Abstract
Protein aggregation and particle formation have been observed when protein solutions contact hydrophobic interfaces, and it has been suggested that this undesirable phenomenon may be initiated by interfacial adsorption and subsequent gelation of the protein. The addition of surfactants, such as polysorbate 20, to protein formulations has been proposed as a way to reduce protein adsorption at silicone oil-water interfaces and mitigate the production of aggregates and particles. In an accelerated stability study, monoclonal antibody formulations containing varying concentrations of polysorbate 20 were incubated and agitated in pre-filled glass syringes (PFS), exposing the protein to silicone oil-water interfaces at the siliconized syringe walls, air-water interfaces, and agitation stress. Following agitation in siliconized syringes that contained an air bubble, lower particle concentrations were measured in the surfactant-containing antibody formulations than in surfactant-free formulations. Polysorbate 20 reduced particle formation when added at concentrations above or below the critical micelle concentration (CMC). The ability of polysorbate 20 to decrease particle generation in PFS corresponded with its ability to inhibit gelation of the adsorbed protein layer, which was assessed by measuring the interfacial diffusion of individual antibody molecules at the silicone oil-water interface using total internal reflectance fluorescence (TIRF) microscopy with single-molecule tracking.
Keywords: PFS, silicone oil, microparticles, protein formulation, protein aggregation, surfactant, adsorption, monoclonal antibody, TIRFM, protein gelation, interfacial diffusion
Introduction
Therapeutic protein molecules may encounter a variety of interfaces (air-liquid, solid-liquid, and liquid-liquid) during their manufacturing, transportation, and storage. Proteins are generally surface active and readily adsorb to many interfaces.1 In some formulations, adsorbed proteins may undergo conformational changes at interfaces,2–9 and they also may form viscoelastic interfacial protein gels.10–13 In turn, formation of interfacial gels may be associated with agitation-induced formation of protein aggregates.12, 13
Interfaces are a particular concern for protein therapeutics formulated in glass prefilled syringes (PFS). In PFS, protein molecules may be exposed to air-water interfaces due to air bubbles that typically remain after syringe filling and stoppering. In addition, because silicone oil is often used as a lubricant on the syringe wall to provide low, smooth glide forces during injection, protein molecules may encounter silicone oil-water interfaces in PFS. Adsorption to air-water interfaces and silicone oil-water interfaces has been shown to foster protein aggregation and particle formation.9, 14–19
A common strategy used by the biopharmaceutical industry to decrease the negative effects associated with protein adsorption to interfaces is to add nonionic surfactants such as polysorbate 20 (Tween 20®) or polysorbate 80 (Tween 80®) to protein formulations.20, 21 The addition of nonionic surfactants has been shown to decrease protein aggregation22–27 and inhibit the formation of visible and sub-visible particles25, 28 in a number of protein formulations subjected to a variety of stress conditions. The protective effects of surfactants are commonly attributed to competitive adsorption of the surfactant to interfaces12, 23, 29–31 or to the formation of surfactant-protein complexes.26, 27, 32 Because of their strong affinity for interfaces, it has been proposed that surfactants may out-compete proteins for adsorption to interfaces, an effect that should correlate with the critical micelle concentration (CMC) of the surfactant.29 Polysorbate 80 has been shown to decrease the amount of lysozyme and Factor VIII that adsorb on hydrophobic silica surfaces,33, 34 and the addition of polysorbate 20 decreased the adsorption of four different model proteins at the silicone oil-water interface.31 Polysorbate 20 is also effective at displacing β-lactoglobulin from the n-hexadecane-water interface.35 Some proteins also form surfactant-protein complexes which inhibit aggregation.32 Polysorbate 20 binds to hydrophobic patches on the surface of recombinant human growth hormone and decreases aggregation at surfactant:protein molar ratios above 2.32 Furthermore, at concentrations below their respective CMC’s, polysorbate 20 and polysorbate 80 inhibit agitation-induced aggregation of Albutropin and darbepoetin alfa due to the formation of surfactant-protein complexes.26, 27
An additional effect of surfactants on proteins adsorbed to interfaces is the ability of surfactants to inhibit gelation of adsorbed protein layers. Polysorbate 20 prevented gelation of β-lactoglobulin at the air-water interface10 and at the n-hexadecane-water interface.35 Addition of polysorbate 20 to formulations of keratinocyte growth factor 2 (KGF-2) also prevented gelation at the air-water interface, and the addition of polysorbate 20 to a pre-formed KGF-2 gel caused the gel to break down.12 Reversal of the gelation process was also observed when sodium dodecyl sulfate (SDS) was added to a pre-formed β-casein gel.11
Recently, several studies attributed agitation-induced aggregation and particle formation in protein formulations to mechanical rupture of the adsorbed protein gel layer at air-water interfaces and at oil-water interfaces.13, 16, 17, 36 Previously, we studied protein aggregation and particle formation in surfactant-free protein formulations in siliconized PFS. We observed that, especially in the presence of air-water interfaces, agitation induced extensive particle formation. We attributed this particle generation to agitation-induced rupture of a gelled protein layer at the silicone oil-water interface.36 In the current study, we hypothesize that the addition of a nonionic surfactant to a protein formulation will inhibit interfacial gel formation at the silicone oil-water interface and thus reduce the number of particles generated in similarly agitated PFS.
To test our hypothesis, we added the nonionic surfactant polysorbate 20 at concentrations that spanned a range above and below the critical micelle concentration (CMC) to formulations of a model monoclonal antibody. These formulations were filled into glass syringes which were subsequently agitated by end-over-end rotation. After agitation, the concentrations of particles in the formulations were measured. In addition, particle generation was monitored in formulations wherein the polysorbate 20:monoclonal antibody molar ratio was varied in order to probe whether protective effects were related to the CMC of polysorbate 20 or to specific binding of polysorbate 20 to the monoclonal antibody. Finally, to assess the ability of polysorbate 20 to inhibit formation of interfacial protein gels, we used total internal reflectance fluorescence (TIRF) microscopy with single-molecule tracking to measure the effect of various bulk concentrations of polysorbate 20 on the interfacial diffusion of single fluorescently-labeled monoclonal antibody molecules adsorbed to silicone oil-water interfaces.
