be used; conical bottom tubes should not be used as the tissue may escape contact with the blade. For a mouse striatum, our laboratory uses a “Tissue Tearor®” homogenizer with a setting of level five (highest is 10) for three 5 s pulses on ice. To assure that no protein is lost in the blades, the homogenizer can be rinsed with a small amount of buffer that will be transferred to the next homogenization step.
Samples are next homogenized by dounce homogenization to assure complete membrane disruption. Samples are transferred to a 1 mL volume homogenizer (or ~5× sample volume); the original sample tube should be rinsed with ~600 μL homogenization buffer to avoid any loss of protein in the transfer (this is a good opportunity to capture any residual protein in the homogenization blades). Glass-on-glass dounce homogenization should proceed for ~20 plunges keeping samples on ice.
Transfer the sample to a 50 mL falcon tube. Rinse dounce homogenizer with homogenization buffer and combine with the sample. Pass the sample 8× through a 26 gauge needle on a 10 mL syringe for a final homogenization step.
Transfer the homogenized tissue suspension to a centrifugation tube that can withstand a 20,000 × g speed centrifugation. Note: If this is a reusable centrifugation tube, it is absolutely critical to make sure that the tube is clean, and a methanol then water rinse step followed by drying is recommended to avoid any residual detergent contamination. Presence of detergent can disrupt subsequent receptor functional signaling.
3.3 Centrifugation
Either a swinging bucket or fixed angle rotor will suffice; centrifugation should be performed under refrigeration at 4 °C for 30 min.
The supernatant is decanted and discarded while the pellet is resuspended by dounce homogenization in homogenization buffer (1 mL in a 1 mL glass-on-glass dounce homogenizer).
Repeat centrifugation at 20,000 × g for 30 min at 4 °C to prepare final membrane pellet. Decant away buffer and remove residual buffer via careful aspiration.
At this point, some elect to store the membrane pellet at −80 °C; however, we find that this comes at a sacrifice of the amplitude of assay signal. Therefore, we recommend continuing through from this point, directly into the assay.
3.4 [35S]-GTPγS Binding Assay
To a portion of the assay buffer, 1 mM DTT is added. (Assay buffer without DTT is used for preparing ligand dilutions for testing.) Assay Buffer with DTT (AB+) is added to the pellet. The volume used to resuspend the pellet should be determined based upon the desired final protein concentration. It is our experience that opioid receptor and cannabinoid CB1 receptor activation can readily be performed using as little as 2.5 μg membrane protein per assay well. Generally we resuspend 1 striatum membrane preparation in 200 μL AB+ and vortex well for final resuspension. Protein concentration should be measured by conventional method of choice (a Lowry or Bradford based assay).
Setting up the assay. For this assay, we will describe setup for a 96-well plate using a Brandel 96-well plate filtration system.
For this paradigm, we use the following final volumes: 50 μL 4× test compound or assay buffer alone; 50 μL 400 pM [35S]-GTPγS in Assay Buffer; 100 μL membrane sample (at 2.5 μg in 100 μL) in Assay Buffer with DTT and GDP.
For a 96-well plate, prepare at least 5 mL of the 400 pM [35S]-GTPγS in Assay Buffer (see Note 5).
Preparation of the test compounds. Compounds to be tested should be prepared at a 4× concentration to cover the concentration ranges desired.
The values observed in cell lines may be used to estimate the range for the concentration response curve, although we find that transfected homogeneous cell lines produce EC50 values that are generally five to ten times lower (relative higher potency) than that observed in the endogenous setting.
If compounds require DMSO for solubility, then compounds should be prepared in <4 % DMSO stock solutions, at each concentration, and then diluted such that the % DMSO is the same across all samples. Any compounds, including the reference agonists, that are to be compared should be prepared in the same manner. The % DMSO in the final concentrations should be less than 1 %. This same dilution of DMSO should be applied to the “vehicle” control wells.
