Abstract
Maternal care is an indispensable component of offspring survival and development in all mammals and necessary for reproductive success. Although brain areas regulating maternal behaviors are innervated by serotonergic afferents, very little is known about the role of this neuro-transmitter in these behaviors. To evaluate the contribution of serotonin to maternal care, we used mice with a null mutation in the gene for tryptophan hydroxylase-2 (TPH2), which results in a genetic depletion of brain serotonin, and tested them in a wide range of maternal behavior paradigms. We found that litters born to and reared by TPH2−/− mothers showed decreased survival, lower weaning weights and increased cannibalization. In addition, TPH2−/− mothers performed poorly in pup retrieval, huddling, nest construction and high-arched back nursing. Aggression in TPH2−/− dams was not triggered by lactation and was steadily high. Survival and weaning weight deficits of TPH2−/− pups were rescued by cross-fostering and in litters of mixed genotype (TPH2−/− and TPH2−/+). However, the maternal behaviors of TPH2−/− dams did not improve when rearing either TPH2+/+ pups or mixed-genotype litters. In addition, TPH2−/− pups significantly worsened the behavior of TPH2+/+ dams with respect to cannibalism, weaning weight and latency to attack. Olfactory and auditory functions of TPH2−/− females or anxiety-like behaviors did not account for these maternal alterations as they were equal to their TPH2+/+ counterparts. These findings illustrate a profound influence of brain serotonin on virtually all elements of maternal behavior and establish that TPH2−/− pups can engender maladaptive mothering in dams of both genotypes.
Keywords: Maternal behavior, pup survival, serotonin, TPH2, TPH2 knockout
Maternal behavior is one of the most important pro-social behaviors of the mammalian female (Bosch 2011) and a highly conserved set of behavioral capacities that are crucial for reproductive success (Leckman & Herman 2002). It is essential for the survival of newborns (Rosenblatt & Lehrman 1963) as it ensures their well-being and development and increases the probability that offspring will reach maturity (Bosch 2011). Maternal behavior includes two distinct complexes, maternal care and maternal aggression, which reflect any offspring-directed behavior and defense against a potential threat (Bosch & Neumann 2012). Successful nurturing requires the coordinate expression of several subcomponents of maternal behavior including pup retrieval, nursing, nest building and defense of offspring (Gammie 2005; Gammie et al. 2007).
Several brain areas are pivotal for maternal behaviors in rodents, such as the medial preoptic area (Gammie 2005), the bed nucleus of the stria terminalis, the lateral septum (Gammie et al. 2009), hippocampus and amygdala (Gammie et al. 2009; Leckman & Herman 2002), all of which are directly innervated by serotonergic afferents arising in the raphe nuclei of the mesencephalon (Steinbusch 1981). The role of serotonin in influencing maternal processes has been primarily associated with increased prolactin secretion (Barofsky et al. 1983; Hyde 1992) but recent studies suggest that serotonin signaling goes beyond indirect regulation (Alenina et al. 2009; Lerch-Haner et al. 2008). For example, studies have associated reductions in serotonin transmission with significant decreases in the growth rate of offspring, pup retrieval and maternal behavior, and increases in pup killing (Alenina et al. 2009; Barofsky et al. 1983; Ieni & Thurmond 1985; Lerch-Haner et al. 2008; Neckers et al. 1975; Van Velzen & Toth 2010). However, the use of non-specific neurotoxins, or surgical ablations in the above mentioned studies often result in alterations in other neurotransmitter systems. Genetic mouse models with mutations in serotonin effectors, while target-specific, only cause partial reductions of serotonin, making it difficult to achieve a more conclusive evaluation of serotonin function in maternal–infant interactions. TPH2 knockout (TPH2−/−) mice have a genetic disruption in the enzyme that synthesizes neural serotonin and are completely devoid of this neurotransmitter in brain. This mouse model offers a clear advantage to study unambiguously the role of serotonin in maternal behaviors and to date, a systematic and complete examination on this matter has not been reported with these mice.
Presently, we find that virtually every facet of maternal behavior is significantly altered in TPH2−/− mice and only offspring survival and weaning weights could be rescued by cross-fostering or in crosses that generate mixed-genotype offspring. These effects are not secondary to anxiety-like behaviors or sensory perception and are influenced in part by pup genotype.
Materials and methods
Animals
TPH2−/− mice were generated by deleting exon 1 of the Tph2 gene as described (Thomas et al. 2010) and are on a mixed C57BL/6J-129S1 genetic background. Genome scanning analysis by the Jackson Laboratory (Bar Harbor, ME, USA) revealed that mice were 94% C57BL/6J. Mature nulliparous TPH2−/−, TPH2−/+ and TPH2+/+ female mice (offspring of two heterozygous breeders) were mated with adult males using the following pairings: TPH2−/− female × TPH2−/− male; TPH2−/− female × TPH2−/+ male; TPH2−/+ female × TPH2−/− male and TPH2+/+ female × TPH2+/+ male. Because the levels of brain serotonin in TPH2−/+ and TPH2+/+ mice are the same (Thomas et al. 2010), crossings using heterozygous mice were limited to TPH2−/+ females × TPH2−/− males and TPH2−/− females × TPH2−/+ males to assess the maternal behavior of dams with and without serotonin, respectively, in fostering offspring with mixed genotypes in the same litter (i.e. TPH2−/+ and TPH2−/−). Mice were housed in a colony room with a 12/12 light/dark schedule and had free access to standard rodent chow and water. With the exception of anxiety-like behaviors and measures of pup retrieval/huddling, independent cohorts of naïve animals were used for each test in order to avoid test-order effects and the stress caused by repeated handling. The date of birth was considered postpartum day 0 (PN0). The Institutional Animal Care and Use Committee of Wayne State University approved the animal care and experimental procedures and all procedures are in compliance with the NIH Guide for the Care and Use of Laboratory Animals.
Cross-fostering and mixed-genotype litters
Two approaches were used to evaluate the contribution of maternal/pup genotype and their interactions in determining the maternal behavior of TPH2−/− dams. First, a cross-fostering approach consisted of transferring TPH2−/− pups to TPH2+/+dams on PN1-3, and vice versa. All fostered pups were littermates and remained with their dams until weaning at PN21. The second strategy involved crosses of TPH2−/+ females × TPH2−/− males and vice versa to produce litters with mixed pup genotypes (i.e. 50% TPH2−/+ pups and 50% TPH2−/− pups).
Pup survival, body weight and litter size
Pup survival, body weight and litter size were assessed in dams for all of the aforementioned pairings. The number of pups delivered by each individual dam was recorded at birth and each pup was weighed daily from PN0 until the day of weaning (PN21). For survival, the following group sizes were used: 52 TPH2+/+ and 43 TPH2−/− dams with their own pups, 16 TPH2+/+ dams with TPH2−/− pups, 24 TPH2−/− dams with TPH2+/+ pups, 16 TPH2−/+ and 14 TPH2−/− dams with mixed litters. For weaning weights, 108 TPH2+/+ and 102 TPH2−/− dams with their own pups, 87 TPH2+/+ dams with TPH2−/− pups, 102 TPH2−/− dams with TPH2+/+ pups, 78 TPH2−/+ and 69 TPH2−/− dams with mixed litters were used.
Cannibalistic behavior
Deceased pups showing signs of bite marks or mutilation were considered cannibalized. Litters were scored for pup cannibalism daily. For measuring cannibalism, the following group sizes were used: 57 TPH2+/+ dams with their own pups, 42 TPH2−/− dams with their own pups, 19 TPH2+/+ dams with TPH2−/− pups, 24 TPH2−/− dams with TPH2+/+ pups, 16 TPH2−/+ dams with mixed litters and 14 TPH2−/− dams with mixed litters.
Pup retrieval and huddling
Dams were removed from their cages on PN0-PN2, nests were disturbed and pups were dispersed evenly throughout the cage. Dams were re-introduced in the cage and the number of pups retrieved was determined in a 20 min session. The number of pups huddled was counted at the end of the 20 min test. Pups were considered huddled if they were in any way touching another pup. Culling of litters was not necessary as the litter size for both genotypes was the same (data not shown) and numbers were normalized to percentages and rates per min. For pup retrieval and huddling, the following group sizes were used: 23 TPH2+/+ and 18 TPH2−/− dams with their own pups, 11 TPH2+/+ dams with TPH2−/− pups, 14 TPH2−/− dams with TPH2+/+ pups, 10 TPH2−/+ and 10 TPH2−/− dams with mixed litters.