Materials and Methods
Materials
Humanized IgG1 monoclonal antibody (molecular weight 146 kDa), here denoted as “3M”, was provided by MedImmune (Gaithersburg, MD).37 The antibody was obtained at a stock concentration of 150 mg/mL in 10 mM L-histidine at pH 6. The antibody 3M is a human IgG1 with three mutations (S239D/A330L/I332E) in the CH2 portion of the Fc. These mutations reduce the thermal stability of 3M,37 which was chosen for the current work because of previous studies36 that showed it to be prone to aggregation when exposed to silicone oil-water interfaces. Polysorbate 20 (>97% purity, Fisher BioReagents) was obtained from Fisher Scientific (Pittsburgh, PA). All buffer salts were of reagent grade or higher, and all solutions were prepared in de-ionized water filtered through a 0.22 μm Millipore filter (Billerica, MA). Silicone oil (Dow Corning 360, 100 cSt) was of medical grade and purchased from Nexeo Solutions (Denver, CO). The syringes used in the incubation studies were BD Hypak SCF 1mL long 27G1/2 (BD Medical-Pharmaceutical Systems, Franklin Lakes, NJ). Glass coverslips, Micro-90, and isopropyl alcohol were obtained from Fisher Scientific (Waltham, MA). Nickel TEM grids (EMS G100-Ni) were obtained from Electron Microscopy Sciences (Hatfield, PA). Teflon® rings were fabricated in-house at the University of Colorado-Boulder.
Incubation of 3M Formulation with Polysorbate 20 (Above CMC) in PFS
A formulation containing 1mg/mL 3M with 0.01% v/v polysorbate 20 in 10 mM L-histidine pH 5 was prepared using the 3M stock (described above) and a 1% v/v stock solution of polysorbate 20 in 10 mM L-histidine pH 5. This 3M formulation was used to fill glass syringes. Prior to filling, the silicone oil coating on some of the syringes was removed, as previously described.36 To prepare syringes containing an air bubble, 1.26 mL of the formulation was pipetted into the syringe, and the syringe was stoppered, creating a headspace containing 30 μL of air. The air-water interfacial area associated with this bubble was approximately 0.5 cm2, or about 5% of the wetted silicone oil-water interfacial area to which protein was exposed to in each syringe. For incubation conditions without headspace, the syringes were stoppered such that no air bubbles remained. Triplicate syringes were prepared for each incubation condition at each time-point. For incubation conditions with agitation, the syringes were rotated end-over-end at 1.5 rpm at room temperature. For quiescent incubation conditions, the syringes were incubated horizontally on the bench top at room temperature. In addition, solutions containing 10 mM L-histidine buffer only (no protein) were incubated in siliconized syringes either with or without headspace.
Agitation of 3M Formulations with Varying Surfactant:Protein Ratios in PFS
To evaluate how the surfactant:protein molar ratio in the formulation affects the number of particles generated by agitation in PFS, protein formulations containing polysorbate 20 at surfactant:protein molar ratios ranging from 0 to 13.1 were prepared by varying the polysorbate 20 concentration and the 3M concentration (Table 1). A volume of 1.26 mL of each formulation was pipetted into siliconized syringes, and the syringes were stoppered such that a headspace containing 30 μL of air remained in the syringe. Triplicate syringes were prepared for each surfactant:protein molar ratio, and the syringes were rotated end-over-end at 1.5 rpm for 24 hours at room temperature.
Table 1.
3M concentrations and polysorbate 20 concentrations corresponding to the polysorbate 20:3M molar ratios used in the formulations tested.a
| Polysorbate 20:3M Molar Ratio | Polysorbate 20 Concentration (% v/v) | 3M Concentration (mg/mL) |
|---|---|---|
| 0.0 | 0.0000 | 1.0 |
| 0.1 | 0.0005 | 7.6 |
| 0.3 | 0.0005 | 2.2 |
| 0.7 | 0.0005 | 1.0 |
| 1.3 | 0.0010 | 1.0 |
| 2.6 | 0.0020 | 1.0 |
| 6.5 | 0.0050 | 1.0 |
| 13.1 | 0.0100 | 1.0 |
| 13.1 | 0.0800 | 7.6 |
The polysorbate 20 CMC is 0.007% v/v (0.06 mM).43
Counting of Particles in Incubated 3M Formulations
Using the same protocol described previously,36 at each time-point during the incubation, syringes were un-stoppered, and the formulation was removed from the flanged end of the syringe using a transfer pipet. The protein formulation was not ejected using the syringe needle to avoid the generation of particles due to plunger movement along the syringe barrel. For each sample, particles between 2 μm to 2 mm (equivalent spherical diameter) were counted using a Fluid Imaging Technologies Benchtop FlowCAM® (Scarborough, ME). The FlowCAM was fitted with a FC100 flow cell, a 10X objective and collimator, and a 0.5 mL syringe. The gain and flash duration were set such that the average intensity mean of the image was consistently between 180 and 200. A sample volume of 0.2 mL was analyzed for each sample at a flow rate of 0.145 mL/min. Particle counts were normalized by dividing the number of particles per sample by the total volume imaged per sample to obtain the particle concentration (#/mL). In addition to the samples incubated in syringes, triplicate samples of a buffer solution and a protein solution (both containing 0.01% v/v polysorbate 20) that had not been incubated in syringes were analyzed by FlowCAM. Furthermore, triplicate samples of buffer solutions and protein solutions containing the polysorbate 20 concentration and the 3M concentration corresponding to each surfactant:protein molar ratio tested were analyzed by FlowCAM without prior incubation.
3M Labeling with Alexa Fluor 555
For TIRF microscopy experiments, 3M was dialyzed into 10 mM sodium acetate pH 5 and was labeled with Alexa Fluor® 555 succinimidyl ester (Molecular Probes, Eugene, OR) following the manufacturer’s protocol (MP 30007). The average labeling efficiency was 9±1 fluorophores per protein molecule and was measured using UV-visible spectroscopy at 280 nm and 555 nm, following the manufacturer’s protocol. After labeling, the protein was dialyzed back into 10 mM L-histidine pH 5 using a Slide-A-Lyzer 10,000 MWCO dialysis cassette (Thermo Scientific, Rockford, IL) before TIRF microscopy experiments were conducted.
After labeling and dialysis, labeled 3M was analyzed by size exclusion chromatography (SEC) using a TSK-GEL G3000SWXL column with guard column (TOSOH Biosciences, Montgomeryville, PA). The flowrate was 0.6 mL/min, and the mobile phase was 0.2 M potassium phosphate monobasic, 0.2 M potassium chloride, and 0.1 g/L sodium azide at pH 7. The absorbance was monitored at 280 nm using a Beckman Coulter (Fullerton, CA) System Gold 166 UV detector. SEC analysis confirmed that labeled 3M was in a monomeric state.