In preparation of the membrane protein solution, the volume of buffer should be increased to a concentration of 4× greater than the final diluted desired concentration using AB+ and GDP at the desired concentration. For example, for a 10 point curve, in duplicate, using 100 μL volume to generate a 2.5 μg/well plate would require at least 2 mL (make at least 2.3 mL) of a 0.05 μg/μL solution. If the starting protein concentration prepared in 1 mL was 0.5 μg/mL, then 1 mL AB+ would be added along with a volume of GDP to produce the final desired concentration of GDP. We find that for brain, for several different GPCRs, that a 20 μM GDP concentration works very well (see Note 6).
Allow the membrane suspension to incubate on ice with GDP for 10–15 min prior to allow for precoupling of the GDP-bound Gα protein. This incubation period is a good time to pipette the other components into the 96-well plates.
Drug dilutions are added first, then the radioactivity as errors are more likely to occur in pipetting the different drug dilutions. If an error occurs in pipetting, it can be corrected prior to adding radioactivity or valuable tissue. It is highly recommended that a multichannel pipette that is calibrated to accuracy be used to avoid error in pipetting (see Notes 7–9).
The reaction begins when the membrane protein solution is added.
The binding assay incubates at room temperature for 1–2 h allowing the reaction to reach equilibrium. We found that 2 h works best for most of our studies. This time and temperature can be modified to optimize the signal to noise ratio.
After incubation, samples are collected by rapid filtration over 96-well glass fiber filters (Whatman filter B) (either plates or sheets) using a Brandel Harvester (this is our choice, we find it provides the most powerful and consistent vacuum pressure). Upon rapid filtration, membranes should be washed multiple times with ice-cold deionized water (see Note 10).
Membranes should be allowed to dry thoroughly before applying scintillation fluid and counting. Residual water on the membranes can interfere with the scintillation producing error. This becomes nontrivial when one considers that the maximal stimulation usually produced is between 0.2- and 2-fold maximal over baseline when assessing endogenous receptor function in tissues.
For a 96-well filter plate, generally, 30–50 μL of scintillation fluid is sufficient for detection, and allowing the plates to sit for at least an hour to overnight can improve detection. Longer times are not recommended.
Filters can be counted on a TopCount NXT HTS microplate scintillation and luminescence counter or similar device.
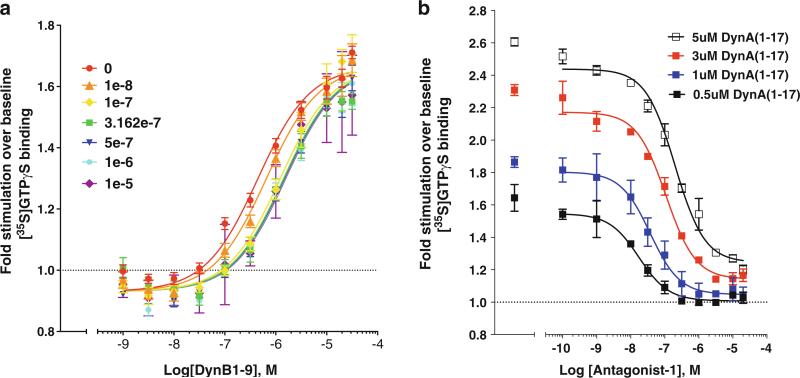
Applications. Since this assay is amenable to a 96-well format, and since we have optimized the procedure to use very little protein, it is possible to analyze full curves of several agonists for comparison on a single plate. This is critical and essential for asking questions of “bias” or whether a compound is an allosteric modulator. For example, from one striatum (one hemisphere) we find we can fill an entire 96-well plate, allowing for 4–6 curves to be run in duplicate. In Fig. 2 we provide examples of agonist, antagonist, and allosteric modulation studies performed on undisclosed test agonists compared to known agonist/antagonist activities.
While we have described these assays for use in mouse tissues, they can be optimized for studying tissue of any source, theoretically, given that the GPCR of interest is present and the integrity of the sample can be preserved. The benefit of performing the G protein signaling assay in the tissue of interest allows for variability associated with context to be reduced, although not eliminated. Utilizing the endogenous tissue, however, does allow for comparison to studies performed in cell-based overexpression systems and can provide a step for verification of relative potency, bias, or allosterism, prior to moving toward interpreting behaviors or complex physiologies in vivo.