Nest construction
Commercially available nestlets (NES3600, Ancare, Bellmore, NY, USA) were placed into cages (1 nestlet per cage) containing a dam and her litter on a single occasion between PN0 and PN2. Dams were allowed to shred the nestlet into a nest and thereafter nests were scored using a modified rating scale (Oxley & Fleming 2000) as follows: 1, nest material untouched; 2, nest material scattered (no nest formed); 3, very rough nest shape (no enclosing walls and gathered loosely); 4, definite nest formed (flat, enclosing walls but no bowl-shape) and 5, definite spherical nest (enclosing walls and bowl-shape). For nest construction, the following group sizes were used: 24 TPH2+/+ and 22 TPH2−/− dams with their own pups, 12 TPH2+/+ dams with TPH2−/− pups, 15 TPH2−/− dams with TPH2+/+ pups, 10 TPH2−/+ and 11 TPH2−/− dams with mixed litters.
Maternal nursing behavior
Dams were observed from PN0-PN5 in two 1 h sessions on the same day at the following Zeitgeber times (ZT): ZT3-4 and ZT8-9 (ZT0 – lights on at 0600 h and ZT12 – lights off at 1800 h). Data from these 2 time points did not differ and were combined for analysis. Dams caring for cross-fostered litters were observed 1–2 days following cross-fostering of pups. Dams and their litters remained undisturbed in their home cages throughout the analysis. High-arched back and low-arched back nursing and time on or off the nest was recording at 1 min intervals. For measuring maternal nursing behaviors, the following group sizes were used: 19 TPH2+/+ dams with their own pups, 9 TPH2−/− dams with their own pups, 9 TPH2+/+ dams with TPH2−/− pups, 6 TPH2−/− dams with TPH2+/+ pups, 10 TPH2−/+ dams with mixed litters and 10 TPH2−/− dams with mixed litters.
Maternal aggression
Aggressive behaviors in dams were assayed starting at PN1. Aggressive behaviors were scored during a 5-min period after introduction of an unfamiliar sexually naïve TPH2+/+ male into the dam's home cage. The latency to the first attack and the number of attacks were recorded. Tail rattling, biting and wrestling were considered aggressive behaviors. Given that maternal aggression is induced by lactation (Gammie & Nelson 1999) and that virgin TPH2−/− females are inherently more aggressive than their TPH2+/+ counterparts (Kane et al. 2012), non-lactating females from both genotypes were tested as controls for the lactating females in this maternal aggression paradigm. This set of non-lactating females consisted of nulliparous, age-matched virgin mice that were tested during the diestrous stage of their cycle. For this test, the following group sizes were used: 16 TPH2+/+ and 13 TPH2−/− virgin mice, 14 TPH2+/+ and 8 TPH2−/− dams with their own pups, 8 TPH2+/+ dams with TPH2−/− pups, 6 TPH2−/− dams with TPH2+/+ pups, 11 TPH2−/+ and 10 TPH2−/− dams with mixed litters.
Olfactory and auditory sensory function
General olfactory function was tested using the olfactory habituation test and the pup odorant/pheromone preference test. The olfactory habituation test used was adapted from Wang and Storm (2011). Briefly, on PN0-3, dams were habituated to the scent of a water-laced kimwipe placed into a plastic tube with open ends. The amount of time spent sniffing was recorded in each of six 2-min trials with a 1 min intertrial interval. Next, another tube containing a kimwipe laced with 25 μl of vanilla extract was introduced and the amount of time spent sniffing the new stimulus during a 2 min dishabituation period was recorded. The pup odorant/pheromone preference test was performed as described previously (Wang & Storm 2011). Pup urination was stimulated by gentle scruffing. Urine was collected and pooled from male and female pups of the same genotype into 1.5 ml Eppendorf tubes at PN5-8. Urine was then stored at −80°C until use. Nursing dams (PN6-9) were removed from their home cages and placed into a cage with the bottom covered by a sheet of filter paper. A 50-μl drop of urine from TPH2−/− pups was applied on one end in a 1 cm2 area and another 50 μl drop of urine from TPH2+/+ pups was applied to the opposite end of the cage. The amount of time spent sniffing each urine spot was recorded by an experienced observer for a 10-min period.
In order to assess auditory function, we measured the auditory brainstem response (ABR) as previously described (Church et al. 2013). Briefly, mice were anesthetized (100 mg/kg ketamine plus 20 mg/kg xylazine, i.p.) and body temperature was carefully monitored and maintained at 37.0 ± 0.5°C using a water circulating heating pad. ABRs were differentially recorded between two subcutaneous platinum needle electrodes in a sound attenuated chamber. Tones were presented in the ascending order of 2, 4, 8, 16, 32 and 45 kHz to encompass the principal hearing range in mice, including ultrasonic frequencies (i.e. 45 Hz). At each of these frequencies, stimulus intensities were varied from 100 to 15 dB in descending order. ABR thresholds were determined by the method of limits (Church et al. 2013). For olfaction studies, 7 TPH2+/+ and 14 TPH2−/− dams were tested; for the pup odorant test, 11 TPH2+/+ and 7 TPH2−/− dams were used and for the ABR, 10 TPH2+/+ and 10 TPH2−/− dams were tested.
Maternal anxiety- and compulsive-like behaviors
To evaluate a possible role of anxiety as a cause for the maternal behaviors evaluated in this study, two commonly used tests for anxiety-like behaviors in rodents [i.e. elevated plus maze (EPM) and light/dark box test (LD)] were carried out. For the EPM, dams were removed from the cages containing their pups on PN0-2 and immediately placed in the EPM (AccuScan Instruments, Omnitech Electronics Inc., Columbus, OH, USA). The maze is set-up under a motion-sensitive digital video camera connected to a computer to track the individual movements (EZ video Software, Automated Tracking System, version 5.61, AccuScan Instruments, Columbus, OH, USA). Mice were placed into the center square of the maze facing one of the open arms and allowed to freely explore all four arms of the maze for a 5-min test period. For each dam, the time spent in each arm and the total distance traveled was recorded. For the LD, dams tested in the EPM were returned to cages containing their pups and allowed to nurse for 5 h before being tested in the LD. The Open Field Box (AccuScan Instruments) was used for this test. Half of the box was covered with a removable opaque ‘dark box’ that had an opening (7 cm long × 3 cm high) in the middle through which the mouse could freely move. During this test, the movement of the mouse was recorded by 16 light beam arrays in the horizontal and vertical axes using the Fusion Software package (AccuScan Instruments). Mice were placed into the lighted section of the box facing away from the darkened section and were allowed to freely explore both halves of the box (darkened and lighted) for a 10 min test period. The total time spent in each half of the box, and the total distance traveled was recorded for each subject. In addition to the lactating TPH2−/− and TPH2+/+ dams, another set of nulliparous, age-matched virgin females was tested during the diestrous stage of their cycle in both the EPM and LD to evaluate baseline levels of anxiety-like behavior. For EPM and LD, the following group sizes were used: 15 TPH2+/+ and 11 TPH2−/− virgin mice, 15 TPH2+/+ dams with their own pups, 14 TPH2−/− dams with their own pups, 7 TPH2+/+ dams with TPH2−/− pups, 6 TPH2−/− dams with TPH2+/+ pups, 11 TPH2−/+ dams with mixed litters and 12 TPH2−/− dams with mixed litters. Maternal compulsive-like digging, climbing and exploring was recorded along with time spent eating and drinking and time spent engaged in self-and pup grooming (see Supporting Information). These behaviors were evaluated in 19 TPH2+/+ and 9 TPH2−/− dams with their own pups, 9 TPH2+/+ dams with TPH2−/− pups, 6 TPH2−/− dams with TPH2+/+ pups, 10 TPH2−/+ and 10 TPH2−/− dams with mixed litters.