3M Molecule Tracking Using Total Internal Reflection Fluorescence (TIRF) Microscopy
TIRF microscopy experiments to examine the effects of added surfactant on the ability of 3M to form interfacial gels were performed using a Nikon Eclipse TI-93 outfitted with a custom illuminator used in conjunction with a 100x oil immersion objective. A cooled CCD camera (Cascade 512B, Photometrics, Tucson, AZ) operating at −80°C was used to capture a sequence of images with a typical acquisition time of 200 ms per image. A Cobolt Samba laser (San Jose, CA) emitting at 532 nm was used as an excitation source; 900 frame movies were captured, corresponding to ca. 3 minutes in duration.
For TIRF microscopy experiments, glass coverslips were cleaned using a cationic surfactant (Micro-90®), Millipore-filtered water to a resistance of 18.2 M-ohm-cm, and isopropyl alcohol and were then dried under a nitrogen stream. Silicone oil droplets with a viscosity of 100 cSt were added to the clean coverslips and stabilized using a nickel transmission electron microscopy (TEM) grid, ensuring a stable planar interface between the silicone oil and the buffer.38–40 A Teflon® ring was then placed in contact with the coverslip, surrounding the silicone oil-filled TEM grid and creating a well to contain a small volume of buffer. A volume of 100 μl of buffer containing 10−6 mg/mL Alexa555-labeled 3M was added to the well, and 15 minutes was allowed for the system to equilibrate, after which time a movie was taken. Then, 100 μl of a solution containing polysorbate 20 and unlabeled 3M at various surfactant:protein molar ratios (Table 1) and doped with 10−6 mg/mL of labeled 3M were added to the well, and again 15 minutes was allowed for the system to reach equilibrium. Upon equilibrium, three movies were captured in various locations on the silicone oil-water the interface.
TIRF Data Analysis
TIRF movies were analyzed using custom-designed molecule identification and tracking algorithms wherein molecules were identified by a fluorescence intensity threshold. After molecules were identified for each frame, molecular trajectories were linked between frames such that an identified object observed within a 0.8 μm radius of an identified object in the previous frame was classified as the same object.41, 42 The diffusion coefficient of each molecule was calculated assuming first-order Fickian diffusion kinetics based on the molecule’s mean squared displacement and the frame rate. From the diffusion coefficient of each individual trajectory, the arithmetic mean was calculated, yielding the mean interfacial diffusion coefficient for 3M molecules adsorbed at the silicone oil-water interface under each condition tested.
Results
Particle Concentrations in 3M Formulation with 0.01% v/v Polysorbate 20 after Incubation in PFS
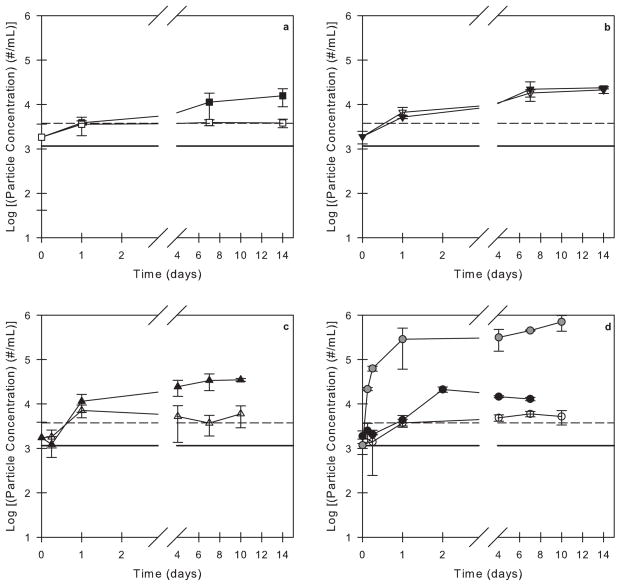
After incubation in PFS, particles of a size greater than 2 μm were detected in all of the 3M and the protein-free formulations that contained 0.01% v/v polysorbate 20. As reported previously36, nearly all particles observed in the protein-free formulations were spherical, as is characteristic of silicone oil droplets. In the presence of protein, mixed particles were observed that contained a combination of spherical droplets (presumed to be silicone oil) associated with irregularly-shaped aggregates characteristic of aggregated protein. In all cases, even after incubation periods of up to two weeks, the particle concentrations did not exceed 100,000 particles/mL (Figure 1).
Figure 1.
Particle concentrations in 3M formulations and buffer solutions with 0.01% v/v polysorbate 20 agitated in PFS as a function of time. Open symbols correspond to syringes incubated with no air bubble and closed symbols correspond to syringes incubated with an air bubble. The particle concentrations in a buffer solution (solid black line) and in a 3M solution (dashed black line) with 0.01% v/v polysorbate 20 that were not incubated in syringes are also shown. The incubation conditions are as follows: (a) L-histidine buffer (no protein) in agitated, siliconized syringes, (b) 3M formulation in quiescent, siliconized syringes, (c) 3M formulation in agitated, un-siliconized syringes, and (d) 3M formulation in agitated, siliconized syringes. For comparison, the gray symbols in panel (d) correspond to a 3M formulation with no surfactant agitated in siliconized syringes with an air bubble (data reproduced from Gerhardt et al.36).