Fig. 2.
Examples demonstrating agonist stimulation (for dynorphin B); allosteric modulation of dynorphin B (1–9) (a), and antagonist effects for a nondisclosed antagonist against Dynorphin A (1–17) (b) at the Kappa Opioid Receptor (KOR). These data are presented for demonstration of how the assays can be utilized and values are not to be derived (sample assays performed in duplicate (a), n = 3 (b))
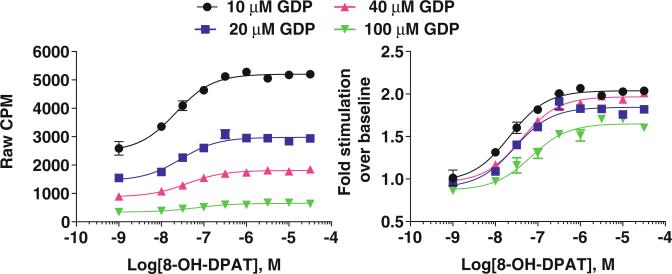
Fig. 3.
Examples demonstrating the effect of GDP concentration of basal signaling levels in rat hippocampus membrane preparations and how this influences the determination of the fold stimulation. In these samples, we opted to work with the 10 μM concentration of GDP. Hippocampus homogenates contained 2 μg protein per well, 100 pM and [35S]-GTPγS
Acknowledgement
NIH/NIDA grants DA0031927, DA033073, DA009158 fund G protein signaling projects in our laboratory.
Footnotes
Given that assay activation windows detected in endogenous tissues downstream of GPCR activation can be very small, it is recommended that only tissues, and not membrane pellets, are frozen prior to use. We find that freezing pellets can lead to a decrease in the maximal amplitudes of stimulation detected and this degree of loss of signaling may prove to be a significant sacrifice.
For agonists, the degree of stimulation will be highly influenced by the integrity of the baseline/buffer only stimulation seen. Therefore, it is very important to obtain a highly accurate reading of the baseline and multiple wells, distributed across the plate, are used to define the baseline. Placing all of the baseline or maximal points in the same position, at the top or to one side of the plate, should be avoided as a “plate-effect” could occur. In such a situation, the readings may be high or low, due not to the degree of stimulation, but due to the position on the plate. This becomes an issue when there is uneven vacuum pressure across the plate, allowing portions of the plate to be washed more thoroughly than others. The harvester should be washed adequately prior to use.
Variations on buffer composition may be optimized to preserve ionic interactions within the activation site of the receptor, but this will be dependent on the receptor and may be best determined empirically.
A thorough rinse at each homogenate transfer step is critical to preserve protein amount in the assay especially for small amount of tissue. Avoid large volume homogenizers and tubes as possible to reduce the contact surface that protein may spray. Also, limit the transfer between different tools, for example, between two spins, there is no need to transfer protein from centrifuge tube to homogenizer and back again.
It is preferable to prepare [35S]-GTPγS and ligand dilutions prior to preparing membranes with GDP. [35S]-GTPγS is currently purchased in our laboratory from Perkin Elmer at a 10 mM solution with a specific activity at 1250 Ci/mmol and 12.5 mCi/mL. To avoid multiple freeze/thaw cycles, the aliquots should be stored at −20 °C until use. Since 35S has a relatively short half-life, we find that the assay works best when the cpm values are greater than 50,000 cpm when 1 μL of the 10 mM stock is diluted to ~1:200 in assay buffer (prior to use).
