Abstract
In previous studies, we have demonstrated that exposure of astroglial cells to A3 adenosine receptor agonists results in dual actions on cell survival, with “trophic” and antiapoptotic effects at nanomolar concentrations and induction of cell death at micromolar agonist concentrations. The protective actions of A3 agonists have been associated with a reinforcement of the actin cytoskeleton, which likely results in increased resistance of cells to cytotoxic stimuli. The molecular mechanisms at the basis of this effect and the signalling pathway(s) linking the A3 receptor to the actin cytoskeleton have never been elucidated. Based on previous literature data suggesting that the actin cytoskeleton is controlled by small GTP-binding proteins of the Rho family, in the study reported here we investigated the involvement of these proteins in the effects induced by A3 agonists on human astrocytoma ADF cells. The presence of the A3 adenosine receptor in these cells has been confirmed by immunoblotting analysis. As expected, exposure of human astrocytoma ADF cells to nanomolar concentrations of the selective A3 agonist 2-chloro-N6-(3-iodobenzyl)-adenosine-5'-N-methyluronamide (Cl-IB-MECA) resulted in formation of thick actin positive stress fibers. Preexposure of cells to the C3B toxin that inactivates Rho-proteins completely prevented the actin changes induced by Cl-IB-MECA. Exposure to the A3 agonist also resulted in significant reduction of Rho-GDI, an inhibitory protein known to maintain Rho proteins in their inactive state, suggesting a potentiation of Rho-mediated effects. This effect was fully counteracted by the concomitant exposure to the selective A3 receptor antagonist MRS1191. These results suggest that the reinforcement of the actin cytoskeleton induced by A3 receptor agonists is mediated by an interference with the activation/inactivation cycle of Rho proteins, which may, therefore, represent a biological target for the identification of novel neuroprotective strategies.
Keywords: Adenosine, A3 receptor, Neuroprotection, Rho proteins
INTRODUCTION
The actions of adenosine are mediated by four G-protein coupled membrane receptors, the A1, A2A, A2B, and A3 receptors.1 Although the existence of the A1, A2A, and A2B receptors was postulated before they were cloned, the A3 receptor was discovered by cloning, initially from rat and subsequently from human tissues.2 The development of selective agonists, such as N6-(3-iodobenzyl)-adenosine-5′-N-methyluronamide (IB-MECA)3 and its 2-chloro-derivative (Cl-IB-MECA),4 and more recently, antagonists, such as MRS1191 (3-ethyl-5-benzyl-2-methyl-6-phenyl-4-phenylethynyl-1,4-(±)-dihydropyridine-3,5-dicarboxyla-te),5 and MRS1220 (9-chloro-2-(2-furyl)-5-phenyl-acetylamino-[1,2,4]-triazolo-[1,5-c]-quinazoline)6 has played a crucial role in defining the putative pathophysiological roles of this receptor.
The A3 receptor is involved in inflammation,2 hypotension and mast cell degranulation,7 ischemic heart preconditioning.2 Although expressed to quite low levels in the brain,8 this receptor has also been implicated in behavioral depression8 and modulation of cerebral ischemic damage.9 Use of selective A3 agonists also revealed that this receptor profoundly affects cell survival, by promoting both cell protection and cell death, depending upon the cell type and/or the agonist concentrations. At low concentrations in the nanomolar range, A3 agonists reduce hypoxic heart damage10 and protect HL-60, U-937 cells, and mammalian astrocytes from apoptosis.11,12 In astroglial cells, increased resistance to apoptosis was associated with a reinforcement of the actin cytoskeleton and with the intracellular redistribution of the antiapoptotic protein Bcl-xL.12,13 At high concentrations in the micromolar range, these same agonists markedly impaired cell cycle progression14 and induced death of cerebellar granule neurons,15 HL-60 cells,16 human lymphocytes,17 and astroglial cells.12 These dual actions confirm the concept that adenosine may represent a signal of both life and death for its target cells, simply depending on specific pathophysiological conditions.18