The presence of an air bubble within PFS caused a small increase in the particle concentrations observed during agitation of 3M formulations in both siliconized and un-siliconized syringes (Figure 1c and 1d). In contrast, in the absence of air-water interfaces, particle counts were roughly constant (Figure 1c and 1d, open symbols), and there was not a noticeable difference in the particle concentrations between syringes that were siliconized and syringes that were un-siliconized. For comparison, Figure 1d also shows particle concentration data collected in a previous study36 for a 3M formulation without polysorbate 20 agitated in siliconized syringes with an air bubble. In the absence of polysorbate 20, the particle counts increased above 100,000 particles/mL after only 1 day of agitation.36
Particle Concentrations in 3M Formulations Containing Various Surfactant:Protein Ratios After Agitation in PFS
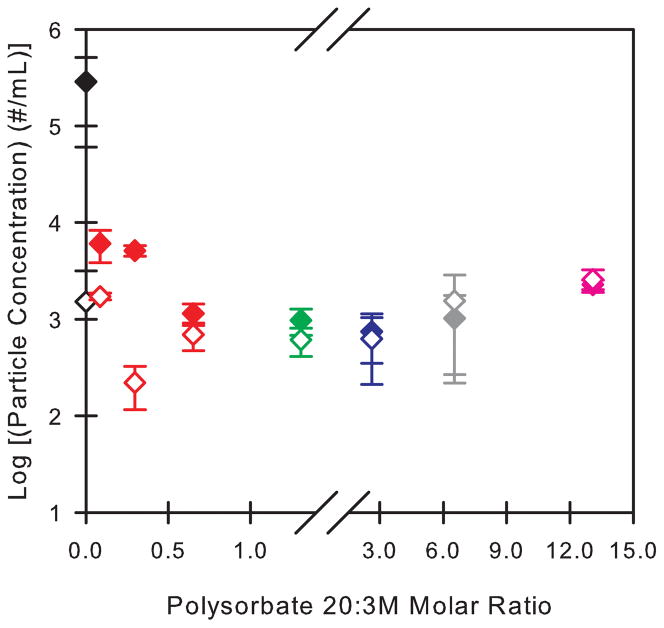
Particle generation in 3M formulations containing polysorbate 20 at various surfactant:protein molar ratios was monitored before and after 24 hours of agitation in siliconized PFS containing a headspace volume of 30 μL (Figure 2). The resulting change in particle concentrations depended on the polysorbate 20:3M molar ratio. The greatest increase in particle concentrations was measured in polysorbate-free 3M formulations. In this case, there was a two order of magnitude increase in the particle concentrations after the 24 hour agitation period (Figure 2). Addition of even very small amounts of polysorbate 20 (e.g. at a polysorbate 20:3M molar ratio of 0.1) reduced the number of particles formed, although particle generation was not completely inhibited at surfactant:protein molar ratios of 0.1 and 0.3. The inhibitory effect of polysorbate 20 on particle formation increased at higher molar ratios, appearing to reach a plateau at polysorbate 20:3M molar ratios greater than ca. 1, where agitation resulted in only minimal increases in particle concentrations.
Figure 2.
Particle concentrations measured in 3M formulations as a function of the polysorbate 20:3M molar ratio in the formulation. Open symbols represent the particle concentrations in 3M formulations that were not incubated. Closed symbols represent the particle concentrations in 3M formulations that were agitated for 24 hours with an air bubble in siliconized syringes. The color of each symbol corresponds to the polysorbate 20 concentration (% v/v) in the formulation: 0% (black), 0.0005% (red), 0.001% (green), 0.002% (blue), 0.005% (gray), 0.01% (pink). At each polysorbate 20:3M ratio, the particle concentration in a buffer solution with the same polysorbate 20 concentration was subtracted from the particle concentration measured in the non-incubated 3M formulation and from that measured in the agitated 3M formulation.
Interfacial Diffusion of Labeled 3M Molecules at the Silicone Oil-Water Interface in Formulations with Varying Surfactant:Protein Molar Ratios
To assess the effects of polysorbate 20 on the formation of 3M gels at the silicone oil-water interface, trace amounts (10−6 mg/mL, ca. 10−11 M) of Alexa Fluor®-labeled 3M were added as reporter molecules to solutions of 3M at various bulk concentrations, and the mean diffusion coefficient of the labeled 3M at the silicone oil-water interface was measured as a function of the polysorbate 20:unlabeled 3M bulk molar ratio (Figure 3) using TIRF microscopy.
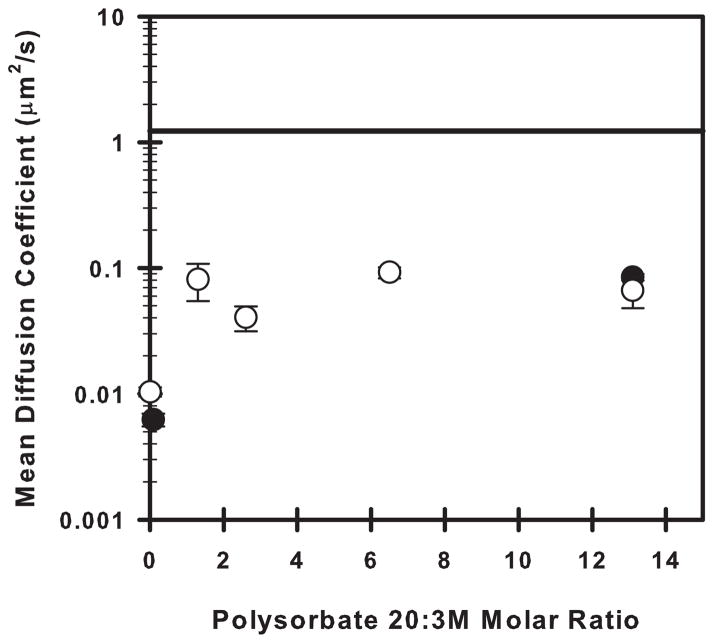
Figure 3.
Mean diffusion coefficients (μm2/s) of labeled 3M at the silicone oil-water interface as a function of the polysorbate 20:unlabeled 3M molar ratio. The open symbols correspond to formulations with a bulk unlabeled 3M concentration of 1.0 mg/mL. The closed symbols correspond to formulations with a bulk unlabeled 3M concentration of 7.6 mg/mL. The solid black line represents the mean diffusion coefficient of interfacially-adsorbed labeled 3M molecules in the absence of polysorbate 20 and without addition of any unlabeled 3M.
In the absence of unlabeled 3M and polysorbate 20, the mean diffusion coefficient of labeled 3M molecules at the silicone oil-water interface was assumed to reflect only protein-interface interactions (Figure 3, solid line). This assumption was made because the bulk concentration (10−6 mg/mL) of labeled 3M was quite low, and the labeled molecules that could be individually observed on the interface appeared to be at low surface density.
In samples that contained 3M at bulk concentrations ranging from 1.0–7.6 mg/mL, the interfacial mean diffusion coefficients of the labeled 3M molecules were significantly reduced compared to the sample that contained only labeled 3M at 10−6 mg/mL (Figure 3). Furthermore, in samples with added unlabeled 3M that did not contain polysorbate 20, gels formed at the silicone oil-water interface, as evidenced by mean diffusion coefficients for the labeled 3M molecules that were almost zero (Figure 3).
Addition of polysorbate 20 inhibited gel formation in samples containing added, unlabeled 3M. Although diffusion coefficients for 3M on the silicone oil-water interface in these samples were approximately four-fold smaller than those measured in samples containing only labeled 3M at a bulk concentration of 10−6 mg/mL (presumably due to hindrance of diffusion by other protein molecules at the crowded interface), diffusion was much more rapid than in corresponding samples without polysorbate 20 (Figure 3). Over the range of 3M bulk concentrations (1.0–7.6 mg/mL) tested, the mean diffusion coefficients were roughly constant when the surfactant:protein molar ratio was above ca. 1.