Statistical analyses
Behavioral tests of investigation time (pup odorant/pheromone preference test) and number of cannibalized pups per litter in mixed-genotype litters were analyzed by Student's t-tests. One-way ANOVAs followed by Tukey's multiple post hoc comparisons were performed to analyze the results for mixed-genotype litters with the exception of the number of cannibalized pups (see above), pup survival and weaning weight (see below). These analyses tested the effect of maternal genotype as the genotype of the pups was not defined (mixed litters). In addition, two-way ANOVAs followed by Tukey's post hoc comparisons were performed to analyze the cross-fostering behavioral tests, survival and weaning weight in mixed litters as well as time sniffing urine, ABR, maternal separation anxiety and digging/eating/grooming behaviors. For these analyses, the effects of maternal genotype × offspring genotype, maternal/progenitor genotype × days (survival over time data) or maternal genotype × lactation status (maternal aggression) and their respective interactions were tested. Olfactory habituation data were analyzed with repeated-measures two-way ANOVA (dam genotype × trial) followed by Bonferroni (between genotypes) and Tukey's (within genotypes) post hoc comparisons. For two-way ANOVAs, the main effects and/or interactions that were not significant were not mentioned in the results section. In most cases, the independent unit of observation was the individual, unless the litter was specified. Values of P < 0.05 were deemed statistically significant. All statistical analyses were carried out using Graphpad Prism version 6.02 for Windows, Graphpad Software, San Diego, CA, USA, www.graphpad.com.
Results
Litters born to TPH2−/− dams show decreased survival, increased cannibalism and reduced body weight at weaning and these deficits are rescued by cross-fostering
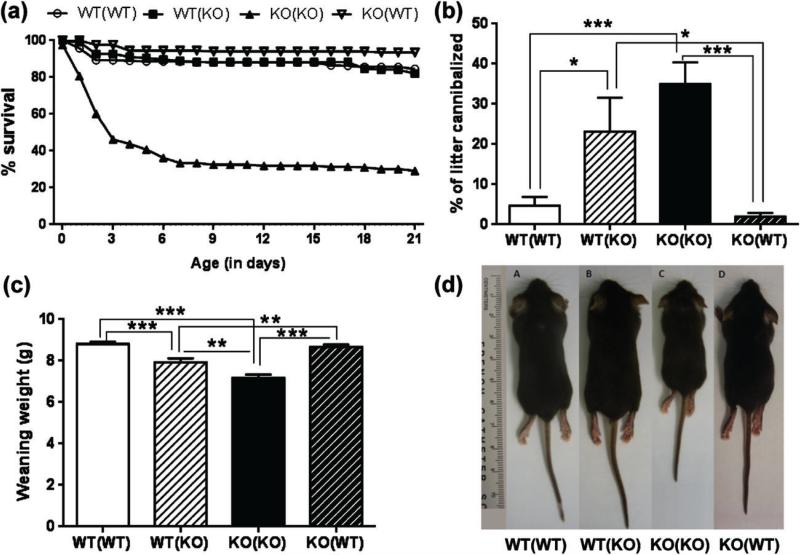
Pups born to TPH2−/− progenitors showed a significant decrease in their survival (29%) from birth to weaning compared to pups born to TPH2+/+ female/male crosses (84%). There was a significant main effect of maternal geno-type (F3,2870 = 640.7, P < 0.0001), time (F21,2870 = 5.016, P < 0.0001) and maternal genotype × time interaction (F63,2870 = 2.456, P < 0.0001) on pup survival. When fostered by TPH2+/+ dams, TPH2−/− pups increased significantly their survival to 82% compared with 29% when reared by their biological mothers (P < 0.0001). Similarly, survival of TPH2+/+ pups increased slightly from 84% to 93% when being reared by TPH2−/− dams compared with their biological mothers (P < 0.0001, Fig. 1a). Survival at weaning was increased for the offspring from TPH2−/+ × TPH2−/+ pairings (TPH2−/− 88%, TPH2+/+ 95% and TPH2−/+ 97%). To further assess the survival deficits of pups, the percentage of litters cannibalized by dams was determined for each genotype. A significant main effect of pup genotype on the percentage of litters cannibalized was present (F3,137 = 32.22, P < 0.0001). Litters of TPH2−/− pups showed a higher percentage of cannibalization compared to litters of TPH2+/+ pups, irrespective of the genotype of the mother (P < 0.05 for TPH2+/+ dams and P < 0.0001 for TPH2−/− dams). Although cannibalization decreased significantly in TPH2−/− mothers fostering TPH2+/+ pups (P < 0.0001), it increased in TPH2+/+ dams fostering TPH2−/− pups (P < 0.05, Fig. 1b), reflecting an influence of TPH2−/− pups on the behavior of TPH2+/+ dams. To support this finding, we measured cannibalization in TPH2−/+ dams mated with TPH2−/+ males. TPH2−/+ dams cannibalized 2% of their litters and only TPH2−/− pups were cannibalized.
Figure 1. Litters born to TPH2−/− dams show decreased survival, increased cannibalism and reduced body weight at weaning and are rescued by cross-fostering.
Litters born to TPH2+/+ (WT) or TPH2−/− (KO) dams were reared by their biological mothers or they were cross-fostered to receptive dams of the opposite genotype immediately after birth. The genotypes of dams and pups (in parentheses) are designated as follows: WT(WT), WT dam rearing their own WT pups; WT(KO), WT dams rearing cross-fostered KO pups; KO(KO), KO dams rearing their own KO pups and KO(WT), KO dams rearing cross-fostered WT pups. Litters were assessed for (a) % pup survival, (b) % litter cannibalized, (c) body weight at weaning and (d) body size at weaning. Data are expressed as the mean ± SEM. *P < 0.05, **P < 0.001 and ***P < 0.0001.
The main effects of maternal (F1,434 = 8.99, P = 0.002) and pup genotype (F1,434 = 62.14, P < 0.0001) and their interaction (F1,434 = 4.02, P = 0.045) were significant on the weight of pups at weaning. A reduced weaning weight was observed in TPH2−/− pups born and reared by TPH2−/− dams compared to pups TPH2+/+ from TPH2+/+ dams at the same age (7.1 g vs. 8.8 g, respectively, P < 0.0001). The presence of milk bands in the stomachs of pups born to TPH2−/− dams was indicative of a normal lactation. As observed for survival, the deficits in weaning weight in TPH2−/− pups reared by their biological mothers disappeared when fostered by TPH2+/+ dams (Fig. 1c,d). These results show that the survival deficits and reduced weaning weights in TPH2−/− pups reared by TPH2−/− dams can be rescued by cross-fostering the pups to TPH2+/+ dams. The weaning weights of TPH2−/+ × TPH2−/+ offspring (TPH2−/− 6.6 g, TPH2+/+ 9.9 g and TPH2−/+ 10.1 g) also indicate that the pup genotype constitutes a major factor determining pup body weight at weaning.