A hypothesis that may reconcile the opposite effects of adenosine on brain cell survival and give them a pathophysiological significance has recently been raised.19 In traumatic and ischemic brain, large amounts of adenosine are released from nucleic acids of dying cells as a result of increased neurotransmitter release and break-down of nucleotides and nucleosides.20 Hence, concentrations of adenosine in the ischemic brain are believed to depend on the extent of cellular damage; that is, to attain their highest values within the ischemic “core” area and to gradually decrease progressively in the direction of the “penumbra” area.19 Hence, within the core itself, adenosine is likely to reach levels that fully activate the A3 receptor, resulting in the full range of its destructive effects to favour the elimination of irreversibly damaged cells and to save space and energy for those cells that retain the ability to recover.19 In contrast, in the penumbral area, the progressively lower concentrations of adenosine would lead to a milder, “subthreshold”, stimulation of the A3 receptor, to result in more benign actions, such as increased resistance of cells to stress, anti-apoptotic effects, and recovery of damaged neurons via a potentiation of the astrocytic support to these cells.19
The molecular mechanisms underlying the protective effects mediated by the A3 receptor remain obscure. As mentioned above, in human astrocytoma cells, the cytoskeletal rearrangement induced by nanomolar A3 agonist concentrations is characterized by a marked increase of F-actin stress fibers.12,13 Since actin polymerization is crucially controlled by small GTP binding proteins of the Rho family,21 in the study reported here we tested whether the cytoprotective effects induced by A3 agonists may be mediated by a specific action on this system.
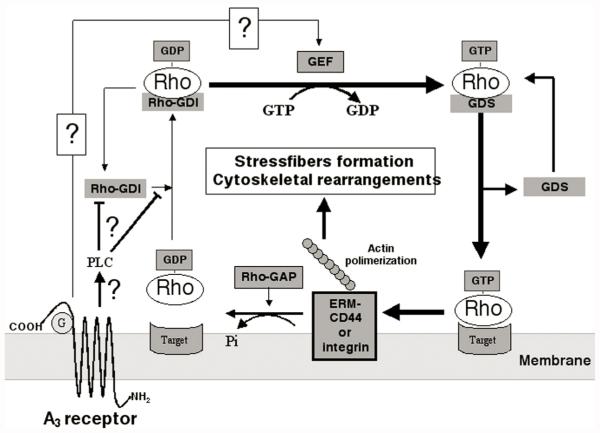
Under basal, unstimulated conditions, Rho proteins are found in the cytoplasm, where they are maintained in their inactive GDP bound state by guanine nucleotide dissociation inhibitors (Rho-GDIs)21 (see Figure 1). Exchange of GDP with GTP results in release of Rho-GDI, binding of Rho to a family of stimulatory proteins (Rho-GDS) and migration of the activated Rho protein to the membrane (Fig. 1). Here, activated Rho can interact with a variety of different targets, mainly kinases, that in turn activate a number of proteins, including those regulating the actin cytoskeleton, such as specific membrane associated proteins (e.g., ERM, Ezrin/Radixin/Moesin).22 As a result of such interactions, actin fibers organize to form filamentous structures, termed stress fibers. Return of Rho proteins to their inactive state is favored by proteins that promote the hydrolysis of GTP to GDP (Rho-GAPs) and binding to Rho-GDI (Fig. 1). The activation/inactivation cycle of Rho proteins is crucially regulated by guanine nucleotide exchange factors (GEFs) that can in turn be activated by several signals merging on Rho proteins. Rho links membrane receptors, activated by extracellular factors such as lysophosphatidic acid (LPA), bombesin, or thrombin, to the formation of actin stress fibers and focal adhesion contacts,22 through various signalling pathways, including tyrosine kinases, cAMP and phospholipaseC/protein kinase.23 It is presently unknown whether activation of the A3 receptor can result in signalling to Rho proteins. However, this receptor has been previously shown to activate phospholipase C (PLC) in the brain.24
FIGURE 1.