Discussion
Particle Generation in Antibody Formulations in PFS
In a previous study,36 large numbers of particles were observed when surfactant-free formulations of 3M were agitated in siliconized syringes containing headspace. We proposed a mechanism wherein surface tension forces at the three-phase (silicone oil-water-air) contact line ruptured layers of adsorbed, gelled proteins leading to the creation of particles.
We hypothesized that the addition of nonionic surfactant would decrease the number of particles generated by agitation in siliconized PFS by inhibiting the formation of a gelled protein layer at the silicone oil-water interface. This hypothesis was first tested in a 3M formulation containing 0.01% v/v polysorbate 20. At this concentration of polysorbate 20, which is above the reported CMC of 0.007% v/v,43 the interfaces present in the pre-filled syringe are expected to be saturated with surfactant. Addition of 0.01% v/v polysorbate 20 inhibited the formation of particles, and there were only minimal differences in particle concentrations between formulations agitated in siliconized and un-siliconized syringes (Figure 1c and 1d, closed symbols). In contrast, our previous study demonstrated that in the absence of polysorbate 20, particle generation was at least one order of magnitude greater in siliconized syringes than in un-siliconized syringes.36
Influence of Polysorbate 20 Concentration on Particle Generation in PFS
In order to investigate further the mechanism by which surfactants inhibit interface-induced particle generation, the polysorbate 20 concentration and the polysorbate 20:3M molar ratio in the protein formulation were varied. For the 1 mg/mL 3M formulation with 0.01% v/v polysorbate 20 used in the first part of this study, the polysorbate 20:3M molar ratio was 13.1 (Table 1), and the polysorbate 20 concentration was above its CMC. For polysorbate 20:3M molar ratios ≥ 0.7, the 3M concentration used in the formulation was 1.0 mg/mL, and the molar ratio was manipulated by changing the polysorbate 20 concentration (Table 1). To obtain polysorbate 20:3M molar ratios < 0.7, the 3M concentration was increased while the polysorbate 20 concentration was held constant at 0.0005% v/v (Table 1).
If saturation of the silicone oil-water interface with surfactant were necessary to inhibit interfacial particle generation in a pre-filled syringe, then particle generation would not be anticipated to be inhibited by the presence of polysorbate 20 at polysorbate 20 concentrations below the CMC, where the interface is not saturated with surfactant. However, we observed that particle generation in 1 mg/ml 3M formulations was almost completely inhibited at polysorbate 20 concentrations well below the CMC (Figure 2). Interestingly, the ability of sub-CMC levels of surfactant to inhibit particle formation decreased as the molar ratio of polysorbate 20:3M decreased. At a constant polysorbate 20 concentration of 0.0005% v/v, particle formation was almost completely inhibited at a polysorbate 20:3M ratios of 0.7 or greater. In contrast, at ratios of 0.1 and 0.3, particle formation, although somewhat less than that observed in polysorbate-free formulations, was not inhibited to the same degree. A potential explanation for this behavior is that polysorbate 20 binding to 3M is responsible for inhibiting 3M aggregation at the silicone oil-water interface.
Influence of Polysorbate 20 Concentration on Gelation of 3M Molecules at the Silicone Oil-Water Interface
We hypothesized that the addition of polysorbate 20 would inhibit the gelation of 3M molecules at the silicone oil-water interface. Therefore, we used TIRF microscopy to directly monitor the interfacial diffusion of fluorescently-labeled 3M molecules on the silicone oil-water interface. As a control, the mean diffusion coefficient of labeled 3M (bulk concentration 10−6 mg/mL) was measured in the absence of polysorbate 20 and without added unlabeled 3M. In the absence of both polysorbate 20 and unlabeled 3M, the mean diffusion coefficient, ca. 0.2 μm2/s, reflected the mobility of 3M molecules at the silicone oil-water interface without any crowding due to the adsorption of polysorbate 20 or other 3M molecules (Figure 3, solid line).
In all other TIRF microscopy experiments, the amount adsorbed at the interface was much higher due to the higher bulk concentrations of polysorbate 20 and unlabeled 3M. At these higher concentrations, the mean diffusion coefficient was slower than that measured for the control due to crowding at the interface and resulting interactions between adsorbed molecules. Furthermore, when the adsorbed layer formed gels, large-scale translational motions of 3M molecules were no longer observed, and the interfacial diffusion coefficients were effectively zero.
In formulations with polysorbate 20:3M molar ratios above ca. 1, observed values of the mean diffusion coefficient of 3M molecules at the silicone oil-water interface were between 0.06–0.08 μm2/s (Figure 3). Because the protein molecules were observed to be diffusing relatively rapidly on the interface, we inferred that, under these conditions, the protein adsorbed to the silicone oil-water interface did not form gels. Furthermore, in the TIRF microscopy method that we used, the motion of any protein molecules that were present in the liquid near, but not adsorbed to, the interface was so fast that these protein molecules could not be tracked individually and registered simply as background fluorescence. On the interface, individual 3M molecules could be observed to diffuse, and thus, we may infer that the presence of polysorbate 20 did not completely prevent 3M molecules from adsorbing to the interface, even at polysorbate 20 concentrations above its CMC. However, interfacial diffusion of 3M in formulations with polysorbate 20:3M molar ratios < 1 was dramatically decreased, suggesting that interfacial gels formed under conditions with very low polysorbate 20:3M ratios. This correlates with the higher particle concentrations observed in agitated 3M formulations with polysorbate 20:3M molar ratios < 1. The number of particles generated in the formulations increased (Figure 2) because polysorbate 20 could not inhibit gelation at polysorbate 20:3M molar ratios < 1 as effectively as it could at molar ratios > 1. In a study by Courthaudon et al., surfactant:protein molar ratios as low as 1 were observed to inhibit gelation of β-lactoglobulin at the n-hexadecane-water interface.35 They also noted that the surfactant:protein molar ratio required to affect protein gelation was much lower than the molar ratio necessary to completely displace protein from the interface. In a later study, Kragel et al. demonstrated the ability of polysorbate 20, SDS, and cetyl trimethyl ammonium bromide (CTAB) to increase the surface diffusion of β-lactoglobulin at the air-water interface at concentrations below the CMC of each surfactant.44
Furthermore, the difference in gelation between the low and high polysorbate 20:3M molar ratios was not due to a difference in 3M concentration between the formulations. In addition to the aforementioned experiments that used a 1 mg/mL 3M formulation, the interfacial diffusion of labeled 3M in a formulation that contained 7.6 mg/mL 3M was measured at both a low and a high polysorbate 20:3M molar ratio. This formulation showed diffusion indicative of a non-gelled protein layer at the high molar ratio but showed diffusion indicative of a gelled protein layer at the low molar ratio (Figure 3, closed symbols).