TPH2−/− dams show defective instinctual maternal behaviors that are resistant to rescue by cross-fostering
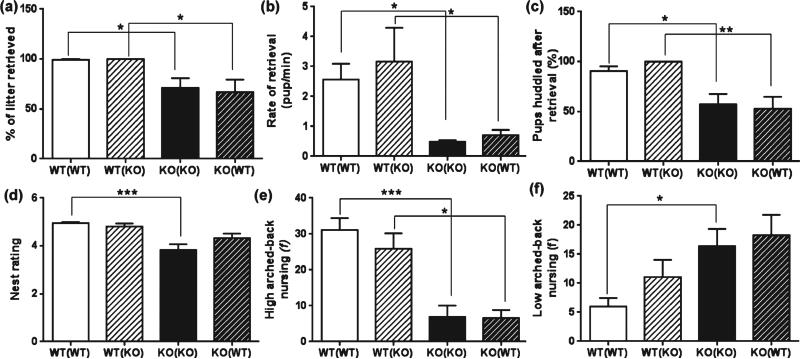
The genotype of the mother had a significant effect on the retrieval of pups (F1,61 = 16.17, P = 0.0002) as shown in Fig. 2. TPH2+/+ dams retrieved 99.2% of their pups within the 20 min test period, which was significantly higher than 71.2% of the pups being retrieved by TPH2−/− dams (P < 0.05). Pup genotype did not have a significant effect on the percentage of pups retrieved by TPH2−/− dams (71% retrieval of their own pups vs. 69% retrieval of cross-fostered pups). Similarly, significant differences were not detected in TPH2+/+ dams as they retrieved cross-fostered TPH2−/− pups to the same extent as their own (99%; Fig. 2a). There was a significant main effect of maternal genotype on the rate of pup retrieval (F1,60 = 17.94, P < 0.0001). The rate of pup retrieval for TPH2+/+ dams (2.5 pups/min) was significantly higher than 0.4 pups/min for the TPH2−/− dams (P = 0.05) but cross-fostering had no rescuing effects, as shown in Fig. 2b. Correspondingly, the percentage of pups huddled after retrieval depended on the genotype of the dam (F1,62 = 22.27, P < 0.0001). Whereas 90% of the TPH2+/+ pups were huddled after being retrieved by their biological mothers, only 57% of the pups retrieved by TPH2−/− dams were huddled (P < 0.05). Cross-fostering did not have any improving effect in pup huddling by TPH2−/− dams nor did it change the performance of TPH2+/+ dams (Fig. 2c). There was a significant main effect of maternal (F1,58 = 30.23, P < 0.0001) and pup (F1,58 = 4.78, P = 0.032) genotype on nest construction. Nests built by TPH2−/− dams were more poorly constructed compared to the well-defined and tightly gathered nests built by TPH2+/+ dams (3.4 vs. 4.9; P < 0.0001). Nest ratings for TPH2−/− dams cross-fostering TPH2+/+ pups did not show any improvement. Nests built by TPH2+/+ dams with cross-fostered TPH2−/− pups did not change significantly compared to nests built for their own pups (Fig. 2d). Although the time spent on/off the nest was the same in TPH2−/− and TPH2+/+ dams (data not shown), the genotype of the mothers had a significant effect on the maternally relevant, high-arched back nursing (F1,39 = 30.71, P < 0.0001) and on the less efficient low-arched back nursing (F1,39 = 10.86, P = 0.0021). High-arched back nursing was significantly lower in TPH2−/− mice relative to TPH2+/+ controls (P < 0.0001, Fig. 2e), and low-arched back nursing was increased in TPH2−/− dams compared to TPH2+/+ mice (P < 0.05). The form of maternal nursing was not modified by cross-fostering in either TPH2+/+ or TPH2−/− dams (Fig. 2f).
Figure 2. TPH2−/− dams show defective instinctual maternal behaviors that are resistant to rescue by cross-fostering.
TPH2+/+ (WT) and TPH2−/− (KO) dams were assessed for maternal behaviors to include (a) % of litter retrieved, (b) pup retrieval rate, (c) pup huddling after retrieval, (d) nest rating, (e) high-arched back nursing and (f) low-arched back nursing. Dam and litter genotypes are designated as described in the legend of Fig. 1. Data are expressed as the mean ± SEM. *P < 0.05, **P < 0.001 and ***P < 0.0001.
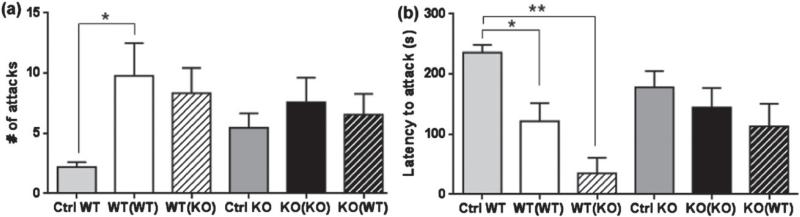
Lactating TPH2−/− dams do not show increased maternally driven aggression to male intruders in the presence of their own or cross-fostered TPH+/+ pups
Females are usually not aggressive toward intruders. Nonetheless, postpartum females display vigorous aggression toward unfamiliar mice. Lactation makes it possible to distinguish general aggression from maternally driven aggression, which aims to protect the offspring from infanticide. Lactation status had a significant effect on the number of attacks (F2,56 = 4.094, P = 0.0219; Fig. 3a) and on the latency to attack (F2,64 = 8.603, P = 0.0005). While maternal aggression was present in the lactating TPH2+/+ females, which showed a significant increase in the number of attacks (P < 0.05, Fig. 3a) and a diminished latency to attack (P < 0.05, Fig. 3b) compared to non-lactating TPH2+/+ females, the TPH2−/− dams did not show increased maternal aggression when compared to non-lactating females of the same genotype (Fig. 3a,b). Although the latency to attack for the TPH2+/+ dams cross-fostering TPH2−/− pups was significantly shorter than the non-lactating controls (P < 0.001, Fig 3b), the number of attacks was not significantly higher. Fostering TPH2+/+ pups did not have any effect on the aggression displayed by TPH2−/− dams (Fig. 3a,b).
Figure 3. Lactating TPH2−/− dams do not show increased aggression to male intruders in the presence of their own or cross-fostered TPH2+/+ pups.
Aggressive behavior shown by lactating TPH2+/+ (WT) or TPH2−/− (KO) dams in the presence of the indicated litters was compared to that of non-lactating WT and KO (Ctrl WT or Ctrl KO) females upon the introduction into their cages of a socialized male intruder. Dam and litter genotypes are designated as described in the legend of Fig. 1. Aggressive behaviors were scored as (a) number of attacks on the intruder and (b) latency to attack the intruder. Data are expressed as the mean ± SEM. *P < 0.05, **P < 0.001.
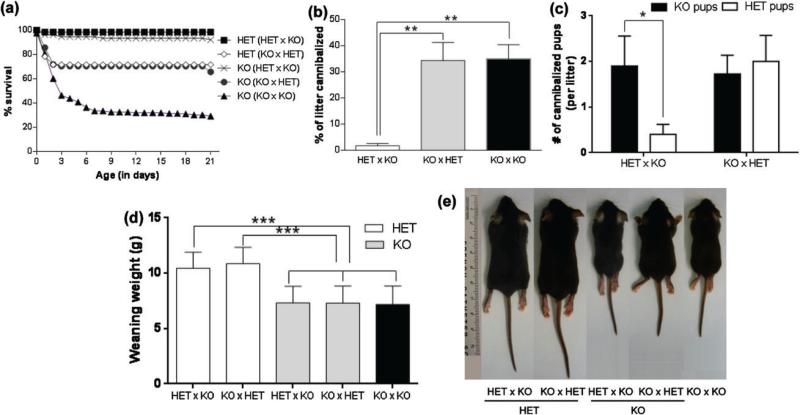
Litters of mixed-genotype born to TPH2−/− dams show decreased survival, increased cannibalism and decreased weaning weights
A significant main effect of progenitor genotype (F4,2178 = 259.9, P < 0.0001) and time (F21,2178 = 2.761, P < 0.0001) was found on the survival of offspring in litters with mixed pup genotype (F4,105 = 149.3; P < 0.0001) as shown in Fig. 4a. TPH2−/− pups born to crosses of TPH2−/− females with TPH2−/+ males significantly increased their percentage of survival to 65% compared to 29% in the crosses of both TPH2−/− progenitors (P < 0.0001). Interestingly, crosses of TPH2−/+ females with TPH2−/− males resulted in an even higher survival rate for both TPH2−/− (92%) and TPH2−/+ (98%) pups (P < 0.0001). Significant differences in survival were not detected in TPH2−/− or TPH2−/+ pups from the same litter. The percentage of litters cannibalized was significantly different for mixed-genotype litters (F2,92 = 5.278; P < 0.001; Fig. 4b). TPH2−/− dams with mixed-genotype litters cannibalized a higher percentage of pups compared to TPH2−/+ mothers (P < 0.001). TPH2−/− mothers showed the same percentage of cannibalism whether they reared litters of TPH2−/− pups or mixed-genotype litters (Fig. 4b). When TPH2−/+ dams did cannibalize pups in mixed litters, they cannibalized significantly more TPH2−/− than TPH2−/+ pups (P < 0.05), whereas TPH2−/− females cannibalized pups of both genotypes equally (Fig. 4c). There was a signifi-cant main effect of the mother's genotype (F2,377 = 183.5; P < 0.0001) and maternal genotype × pup genotype interaction (F2,377 = 174.6, P < 0.0001) on the weaning weights of their pups as shown in Fig. 4d,e. However, the weaning weights of TPH2−/− pups from mixed-genotype litters remained similar whether the mother was TPH2−/− or TPH2−/+ (7.3 g, and 7.6 g, respectively).