Protein quantity needed for each reaction needs to be tested and evaluated in the system. From our experience, too high of protein quantity does not help to increase the fold over basal stimulation as the background reading also increases. Actually, lower protein concentrations provide a better signal to noise ratio allowing for a higher fold stimulation over vehicle or buffer only wells. Lower protein concentrations also allow for more data points to be acquired using the same amount of tissue (essential for comparing ligands for bias or allosteric activity). Tissues from receptor knockout mice as well as antagonists are highly recommended to test agonist selectivity in the brain tissue as multiple receptors are present in the same preparation. The assay buffer must be empirically determined for particular receptors. In general, the assay buffer will contain a reducing agent, such as DTT, a chelating agent such as EDTA, and Mg2+ as this ion is required for agonist activity at certain receptors.
Importantly, GDP is added to promote a population of Gα proteins in the GDP-bound found form. The concentration of GDP added to the samples will depend on the relative basal activity of the system. Therefore, it is highly recommended that at the start of an assay, that stimulation curves are performed using a known efficacious agonist and varying concentrations of GDP to determine what will be best for achieving a good separation of signal to background activity. See Fig. 3 for an example of the effect of GDP concentration on serotonin 1A receptor signaling in rat hippocampus.
If we find that an assay no longer performs as we have previously seen, we find that it is usually due to the degradation of GDP. Replacing the GDP often restores the assay integrity.
A dirty harvester will produce random and nonconverging curves. We recommend using a dilute bleach solution (5 %) followed by multiple water washes, a 20 % methanol wash, and several additional washes with distilled-deionized water should be performed prior to use.
Detergent should never be used in the harvester. Drying of the plates prior to adding the aqueous scintillation cocktail and counting will improve the signal to noise ratio and the overall magnitude of the CPM read.
References
- 1.Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov. 2010;9:373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wisler J, Xiao K, Thomsen A, Lefkowitz R. Recent developments in biased agonism. Curr Opin Cell Biol. 2014;27:18. doi: 10.1016/j.ceb.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou L, Lovell KM, Frankowski KJ, Slauson SR, Phillips AM, Streicher JM, Stahl E, Schmid CL, Hodder P, Madoux F. Development of functionally selective, small molecule agonists at kappa opioid receptors. J Biol Chem. 2013;288:36703–36716. doi: 10.1074/jbc.M113.504381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahmeh R, Damian M, Cottet M, Orcel H, Mendre C, Durroux T, Sharma KS, Durand G, Pucci B, Trinquet E. Structural insights into biased G protein-coupled receptor signaling revealed by fluorescence spectroscopy. Proc Natl Acad Sci. 2012;109:6733–6738. doi: 10.1073/pnas.1201093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rivero G, Llorente J, McPherson J, Cooke A, Mundell SJ, McArdle CA, Rosethorne EM, Charlton SJ, Krasel C, Bailey CP. Endomorphin-2: a biased agonist at the μ-opioid receptor. Mol Pharmacol. 2012;82:178–188. doi: 10.1124/mol.112.078659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hurowitz EH, Melnyk JM, Chen Y-J, KourosMehr H, Simon MI, Shizuya H. Genomic characterization of the human heterotrimeric G protein α, β, and γ subunit genes. DNA Res. 2000;7:111–120. doi: 10.1093/dnares/7.2.111. [DOI] [PubMed] [Google Scholar]