Effects of Surfactants on Protein Gelation at Interfaces
Surfactants have been observed to protect proteins against interface-induced aggregation through several different mechanisms. The most commonly cited mechanism is preferential adsorption of the surfactant to an interface which inhibits protein adsorption at that same interface. In several different systems, surfactants at concentrations above their CMC’s were shown to decrease protein aggregation, and these protective effects were attributed to the preferential adsorption mechanism.12, 23, 29, 43 This explanation is reasonable because, at concentrations above their CMC, surfactants will saturate the interface. Furthermore, at the silicone oil-water interface specifically, Ludwig et al. observed significantly less adsorption of four different proteins to the interface in the presence of polysorbate 20 above its CMC.31
The other common mechanism by which surfactants may protect proteins against aggregation at interfaces is the stabilization of protein conformation that may result from surfactant binding to protein native-state structures. Binding of nonionic surfactants to proteins has been documented in several studies.26, 27, 32 Surfactant molecules can interact with hydrophobic patches on a protein’s surface which then inhibits the protein-protein interactions that lead to aggregation.32 Alternatively, preferential binding of surfactants to a protein molecule’s native state (as opposed to binding to non-native, unfolded states) stabilizes the native state and increases the protein’s free energy of unfolding.26 The formation of surfactant-protein complexes has been observed to protect protein formulations against interfacial damage at surfactant concentrations below the CMC26, 27, 32 because, in this mechanism, the interface does not need to be saturated with surfactant. However, these interactions between surfactant and protein that provide protective effects have not been observed for every surfactant-protein system,12, 29, 30 and in systems where surfactant-protein complexes are not formed, it has been observed that the surfactant must be present above the CMC in order to decrease interface-induced protein aggregation by the preferential adsorption mechanism.
In addition, surfactants will affect the gelation of an adsorbed protein layer. Liu, et al. showed that KGF-2 did not form an interfacial gel in the presence of 0.01% w/v polysorbate 20 because polysorbate 20 inhibited adsorption of KGF-2 to the air-water interface.12 In this case, polysorbate 20 was present above its CMC, and binding of polysorbate 20 to KGF-2 was not observed. However, other studies have shown that surfactants affect the diffusion of protein molecules at an interface at concentrations well below their CMC’s.10, 11, 35, 44–46 In these cases, the protein was adsorbed at the interface, but it did not form a gel. Instead of complete displacement of protein by surfactant at the interface, the inhibition of gelation at sub-CMC levels was likely due to the interaction of surfactant molecules with hydrophobic patches on the protein’s surface which inhibited the protein-protein interactions necessary for interfacial gel formation.
In our agitated, siliconized syringe system, particles were formed by rupture of the protein gel layer.36 Therefore, inhibition of protein gelation was hypothesized to decrease particle formation in this system. The protective effects of polysorbate 20 were seen at concentrations both above and below the CMC of polysorbate 20. At surfactant:protein molar ratios > 1, interfacial diffusion coefficient measurements indicated that the adsorbed protein layer was not gelled, but protein was still present at the silicone oil-water interface. Thus, at sub-CMC levels, it was likely that gelation was inhibited, not by complete displacement of the protein from the interface, but by the interaction of polysorbate 20 with 3M which inhibited the protein-protein interactions required for gelation. Even at polysorbate 20 concentrations above the CMC, interfacial diffusion coefficient measurements indicated that 3M molecules were still present at the silicone oil-water interface. This was consistent with quartz crystal microbalance measurements of the adsorption and viscoelastic nature of an Fc-fusion protein and polysorbate 20 at the silicone oil-water interface.47 For the Fc-fusion protein-polysorbate 20 system, both the protein and the surfactant adsorbed to the silicone oil-water interface in solutions with a polysorbate 20 concentration of 0.02% w/v (above the CMC). However, in the presence of 0.02% w/v polysorbate 20, the viscoelastic nature of the adsorbed protein layer was significantly different than the viscoelasticity of the adsorbed protein layer in the absence of polysorbate 20.47
In earlier work13 with the same mAb, we showed that, when formulated at a surfactant:protein molar ratio of approximately 13, both polysorbate 20 and polysorbate 80 were effective at reducing the number of particles formed when silicone oil-water interfaces were mechanically ruptured. At a surfactant:protein ratio of about 1.3, both surfactants reduced the number of particles that were formed but to a lesser degree than at the higher ratio. Interestingly, at a surfactant:protein ratio of 1.3, the more hydrophobic polysorbate 80 (which would likely bind more effectively to hydrophobic patches on a protein’s surface) reduced particle formation to a greater degree than did polysorbate 20. These results are consistent with our current suggestion that polysorbate protects against gelation and subsequent particle formation by binding to 3M.
Conclusions
Polysorbate 20, at concentrations above and below the CMC, was observed to decrease particle generation in formulations of a model antibody that were agitated in siliconized syringes with headspace. In PFS filled with formulations that contained polysorbate 20:antibody molar ratios above ca. 1, no increase in particle concentration could be detected after 24 hours of agitation. Also, interfacial diffusion coefficient measurements showed that, at polysorbate 20:antibody molar ratios above ca. 1, the presence of polysorbate 20 inhibited the gelation of antibody molecules adsorbed at the silicone oil-water interface. At polysorbate 20:antibody molar ratios below ca. 1, polysorbate 20 was not as effective in inhibiting gelation of adsorbed protein molecules at the silicone oil-water interface, and therefore particle generation was not completely inhibited at these ratios.
The lack of correlation between the CMC of the surfactant and its protective effect against aggregation, the apparent stoichiometric dependence of the polysorbate-induced inhibition of aggregation, as well as the TIRF observations of antibody adsorbed to the silicone oil-water interface even at surfactant concentrations above the CMC suggest that polysorbate 20 does not inhibit gelation solely by displacing the protein from the interface. Rather, we speculate that polysorbate 20 binding to the protein interferes with protein-protein interactions required for protein gelation at the silicone oil-water interface.
Acknowledgments
The authors gratefully acknowledge support from MedImmune and the National Institutes of Health grant RO1 EB006006.