Figure 4. Litters of mixed-genotype born to TPH2−/− dams show decreased survival, increased cannibalism and decreased weaning weights.
Matings between TPH2−/+ (HET) females and TPH2−/− (KO) males (HET × KO) or between KO females and HET males (KO × HET) were arranged to produce litters containing pups of mixed genotypes (i.e. 50% KO and 50% HET). Independent matings of KO females to KO males were also carried out to produce litters containing 100% KO pups (KO × KO). The maternal performance of HET and KO dams was scored for (a) % pup survival, (b) % of litter cannibalized, (c) % of pups cannibalized by HET or KO dams; (d) body weight at weaning and (e) body size at weaning. Data are expressed as the mean ± SEM. *P < 0.05, **P < 0.001 and ***P < 0.0001.
TPH2−/− dams show defective instinctual maternal behavior when rearing litters containing pups of mixed genotype
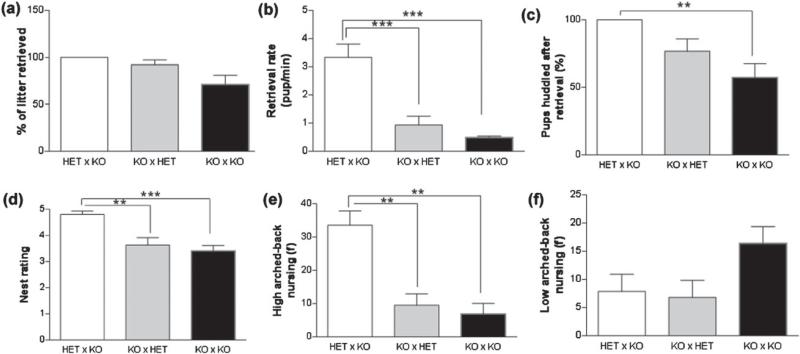
Dam genotype did not have an effect on the extent of pup retrieval (Fig. 5a) but a significant main effect of genotype was seen on retrieval rate as shown in Fig. 5b (F2,34 = 32.36, P < 0.001). TPH2−/+dams had a significantly higher rate compared to TPH2−/− mothers irrespective of having all TPH2−/− pups or mixed litters (P < 0.0001). Although the rate for TPH2−/− mothers to retrieve pups of mixed genotype was higher compared to their retrieval of TPH2−/− pups, the difference was not significant. Pups huddled by TPH2−/− mothers with mixed litters increased to 77% compared with 57% with only TPH2−/− pups, but this difference was not statistically significant. TPH2−/+ and TPH2−/− mothers with mixed-genotype litters huddled their pups similarly but only TPH2−/+ dams showed significantly greater huddling than that displayed by TPH2−/− dams with TPH2−/− pups (P < 0.001, Fig. 5c). There was a significant main effect of the genotype of the mother on nest ratings (F2,40 = 9.267, P < 0.001; Fig. 5d). While TPH2−/− dams with mixed litters were not different from the score of the nest when they reared only TPH2−/− pups, TPH2−/+ dams with mixed-genotype pups showed significantly higher nest scores than TPH2−/− mothers (P < 0.001 in TPH2−/− × TPH2−/+ crosses and P < 0.0001 in TPH2−/− × TPH2−/− crosses). Similarly, a significant main effect of dam genotype was found in the maternally relevant high-arched back type of nursing (F2,25 = 15.98, P < 0.0001) but not for low-arched back type (Fig. 5e,f). High-arched back nursing was higher in TPH2−/+ dams compared to TPH2−/− dams fostering both mixed-genotype (P < 0.0001) and TPH2−/− (P < 0.00001) litters. Low-arched back nursing frequency was the same regardless of the genotype of the mom and the litter.
Figure 5. TPH2−/− dams show defective instinctual maternal behaviors when rearing litters of mixed genotype.
Matings were arranged so that TPH2−/+ (HET) or TPH2−/− (KO) dams reared litters of mixed-genotype pups (50% HET, 50% KO) as described in the legend of Fig. 4. HET and KO dams were assessed for maternal behaviors to include (a) % of litter retrieved, (b) pup retrieval rate, (c) pup huddling after retrieval, (d) nest rating, (e) high-arched back nursing and (f) low-arched back nursing. Data are expressed as the mean ± SEM. **P < 0.001 and ***P < 0.0001.
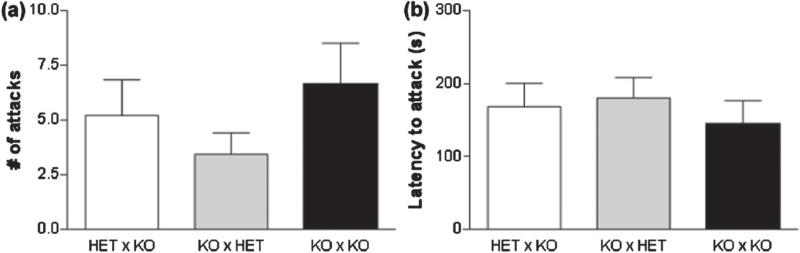
TPH2−/− dams do not show maternal aggression when rearing litters containing pups of mixed genotype
An effect of maternal genotype was not seen on either the number of attacks or the latency to attack for TPH2−/− or TPH2−/+ dams raising mixed-genotype litters or when compared to TPH2−/− dams rising only knockout pups (Fig. 6).
Figure 6. TPH2−/− dams do not show maternal aggression when rearing litters of mixed genotype.
Matings were arranged so that TPH2−/+ (HET) or TPH2−/− (KO) dams reared litters of mixed-genotype pups (50% HET, 50% KO) as described in the legend of Figure 4. Aggressive behaviors were scored as (a) number of attacks on the intruder and (b) latency to attack the intruder. Data are expressed as the mean ± SEM for groups containing 10–11 mice per group.
General olfactory and auditory sensory function is normal in TPH2−/− dams
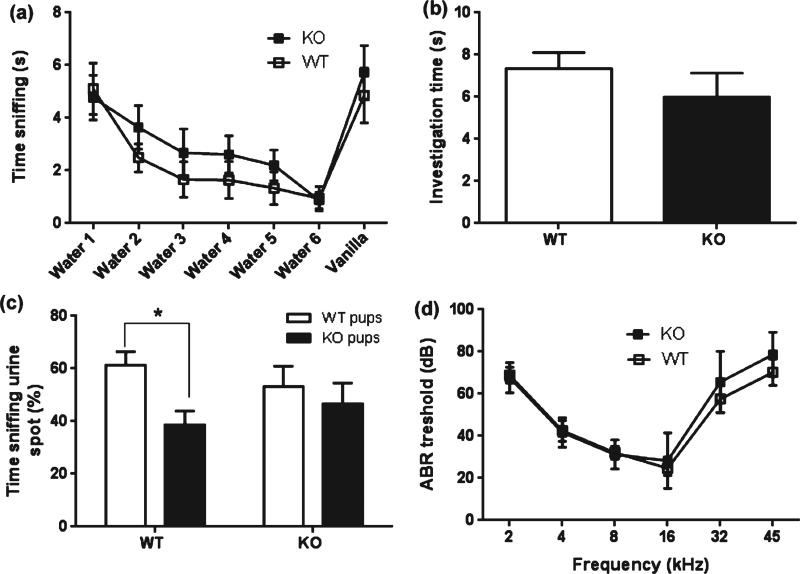
A gradual habituation to water-scented filters followed by a dishabituation response to a novel vanilla odor was displayed by both TPH2+/+ and TPH2−/− dams (F6,60 = 20.64, P < 0.0001) in the olfactory habituation test (Fig. 7a). In the pup odorant preference test, no differences were detected in the total time investigating the urine-containing spots by TPH2+/+ and TPH2−/− dams (Fig. 7b). A significant main effect of pup genotype was observed for time spent sniffing (F1,30 = 5.2, P = 0.029), shown in Fig. 7c. TPH2+/+ dams showed a clear and significant preference for the urine of their own pups vs. urine of TPH2−/− pups (P < 0.05), whereas the TPH2−/− mothers did not show any preference for the urine of their own offspring compared to TPH2+/+ pups (Fig. 7c). Auditory function in TPH2−/− dams, as assessed by determination of ABR thresholds, was not different from that of TPH2+/+ mice over the range of 2–45 kHz as shown in Fig. 7d.