- 7.Baltoumas FA, Theodoropoulou MC, Hamodrakas SJ. Interactions of the α-subunits of heterotrimeric G-proteins with GPCRs, effectors and RGS proteins: a critical review and analysis of interacting surfaces, conformational shifts, structural diversity and electrostatic potentials. J Struct Biol. 2013;182:209–218. doi: 10.1016/j.jsb.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Schmid CL, Streicher JM, Groer CE, Munro TA, Zhou L, Bohn LM. Functional selectivity of 6′-guanidinonaltrindole (6′-GNTI) at κ-opioid receptors in striatal neurons. J Biol Chem. 2013;288:22387–22398. doi: 10.1074/jbc.M113.476234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin F-T. Enhanced morphine analgesia in mice lacking β-arrestin 2. Science. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- 10.Dennis I, Whalley BJ, Stephens GJ. Effects of Δ9-tetrahydrocannabivarin on [35S] GTPγS binding in mouse brain cerebellum and piriform cortex membranes. Br J Pharmacol. 2008;154:1349–1358. doi: 10.1038/bjp.2008.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hungund B, Vinod K, Kassir S, Basavarajappa B, Yalamanchili R, Cooper T, Mann J, Arango V. Upregulation of CB1 receptors and agonist-stimulated [35S]GTPγS binding in the prefrontal cortex of depressed suicide victims. Mol Psychiatry. 2004;9:184–190. doi: 10.1038/sj.mp.4001376. [DOI] [PubMed] [Google Scholar]
- 12.Jin LQ, Wang HY, Friedman E. Stimulated D1 dopamine receptors couple to multiple Gα proteins in different brain regions. J Neurochem. 2001;78:981–990. doi: 10.1046/j.1471-4159.2001.00470.x. [DOI] [PubMed] [Google Scholar]
- 13.Márki Á , Monory K, Ötvös F, Tóth G, Krassnig R, Schmidhammer H, Traynor JR, Roques BP, Maldonado R, Borsodi A. μ-Opioid receptor specific antagonist cyprodime: characterization by in vitro radioligand and [35S] GTPγS binding assays. Eur J Pharmacol. 1999;383:209–214. doi: 10.1016/s0014-2999(99)00610-x. [DOI] [PubMed] [Google Scholar]
- 14.Panchalingam S, Undie AS. Optimized binding of [35S] GTPγS to Gq-like proteins stimulated with dopamine D1-like receptor agonists. Neurochem Res. 2000;25:759–767. doi: 10.1023/a:1007553004615. [DOI] [PubMed] [Google Scholar]
- 15.Szekeres PG, Traynor JR. Delta opioid modulation of the binding of guanosine-5′-O- (3-[35S] thio) triphosphate to NG108–15 cell membranes: characterization of agonist and inverse agonist effects. J Pharmacol Exp Ther. 1997;283:1276–1284. [PubMed] [Google Scholar]
- 16.Odagaki Y, Toyoshima R. Dopamine D 2 receptor-mediated G protein activation assessed by agonist-stimulated [35S] guanosine 5′-O-(γ-thiotriphosphate) binding in rat striatal membranes. Prog Neuro-Psychopharmacol Biol Psychiatr. 2006;30:1304–1312. doi: 10.1016/j.pnpbp.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Tang K, Inan S, Siebert D, Holzgrabe U, Lee DY, Huang P, Li J-G, Cowan A, Liu-Chen L-Y. Comparison of pharmacological activities of three distinct κ ligands (salvinorin A, TRK-820 and 3FLB) on κ opioid receptors in vitro and their antipruritic and antinociceptive activities in vivo. J Pharmacol Exp Ther. 2005;312:220–230. doi: 10.1124/jpet.104.073668. [DOI] [PubMed] [Google Scholar]
- 18.Savinainen JR, Järvinen T, Laine K, Laitinen JT. Despite substantial degradation, 2- arachidonoylglycerol is a potent full efficacy agonist mediating CB1 receptor-dependent G- protein activation in rat cerebellar membranes. Br J Pharmacol. 2001;134:664–672. doi: 10.1038/sj.bjp.0704297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sim-Selley L, Daunais J, Porrino L, Childers S. Mu and kappa 1 opioid-stimulated [35S] guanylyl-5′-O-(γ-THIO)-triphosphate binding in cynomolgus monkey brain. Neuroscience. 1999;94:651–662. doi: 10.1016/s0306-4522(99)00344-9. [DOI] [PubMed] [Google Scholar]