References
- 1.Magdassi S, editor. Surface Activity of Proteins. 1. Boca Raton: CRC Press; 1996. [Google Scholar]
- 2.Dickinson E. Adsorbed protein layers at fluid interfaces: interactions, structure and surface rheology. Colloids and Surfaces. 1999;15:161–176. [Google Scholar]
- 3.Vermeer AW, Bremer MG, Norde W. Structural changes of IgG induced by heat treatment and by adsorption onto a hydrophobic Teflon surface studied by circular dichroism spectroscopy. Biochim Biophys Acta. 1998;1425:1–12. doi: 10.1016/s0304-4165(98)00048-8. [DOI] [PubMed] [Google Scholar]
- 4.Sluzky V, Tamada JA, Klibanov AM, Langer R. Kinetics of insulin aggregation in aqueous solutions upon agitation in the presence of hydrophobic surfaces. Proc Natl Acad Sci U S A. 1991;88:9377–9381. doi: 10.1073/pnas.88.21.9377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roach P, Farrar D, Perry CC. Interpretation of protein adsorption: surface-induced conformational changes. J Am Chem Soc. 2005;127:8168–8173. doi: 10.1021/ja042898o. [DOI] [PubMed] [Google Scholar]
- 6.Wong BT, Zhai J, Hoffmann SV, Aguilar MI, Augustin M, Wooster TJ, Day L. Conformational changes to deamidated wheat gliadins and β-casein upon adsorption to oil water emulsion interfaces. Food Hydrocoll. 2012;27:91–101. [Google Scholar]
- 7.Britt K, Schwartz D, Wurth C, Mahler H, Carpenter JF, Randolph TW. Excipient Effects on Humanized Monoclonal Antibody Interactions with Silicone Oil Emulsions. J Pharm Sci. 2012;101:4419–4432. doi: 10.1002/jps.23318. [DOI] [PubMed] [Google Scholar]
- 8.Dixit N, Maloney KM, Kalonia DS. Application of quartz crystal microbalance to study the impact of pH and ionic strength on protein-silicone oil interactions. Int J Pharm. 2011;412:20–27. doi: 10.1016/j.ijpharm.2011.03.062. [DOI] [PubMed] [Google Scholar]
- 9.Gerhardt A, Bonam K, Bee JS, Carpenter JF, Randolph TW. Ionic strength affects tertiary structure and aggregation propensity of a monoclonal antibody adsorbed to silicone oil water interfaces. J Pharm Sci. 2013;102:429–440. doi: 10.1002/jps.23408. [DOI] [PubMed] [Google Scholar]
- 10.Petkov J, Gurkov T, Campbell B, Borwankar R. Dilatational and shear elasticity of gel-like protein layers on air/water interface. Langmuir. 2000;16:3703–3711. [Google Scholar]
- 11.Bantchev G, Schwartz D. Surface Shear Rheology of Beta-Casein Layers at the Air/Solution Interface: Formation of a Two-Dimensional Physical Gel. Langmuir. 2003;19:2673–2682. [Google Scholar]
- 12.Liu L, Qi W, Schwartz D, Randolph TW, Carpenter JF. The effects of excipients on protein aggregation during agitation: An interfacial shear rheology study. J Pharm Sci. 2013;102:2460–2470. doi: 10.1002/jps.23622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta SB, Lewus R, Bee JS, Randolph TW, Carpenter JF. Gelation of a monoclonal antibody at the silicone oil-water interface and subsequent rupture of the interfacial gel results in aggregation and particle formation. J Pharm Sci. 2015 doi: 10.1002/jps.24358. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Pinnamaneni S, Quan Y, Jaiswal A, Andersson FI, Zhang X. Mechanistic understanding of protein-silicone oil interactions. Pharm Res. 2012;29:1689–1697. doi: 10.1007/s11095-012-0696-6. [DOI] [PubMed] [Google Scholar]
- 15.Felsovalyi F, Janvier S, Jouffray S, Soukiassian H, Mangiagalli P. Silicone-oil-based subvisible particles: Their detection, interactions, and regulation in prefilled container closure systems for biopharmaceuticals. J Pharm Sci. 2012;101:4569–4583. doi: 10.1002/jps.23328. [DOI] [PubMed] [Google Scholar]
- 16.Bee JS, Schwartz DK, Trabelsi S, Freund E, Stevenson JL, Carpenter JF, Randolph TW. Production of particles of therapeutic proteins at the air water interface during compression/dilation cycles. Soft Matter. 2012;8:10329–10335. [Google Scholar]
- 17.Rudiuk S, Cohen-Tannoudji L, Huille S. Importance of the dynamics of adsorption and of a transient interfacial stress on the formation of aggregates of IgG antibodies. Soft Matter. 2012;8:2651–2661. [Google Scholar]
- 18.Badkar A, Wolf A, Bohack L, Kolhe P. Development of Biotechnology Products in Pre-filled Syringes: Technical Considerations and Approaches. AAPS PharmSciTech. 2011;12:564–572. doi: 10.1208/s12249-011-9617-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Majumdar S, Ford BM, Mar KD, Sullivan VJ, Ulrich RG, D’Souza AJM. Evaluation of the effect of syringe surfaces on protein formulations. J Pharm Sci. 2011;100:2563–2573. doi: 10.1002/jps.22515. [DOI] [PubMed] [Google Scholar]
- 20.Randolph T, Jones L. Surfactant-protein interactions. In: Carpenter JF, Manning MC, editors. Rational Design of Stable Protein Formulations. New York: Springer; 2002. pp. 159–175. [Google Scholar]
- 21.Lee HJ, McAuley A, Schilke KF, McGuire J. Molecular origins of surfactant-mediated stabilization of protein drugs. Adv Drug Deliv Rev. 2011;63:1160–1171. doi: 10.1016/j.addr.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 22.Kiese S, Papppenberger A, Friess W, Mahler H-C. Shaken, Not Stirred: Mechanical Stress Testing of An IgG1 Antibody. J Pharm Sci. 2008;97:4347–4366. doi: 10.1002/jps.21328. [DOI] [PubMed] [Google Scholar]
- 23.Thirumangalathu R, Krishnan S, Ricci MS, Brems DN, Randolph TW, Carpenter JF. Silicone Oil- and Agitation-Induced Aggregation of a Monoclonal Antibody in Aqueous Solution. J Pharm Sci. 2009;98:3167–3181. doi: 10.1002/jps.21719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bam N, Randolph T, Cleland J. Stability of Protein Formulations: Investigation of Surfactant Effects by a Novel EPR Spectroscopic Technique. Pharm Res. 1995;12:2–11. doi: 10.1023/a:1016286600229. [DOI] [PubMed] [Google Scholar]