Figure 7. General olfactory and auditory sensory function is normal in TPH2−/− mice.
General olfactory function was assessed in TPH2+/+ (WT) and TPH2−/− (KO) female mice using (a) the olfactory habituation test and (b, c) the pup odorant/pheromone preference test. The ABR-threshold served as a measure of auditory responsiveness in TPH2−/− and TPH2+/+ females (d). Data are expressed as the mean ± SEM. *P < 0.05.
TPH2−/− females do not exhibit an increase in anxiety-like behaviors upon separation from their own or cross-fostered pups
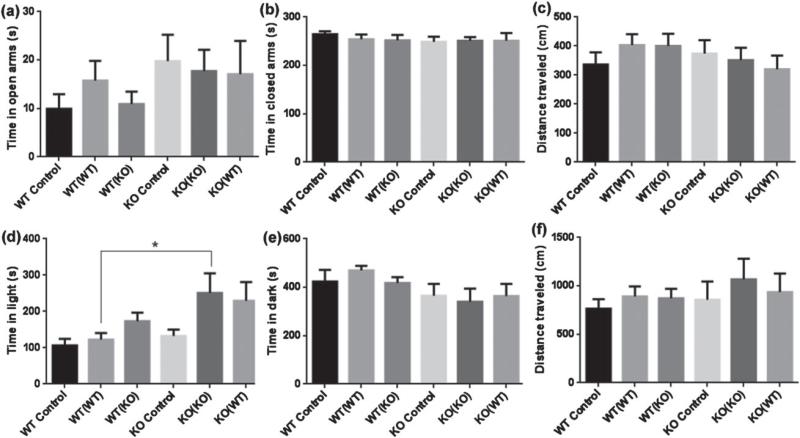
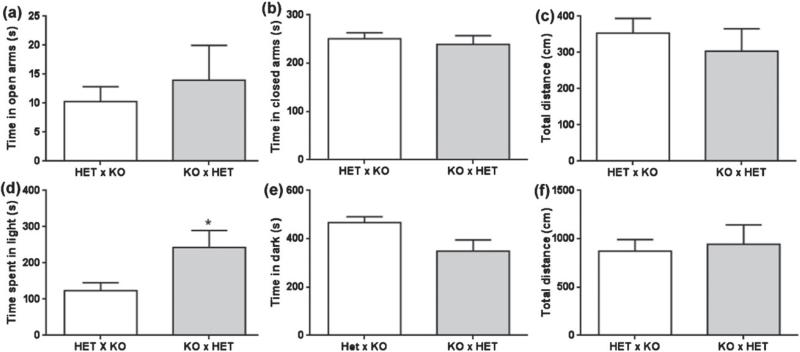
Neither TPH2−/− nor TPH2+/+ dams showed increased anxiety-like behaviors when separated from their own or cross-fostered pups (Fig. 8a–c). In fact, TPH2−/− dams spent significantly more time in the light compartment of the LD than TPH2+/+ dams (F1,56 = 7.29, P = 0.009), an index of lowered anxiety. Likewise, an increase in anxiety-like behaviors was not induced either in TPH2−/− or TPH2−/+ dams with mixed litters upon removal of pups from the cage. Time spent in the open or closed arms on the EPM (Fig. 9a,b) was the same regardless of the genotype of the mother or the pups. Whereas no differences were found in the amount of time spent by TPH2−/+ and TPH2−/− dams in the dark compartment of the LD, the latter spent significantly more time in the light side of the LD compared to TPH2+/− mothers (t22 = 2.176, P < 0.05; Fig. 9d,e). No differences were detected in the total distance traveled by TPH2−/+ and TPH2−/− dams raising mixed litters (Fig. 9c,f).
Figure 8. TPH2−/− dams do not show anxiety-like behavior upon separation from their own or cross-fostered pups.
TPH2+/+ (WT) and TPH2−/− (KO) dams raising their own or cross-fostered pups were assessed for anxiety-like behaviors using the EPM (a–c) or the LD (d–f). Dams and pup genotypes are designated in the legend of Figure 1. In the elevated plus maze, dams were assessed for (a) time spent in the open arms, (b) time spent in the closed arms and (c) distance traveled. In the light–dark box, dams were assessed for (a) time spent in the lighted compartment, (b) time spent in the dark compartment and (f) distance traveled. Data are expressed as the mean ± SEM. *P < 0.05.
Figure 9. TPH2−/− dams do not show anxiety-like behavior upon separation from litters of mixed genotype.
Matings between TPH2−/+ (HET) females and TPH2−/− (KO) males (HET × KO) or between KO females and HET males were arranged to produce litters containing pups of mixed genotypes (i.e. 50% KO and 50% HET). Tests of anxiety-like behaviors were carried out using the elevated plus maze (a–c) and the light–dark box (d–f). In the elevated plus maze, dams were assessed for (a) time spent in the open arms, (b) time spent in the closed arms and (c) distance traveled. In the light–dark box, dams were assessed for (d) time spent in the lighted compartment, (e) time spent in the dark compartment and (f) distance traveled. Data are expressed as the mean ± SEM. *P < 0.05.
TPH2−/− dams show increased compulsive-like digging and climbing behaviors but normal consummatory and grooming behaviors in the presence of their own or cross-fostered pups
TPH2−/− mice show significantly increased levels of compulsive-like digging when compared to TPH2+/+ dams (Figs. S1 and S2, Supporting Information). TPH2−/− dams did not differ from TPH2+/+ dams on expression of any other recorded in-cage behavior (see Supporting Information).
Discussion
Our results document a profound deficit in survival of TPH2−/− pups born to TPH2+/+ dams that was successfully rescued by cross-fostering to TPH2+/+ dams. Previous studies have shown a significant survival reduction in off-spring with central serotonin deficiencies (Alenina et al. 2009, Lerch-Haner et al. 2008) but no effects of cross-fostering (Alenina et al. 2009). Survival of TPH2−/− pups was similarly improved by increasing the serotonergic profile of their mother using TPH2−/+ dams. It was previously demonstrated in TPH2−/+ mice that at least one allele of the Tph2 gene accounts for a serotonin production equivalent to that of TPH2+/+ mice (Thomas et al. 2010). Surprisingly, a slight but significant increase in survival of TPH2+/+ pups occurred when being reared by TPH2−/− dams compared to their biological mothers. These results cannot be explained through single effects of maternal or pup genotype and suggest very complex maternal–infant interactions. The absence of brain serotonin does not result in a lethal phenotype. Although cannibalism was not the only factor to explain the drop in survival of TPH2−/− pups, it had a significant contribution. TPH2−/− dams had greater cannibalization rates than TPH2+/+ or TPH2−/+ dams and TPH2−/− pups were more selectively cannibalized when fostered by TPH2+/+ or TPH2−/− dams. This indicates that pup genotype has a determinant effect on their cannibalization, suggesting a negative feature present in TPH2−/− pups that affects their interaction with dams of either genotype.