- 20.Rinken A, Finnman U-B, Fuxe K. Pharmacological characterization of dopamine-stimulated [35S]-guanosine 5′-(γ-thiotriphosphate)([35S] GTPγS) binding in rat striatal membranes. Biochem Pharmacol. 1999;57:155–162. doi: 10.1016/s0006-2952(98)00287-1. [DOI] [PubMed] [Google Scholar]
- 21.Albrecht E, Samovilova NN, Oswald S, Baeger I, Berger H. Nociceptin (orphanin FQ): high-affinity and high-capacity binding site coupled to low-potency stimulation of guanylyl-5′-O-(γ-thio)-triphosphate binding in rat brain membranes. J Pharmacol Exp Ther. 1998;286:896–902. [PubMed] [Google Scholar]
- 22.Zhu J, Luo L-Y, Li J-G, Chen C, Liu-Chen L-Y. Activation of the cloned human kappa opioid receptor by agonists enhances [35S] GTPγS binding to membranes: determination of potencies and efficacies of ligands. J Pharmacol Exp Ther. 1997;282:676–684. [PubMed] [Google Scholar]
- 23.Selley DE, Sim LJ, Xiao R, Liu Q, Childers SR. μ-Opioid receptor-stimulated guanosine-5′-O-(γ-thio)-triphosphate binding in rat thalamus and cultured cell lines: signal transduction mechanisms underlying agonist efficacy. Mol Pharmacol. 1997;51:87–96. doi: 10.1124/mol.51.1.87. [DOI] [PubMed] [Google Scholar]
- 24.Traynor JR, Nahorski SR. Modulation by mu-opioid agonists of guanosine-5′-O-(3-[35S] thio) triphosphate binding to membranes from human neuroblastoma SH-SY5Y cells. Mol Pharmacol. 1995;47:848–854. doi: 10.1016/S0026-895X(25)08634-1. [DOI] [PubMed] [Google Scholar]
- 25.Sim LJ, Selley DE, Childers SR. In vitro autoradiography of receptor-activated G proteins in rat brain by agonist-stimulated guanylyl 5′-[gamma-[35S] thio]-triphosphate binding. Proc Natl Acad Sci. 1995;92:7242–7246. doi: 10.1073/pnas.92.16.7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wettschureck N, Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol Rev. 2005;85:1159–1204. doi: 10.1152/physrev.00003.2005. [DOI] [PubMed] [Google Scholar]
- 27.Hinton DR, Blanks JC, Fong H, Casey PJ, Hildebrandt E, Simons MI. Novel localization of a G protein, Gz-alpha, in neurons of brain and retina. J Neurosci. 1990;10:2763–2770. doi: 10.1523/JNEUROSCI.10-08-02763.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matesic D, Manning D, Wolfe B, Luthin G. Pharmacological and biochemical characterization of complexes of muscarinic acetylcholine receptor and guanine nucleotide-binding protein. J Biol Chem. 1989;264:21638–21645. [PubMed] [Google Scholar]
- 29.Law SF, Manning D, Reisine T. Identification of the subunits of GTP-binding proteins coupled to somatostatin receptors. J Biol Chem. 1991;266:17885–17897. [PubMed] [Google Scholar]
- 30.Law S, Reisine T. Agonist binding to rat brain somatostatin receptors alters the interaction of the receptors with guanine nucleotide-binding regulatory proteins. Mol Pharmacol. 1992;42:398–402. [PubMed] [Google Scholar]
- 31.Chalecka-Franaszek E, Weems HB, Crowder AT, Cox BM, Côté TE. Immunoprecipitation of high-affinity, guanine nucleotide-sensitive, solubilized μ-opioid receptors from rat brain. J Neurochem. 2000;74:1068–1078. doi: 10.1046/j.1471-4159.2000.0741068.x. [DOI] [PubMed] [Google Scholar]
- 32.Sidhu A, Kimura K, Uh M, White BH, Patel S. Multiple coupling of human D5 dopa-mine receptors to guanine nucleotide binding proteins Gs and Gz. J Neurochem. 1998;70:2459–2467. doi: 10.1046/j.1471-4159.1998.70062459.x. [DOI] [PubMed] [Google Scholar]
- 33.Georgoussi Z, Milligan G, Zioudrou C. Immunoprecipitation of opioid receptor-Go-protein complexes using specific GTP-binding-protein antisera. Biochem J. 1995;306:71–75. doi: 10.1042/bj3060071. [DOI] [PMC free article] [PubMed] [Google Scholar]