- 25.Mahler H-C, Muller R, Friess W, Delille A, Matheus S. Induction and analysis of aggregates in a liquid IgG1-antibody formulation. Eur J Pharm Biopharm. 2005;59:407–417. doi: 10.1016/j.ejpb.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Chou DK, Krishnamurthy R, Randolph TW, Carpenter JF, Manning MC. Effects of Tween 20 and Tween 80 on the stability of Albutropin during agitation. J Pharm Sci. 2005;94:1368–1381. doi: 10.1002/jps.20365. [DOI] [PubMed] [Google Scholar]
- 27.Deechongkit S, Wen J, Narhi LO, Jiang Y, Park SS, Kim J, Kerwin BA. Physical and Biophysical Effects of Polysorbate 20 and 80 on Darbepoetin Alfa. J Pharm Sci. 2009;98:3200–3217. doi: 10.1002/jps.21740. [DOI] [PubMed] [Google Scholar]
- 28.Mahler H, Senner F, Maeder K. Surface activity of a monoclonal antibody. J Pharm Sci. 2009;98:4525–4533. doi: 10.1002/jps.21776. [DOI] [PubMed] [Google Scholar]
- 29.Krielgaard L, Jones L, Randolph T, Frokjaer S, Flink J, Manning M, Carpenter J. Effect of Tween 20 on Freeze-Thawing- and Agitation-Induced Aggregation of Recombinant Human Factor XIII. J Pharm Sci. 1998;87:1597–1603. doi: 10.1021/js980126i. [DOI] [PubMed] [Google Scholar]
- 30.Kerwin BA, Heller MC, Levin SH, Randolph TW. Effects of Tween 80 and sucrose on acute short-term stability and long-term storage at −20 degrees C of a recombinant hemoglobin. J Pharm Sci. 1998;87:1062–1068. doi: 10.1021/js980140v. [DOI] [PubMed] [Google Scholar]
- 31.Ludwig DB, Carpenter JF, Hamel J-B, Randolph TW. Protein adsorption and excipient effects on kinetic stability of silicone oil emulsions. J Pharm Sci. 2010;99:1721–1733. doi: 10.1002/jps.21982. [DOI] [PubMed] [Google Scholar]
- 32.Bam NB, Cleland JL, Yang J, Manning MC, Carpenter JF, Kelley RF, Randolph TW. Tween protects recombinant human growth hormone against agitation-induced damage via hydrophobic interactions. J Pharm Sci. 1998;87:1554–1559. doi: 10.1021/js980175v. [DOI] [PubMed] [Google Scholar]
- 33.Joshi O, McGuire J, Wang D. Adsorption and function of recombinant factor VIII at solid-water interfaces in the presence of Tween 80. J Pharm Sci. 2008;97:4741–4755. doi: 10.1002/jps.21333. [DOI] [PubMed] [Google Scholar]
- 34.Joshi O, Chu L, Mcguire J, Wang D. Adsorption and Function of Recombinant Factor VIII at the Air Water Interface in the Presence of Tween 80. J Pharm Sci. 2009;98:3099–3107. doi: 10.1002/jps.21569. [DOI] [PubMed] [Google Scholar]
- 35.Courthaudon J-L, Dickinson E, Matsumura Y, Clark DC. Competitive adsorption of β-lactoglobulin + tween 20 at the oil-water interface. Colloids and Surfaces. 1991;56:293–300. [Google Scholar]
- 36.Gerhardt A, McGraw NR, Schwartz DK, Bee JS, Carpenter JF, Randolph TW. Protein Aggregation and Particle Formation in Pre-filled Glass Syringes. J Pharm Sci. 2014;103:1601–1612. doi: 10.1002/jps.23973. [DOI] [PubMed] [Google Scholar]
- 37.Oganesyan V, Damschroder MM, Leach W, Wu H, Dall’Acqua WF. Structural characterization of a mutated, ADCC-enhanced human Fc fragment. Mol Immunol. 2008;45:1872–1882. doi: 10.1016/j.molimm.2007.10.042. [DOI] [PubMed] [Google Scholar]
- 38.Meli M-V, Lin I-H, Abbott NL. Preparation of microscopic and planar oil-water interfaces that are decorated with prescribed densities of insoluble amphiphiles. J Am Chem Soc. 2008;130:4326–4333. doi: 10.1021/ja077379a. [DOI] [PubMed] [Google Scholar]
- 39.Walder R, Schwartz DK. Single molecule observations of multiple protein populations at the oil-water interface. Langmuir. 2010;26:13364–13367. doi: 10.1021/la1023357. [DOI] [PubMed] [Google Scholar]
- 40.Brake JM, Daschner MK, Luk Y-Y, Abbott NL. Biomolecular interactions at phospholipid-decorated surfaces of liquid crystals. Science. 2003;302:2094–2097. doi: 10.1126/science.1091749. [DOI] [PubMed] [Google Scholar]
- 41.Kastantin M, Walder R, Schwartz DK. Identifying mechanisms of interfacial dynamics using single-molecule tracking. Langmuir. 2012;28:12443–12456. doi: 10.1021/la3017134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walder R, Kastantin M, Schwartz DK. High throughput single molecule tracking for analysis of rare populations and events. Analyst. 2012;137:2987–2996. doi: 10.1039/c2an16219a. [DOI] [PubMed] [Google Scholar]
- 43.Kerwin BA. Polysorbates 20 and 80 Used in the Formulation of Protein Biotherapeutics: Structure and Degradation Pathways. J Pharm Sci. 2008;97:2924–2935. doi: 10.1002/jps.21190. [DOI] [PubMed] [Google Scholar]
- 44.Kragel J, Wustneck R, Husband F, Wilde P, Makievski A, Grigoriev D, Li J. Properties of mixed protein/surfactant adsorption layers. Colloids Surfaces B Biointerfaces. 1999;12:399–407. [Google Scholar]
- 45.Clark DC, Mackie AR, Wilde PJ, Wilson DR. Differences in the structure and dynamics of the adsorbed layers in protein-stabilized model foams and emulsions. Faraday Discuss. 1994;98:253–262. [Google Scholar]
- 46.Jones D, Middelberg A. Direct determination of the mechanical properties of an interfacially adsorbed protein film. Chem Eng Sci. 2002;57:1711–1722. [Google Scholar]
- 47.Dixit N, Maloney KM, Kalonia DS. The effect of Tween 20 on silicone oil-fusion protein interactions. Int J Pharm. 2012;429:158–167. doi: 10.1016/j.ijpharm.2012.03.005. [DOI] [PubMed] [Google Scholar]