The role of central serotonin in regulating body weight is not clear but the depletion of this neurotransmitter has been reported to be associated with growth retardation in rats (Leshem & Kreider 1987) and mice (Alenina et al. 2009; Kane et al. 2012; Lerch-Haner et al. 2008; Narboux-Neme et al. 2013). Although lower levels of insulin-like growth factor have been reported in serum of TPH2−/− mice until 2 months of age, it is possible that this decrease is secondary to malnutrition (Alenina et al. 2009). Moreover, early studies have estimated that postnatal maternal influence on body weight accounts for about 69% of the variance at 21 days of age (White et al. 1968). Nonetheless, TPH2−/− mothers nursed their pups and milk bands were present in the newborns suggesting normal lactation. Maternal genotype did not have a direct influence on the growth deficit because TPH2−/− pups born to TPH2−/+ dams still showed the same reduction in body weight. Competition with pup littermates can also be ruled out as a cause for lower weights in TPH2−/− pups because pure knockout litters had the same pheno-type as TPH2−/− pups reared in mixed-genotype litters. Our results showing that cross-fostering to TPH2+/+ mothers was effective in ameliorating the growth retardation observed in TPH2−/− pups suggest that maternal–pup interactions exert great influence on the thriving of these mice.
Successful rearing and survival of offspring in rodents involves the expression of a number of maternal behaviors, including nursing, pup retrieval (Champagne et al. 2007) and huddling (Lerch-Haner et al. 2008), nest building and maternal aggression (Gammie et al. 2007). These behaviors are considered indexes of maternal motivation (Numan & Stolzenberg 2009) and maternal responsivity, both of which are essential for offspring viability (Wang & Storm 2011), protection against predators, temperature maintenance (Sauce et al. 2012) and facilitation of feeding (Lerch-Haner et al. 2008). After an extensive assessment of maternal performance we found that TPH2−/− dams show clear deficiencies in pup retrieval, huddling and nest construction. This maternal neglect shown by TPH2−/− dams involved a decrease in the active high-arched back nursing position accompanied by an increase in the less engaged low-arched back nursing. The genotype of the pups did not have a direct effect on the expression of these maternal behaviors because the neglect displayed by TPH2−/− dams took place when rearing either cross-fostered TPH2+/+ pups or litters with mixed genotypes. Several brain areas suggested to be pivotal for maternal behaviors include the medial preoptic area (Numan & Stolzenberg 2009), the bed nucleus of the stria terminalis, the lateral septum, the hippocampus and amygdala (Ruthschilling et al. 2012), all of which are directly innervated by serotonergic afferents arising in the dorsal and median raphé and B9 nucleus (Lerch-Haner et al. 2008). The maternal neglect observed in TPH2−/− mothers could be the result of the altered formation of circuitry governing maternal motivation and reward due to the absence of serotonin or other serotonin-related signals in the brain areas driving maternal care, as suggested by other mouse models of altered maternal behaviors with a decrease in brain serotonin (Lerch-Haner et al. 2008).
Besides maternal retrieval and care, maternal defense is a behavior exhibited by all dams to defend their litter against a potential threat from other conspecifics (Erskine et al. 1978; Lonstein & Gammie 2002; Neumann et al. 2001; Rosenblatt 1994). We found that lactating TPH2−/− dams were as aggressive as TPH2+/+ dams in a maternal aggression paradigm. However, a previous finding that TPH2−/− mice are inherently more aggressive than their TPH2+/+ counterparts (Kane et al. 2012) created a confounding factor that was differentiated by testing non-lactating females of both genotypes in the same paradigm. The result showed that lactation did not change the patterns of elevated aggression of TPH2−/− females whereas lactating TPH2+/+ females had greater number of attacks and shorter latencies to attack than their non-lactating counterparts. It is not clear if the elevated aggression that the TPH2−/− dams display in general contexts has a ceiling point and makes it difficult for them to show even more aggression in a maternal context. Studies have found that the sensory input produced by the suckling of pups plays a role in activating and maintaining maternal aggression and this action may result from suckling-induced increases in central serotonin production and release (Gammie & Nelson 1999). The lack of central serotonin in the TPH2−/− dams could explain their deficiencies in maternally driven aggression while the steadily increased aggression is more consistent with decreased serotonergic activity (Popova 2006).
In addition to the expression of maternal behaviors, another adaptation in lactation is reduced anxiety (Bosch 2011). Furthermore, serotonin deficiencies have been largely associated with anxiety (Griebel & Holmes 2013) and depression (Albert 2012). Our results show that the lack of serotonin does not cause an increase in anxiety-like behaviors in non-lactating or lactating TPH2−/− females. This suggests that the maternal neglect showed by TPH2−/− dams is not a result of an increased anxiety phenotype. The high degree of compulsivity characteristic of the TPH2−/− mice (Angoa-Perez et al. 2012) was also present in lactating females as observed by increased digging, climbing and exploring behavior (Figs. S1 and S2). This suggests that elevated compulsivity rather than anxiety exerts more influence on the maternal impairments displayed by TPH2−/− mothers. Compulsive-like traits (i.e. repetitive behaviors) have been modeled in mice as part of a repertoire of maladaptive behaviors that resemble obsessive compulsive disorders in humans. These disorders are associated with an inability to stop unwanted actions in the affected individual, leading to deleterious effects (Kas et al. 2010). The increase in the frequency of repetitive behaviors (digging, climbing and exploring) observed in the TPH2−/− dams could be associated with lack of an ability to focus on relevant tasks at the moment, such as the nurturing of their offspring.
Adult mice are able to discriminate between their own and alien offspring based upon olfactory and gustatory cues (Ostermeyer & Elwood 1983). TPH2−/− dams did not show any olfactory alterations that could account for their poor maternal behaviors. However, the urine preference results show that TPH2−/− dams were not able to differentiate TPH2+/+ from TPH2−/− pups, whereas TPH2+/+ dams were (Fig. 7c). This lack of an ability of TPH2−/− dams to discriminate their related offspring suggests that other systems beyond olfaction involved in pheromone detection could be affected by the depletion of serotonin. Infant crying is an important cue for mothers to respond adequately. In rodents, the pup-mother interaction largely depends on pup vocalizations. Mouse pups emit high frequency to ultrasonic vocalizations (2–90 kHz) to communicate with their dam for maternal care (Wu et al. 2009). Although we did not measure pup vocalizations in this study, it has been reported that TPH2−/− pups vocalize equally as controls when separated from their mothers (Alenina et al. 2009). Studies in mice have associated hearing impairments in the dams with deficits in their maternal behaviors (Wu et al. 2009). However, no differences were found in TPH2−/− females compared to controls over a range of auditory stimuli from 2 to 45 Hz that includes ultrasonic frequencies, suggesting that hearing impairments are not a contributor to the maternal neglect exhibited by TPH2−/− dams.
Overall, these results show that up to weaning, certain elements of the maternal behavior repertoire as well as other indicators of pup thriving have differential influences. Pup retrieval and nursing behaviors were determined by maternal genotype, whereas cannibalization and urine preference were determined by the genotype of the pups. Survival, weaning weight and nest building were influenced by both maternal and pup genotype interactions. Lactation made it possible to distinguish general aggression from maternal aggression in TPH2+/+dams. This suggests that not only maternal care but also pup attributes and interactions with their mother represent crucial components for reproductive success.
The present study illustrates the key involvement of brain serotonin in maternal behaviors that are essential for off-spring survival and nurturing and it opens the question of exploring the impact of poor maternal care on the progeny development and behavior in adulthood.
Supplementary Material
Footnotes
Supporting Information
Additional supporting information may be found in the online version of this article at the publisher's web-site:
Appendix S1: Supporting methods
References
- Albert PR. Transcriptional regulation of the 5-HT1A receptor: implications for mental illness. Philos Trans R Soc Lond B Biol Sci. 2012;367:2402–2415. doi: 10.1098/rstb.2011.0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alenina N, Kikic D, Todiras M, Mosienko V, Qadri F, Plehm R, Boye P, Vilianovitch L, Sohr R, Tenner K, Hortnagl H, Bader M. Growth retardation and altered autonomic control in mice lacking brain serotonin. Proc Natl Acad Sci U S A. 2009;106:10332–10337. doi: 10.1073/pnas.0810793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angoa-Perez M, Kane MJ, Briggs DI, Sykes CE, Shah MM, Francescutti DM, Rosenberg DR, Thomas DM, Kuhn DM. Genetic depletion of brain 5HT reveals a common molecular pathway mediating compulsivity and impulsivity. J Neurochem. 2012;121:974–984. doi: 10.1111/j.1471-4159.2012.07739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barofsky AL, Taylor J, Tizabi Y, Kumar R, Jones-Quartey K. Specific neurotoxin lesions of median raphe serotonergic neurons disrupt maternal behavior in the lactating rat. Endocrinology. 1983;113:1884–1893. doi: 10.1210/endo-113-5-1884. [DOI] [PubMed] [Google Scholar]
- Bosch OJ. Maternal nurturing is dependent on her innate anxiety: the behavioral roles of brain oxytocin and vasopressin. Horm Behav. 2011;59:202–212. doi: 10.1016/j.yhbeh.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: from central release to sites of action. Horm Behav. 2012;61:293–303. doi: 10.1016/j.yhbeh.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Curley JP, Keverne EB, Bateson PP. Natural variations in postpartum maternal care in inbred and out-bred mice. Physiol Behav. 2007;91:325–334. doi: 10.1016/j.physbeh.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Church MW, Zhang JS, Langford MM, Perrine SA. ‘Ecstasy’ enhances noise-induced hearing loss. Hear Res. 2013;302:96–106. doi: 10.1016/j.heares.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erskine MS, Denenberg VH, Goldman BD. Aggression in the lactating rat: effects of intruder age and test arena. Behav Biol. 1978;23:52–66. doi: 10.1016/s0091-6773(78)91148-3. [DOI] [PubMed] [Google Scholar]
- Gammie SC. Current models and future directions for understanding the neural circuitries of maternal behaviors in rodents. Behav Cogn Neurosci Rev. 2005;4:119–135. doi: 10.1177/1534582305281086. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Nelson RJ. Maternal aggression is reduced in neuronal nitric oxide synthase-deficient mice. J Neurosci. 1999;19:8027–8035. doi: 10.1523/JNEUROSCI.19-18-08027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammie SC, Bethea ED, Stevenson SA. Altered maternal profiles in corticotropin-releasing factor receptor 1 deficient mice. BMC Neurosci. 2007;8:17. doi: 10.1186/1471-2202-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammie SC, D'Anna KL, Gerstein H, Stevenson SA. Neurotensin inversely modulates maternal aggression. Neuro-science. 2009;158:1215–1223. doi: 10.1016/j.neuroscience.2008.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Holmes A. 50 years of hurdles and hope in anxiolytic drug discovery. Nat Rev Drug Discov. 2013;12:667–687. doi: 10.1038/nrd4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde JF. Effects of phencyclidine on 5-hydroxytryptophanand suckling-induced prolactin release. Brain Res. 1992;573:204–208. doi: 10.1016/0006-8993(92)90764-z. [DOI] [PubMed] [Google Scholar]
- Ieni JR, Thurmond JB. Maternal aggression in mice: effects of treatments with PCPA, 5-HTP and 5-HT receptor antagonists. Eur J Pharmacol. 1985;111:211–220. doi: 10.1016/0014-2999(85)90758-7. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Angoa-Perez M, Briggs DI, Sykes CE, Frances-cutti DM, Rosenberg DR, Kuhn DM. Mice genetically depleted of brain serotonin display social impairments, communication deficits and repetitive behaviors: possible relevance to autism. PLoS One. 2012;7:e48975. doi: 10.1371/journal.pone.0048975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kas MJ, Gelegen C, van Nieuwerburgh F, Westenberg HG, Deforce D, Denys D. Compulsivity in mouse strains homologous with chromosomes 7p and 15q linked to obsessive-compulsive disorder. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:252–259. doi: 10.1002/ajmg.b.30994. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Herman AE. Maternal behavior and developmental psychopathology. Biol Psychiatry. 2002;51:27–43. doi: 10.1016/s0006-3223(01)01277-x. [DOI] [PubMed] [Google Scholar]
- Lerch-Haner JK, Frierson D, Crawford LK, Beck SG, Deneris ES. Serotonergic transcriptional programming determines maternal behavior and offspring survival. Nat Neurosci. 2008;11:1001–1003. doi: 10.1038/nn.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshem M, Kreider M. Brain serotonin depletion and nipple attachment in rat pups. Pharmacol Biochem Behav. 1987;27:7–14. doi: 10.1016/0091-3057(87)90469-2. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Gammie SC. Sensory, hormonal, and neural control of maternal aggression in laboratory rodents. Neurosci Biobehav Rev. 2002;26:869–888. doi: 10.1016/s0149-7634(02)00087-8. [DOI] [PubMed] [Google Scholar]
- Narboux-Neme N, Angenard G, Mosienko V, Klempin F, Pity-choutis PM, Deneris E, Bader M, Giros B, Alenina N, Gaspar P. Postnatal growth defects in mice with constitutive depletion of central serotonin. ACS Chem Neurosci. 2013;4:171–181. doi: 10.1021/cn300165x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckers LM, Zarrow MX, Myers MM, Denenberg VH. Influence of olfactory bulbectomy and the serotonergic system upon intermale aggression and maternal behavior in the mouse. Pharmacol Biochem Behav. 1975;3:545–550. doi: 10.1016/0091-3057(75)90170-7. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Toschi N, Ohl F, Torner L, Kromer SA. Maternal defence as an emotional stressor in female rats: correlation of neuroendocrine and behavioural parameters and involvement of brain oxytocin. Eur J Neurosci. 2001;13:1016–1024. doi: 10.1046/j.0953-816x.2001.01460.x. [DOI] [PubMed] [Google Scholar]
- Numan M, Stolzenberg DS. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Front Neuroendocrinol. 2009;30:46–64. doi: 10.1016/j.yfrne.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Ostermeyer MC, Elwood RW. Pup recognition in Mus musculus: parental discrimination between their own and alien young. Dev Psychobiol. 1983;16:75–82. doi: 10.1002/dev.420160202. [DOI] [PubMed] [Google Scholar]
- Oxley G, Fleming AS. The effects of medial preoptic area and amygdala lesions on maternal behavior in the juvenile rat. Dev Psychobiol. 2000;37:253–265. [PubMed] [Google Scholar]
- Popova NK. From genes to aggressive behavior: the role of serotonergic system. Bioessays. 2006;28:495–503. doi: 10.1002/bies.20412. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS. Psychobiology of maternal behavior: contribution to the clinical understanding of maternal behavior among humans. Acta Paediatr Suppl. 1994;397:3–8. doi: 10.1111/j.1651-2227.1994.tb13259.x. [DOI] [PubMed] [Google Scholar]
- Rosenblatt J, Lehrman D. Maternal behavior in the laboratory rat. In: Rheingold HL, editor. Maternal Behavior in Mammals. John Wiley and Sons; New York, NY: 1963. pp. 8–57. [Google Scholar]
- Ruthschilling CA, Albiero G, Lazzari VM, Becker RO, de Moura AC, Lucion AB, Almeida S, Veiga AB, Giovenardi M. Analysis of transcriptional levels of the oxytocin receptor in different areas of the central nervous system and behaviors in high and low licking rats. Behav Brain Res. 2012;228:176–184. doi: 10.1016/j.bbr.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Sauce B, de Brito RA, Peripato AC. Genetic architecture of nest building in mice LG/J x SM/J. Front Genet. 2012;3:90. doi: 10.3389/fgene.2012.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Angoa Perez M, Francescutti-Verbeem DM, Shah MM, Kuhn DM. The role of endogenous serotonin in methamphetamine-induced neurotoxicity to dopamine nerve endings of the striatum. J Neurochem. 2010;115:595–605. doi: 10.1111/j.1471-4159.2010.06950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Velzen A, Toth M. Role of maternal 5-HT(1A) receptor in programming offspring emotional and physical development. Genes Brain Behav. 2010;9:877–885. doi: 10.1111/j.1601-183X.2010.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Storm DR. Maternal behavior is impaired in female mice lacking type 3 adenylyl cyclase. Neuropsychopharmacology. 2011;36:772–781. doi: 10.1038/npp.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JM, Legates JE, Eisen EJ. Maternal effects among lines of mice selected for body weight. Genetics. 1968;60:395–408. doi: 10.1093/genetics/60.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WL, Wang CH, Huang EY, Chen CC. Asic3(−/−) female mice with hearing deficit affects social development of pups. PLoS One. 2009;4:e6508. doi: 10.1371/journal.pone.0006508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.