Schematic representation of the putative relationship between the adenosine A3 receptor and Rho proteins. Under basal, unstimulated conditions, Rho proteins are maintained in their GDP bound inactive state by Rho-GDI. Stimulation of GDP exchange with GTP via guanine nucleotide exchange factors (GEF) results in release of Rho-GDI, binding of Rho to Rho-GDS and migration of the activated Rho protein to the membrane. Here, Rho can interact with its targets (mainly kinases) that in turn activate a number of proteins, including integrins, CD44, and proteins involved in regulation of the actin cytoskeleton, such as ERM (Ezrin/Radixin/Moesin). Interaction with actin promotes its polymerization and the formation of F-actin stress fibers (as an example, see FIG. 3 b). Return of Rho protein to its inactive state is favoured by proteins that promote the hydrolysis of GTP to GDP (Rho-GAPs) and binding to Rho-GDI. These results suggest that stimulation of the A3 receptor, likely through PLC,24 can influence the activation/inactivation cycle of Rho proteins. This could be achieved by either a direct stimulation of GEFs and/or by modulation of Rho-GDI. Data show that exposure to A3 agonists reduces Rho-GDI availability, by either reducing its expression or by promoting conformational changes that decrease its ability to bind to Rho. This would lead to a potentiation of Rho-mediated effects (thick arrows). See text for further details.
MATERIALS AND METHODS
Cell Culture and Treatment
Human astrocytoma ADF cells were grown at 37°C in humidified atmosphere as previously described.25 After 24 h in culture, cells were exposed to Cl-IB-MECA in the absence or presence of MRS 1191 for 48 h prior to analysis. In selected experiments, before addition of Cl-IB-MECA, cells were exposed to the chimeric toxin C3B (0.5 microg/ml) for three hour, and then the toxin was maintained in the medium for the remainder of the experiment (which lasted 48 h). This toxin consists of the C3 isoenzyme linked to the binding portion of the diphtheria toxin, and has been shown to specifically ADP-ribosylate Rho, rendering it inactive (for more details on the effects of C3B, see Refs. 23 and 26) The A3 selective agonist Cl-IB-MECA and antagonist MRS1191 were synthesized as described elsewhere.4,5
Analytical Cytology
For actin staining, ADF cells were fixed with 3.7% formaldehyde in PBS (pH 7.4) for 20 min at room temperature. After washing in the same buffer, cells were permeabilized with 0.5% Triton X-100 (Sigma) in PBS for five minute at room temperature. Cells were stained with fluorescein–phalloidin (Sigma) at 37°C for 30 min. Finally, after washing, all the samples were mounted with glycerol-PBS (2: 1) and observed with a Nikon Microphot fluorescence microscope.
Western Blot Analysis
Detection of the A3 Adenosine Receptor
Cells at subconfluency were washed, scraped and incubated in lysis buffer (9.1 mM Na2H2PO4; 1.7 mM Na2HPO4; 150 mM NaCl, pH 7.4; 0.5% sodium deoxycholate; 1% Nonidet P-40; 0.1% SDS, containing proteinase inhibitors) for 60 min at 4°C. After centrifugation, proteins were assayed in the soluble fraction and lysates (1 mg) incubated overnight at 4°C with an antibody raised against the human A3 receptor (Alfa-Diagnostic, San Antonio, TX, U.S.A.; 4 μg/ml). The immunocomplex was precipitated with Protein-A sepharose (50 μg) for two hours at 4°C. The beads were washed with buffer (150 mM NaCl, 10 mM Tris, 1% NP-40) three times, and bound proteins solubilized in Laemmli buffer. For immunoblotting, equivalent amounts of protein (typically 100 μg/samples) were resolved on 12% (w/v) sodiumdodecylsulphate (SDS) polyacrilamide gels. Resolved proteins were transferred to nitrocellulose and incubated with the primary anti-A3 receptor antibody (1 μg/ml overnight at 4°C). After extensive washing with TBS (10 mM Tris-HCL, 150 mM NaCl, pH 8) containing 0.05% Tween-20, the nitrocellulose membrane was incubated for 120 min at room temperature with horseradish peroxidase (HRP) goat anti-rabbit conjugated secondary antibody diluted to 1 : 2,000 in Blotto. After several washes, reactive proteins were visualized by an enhanced chemiluminescence protocol ECL (Amersham Pharmacia Biotech). Immunoblotting was quantified by densitometric scanning of films exposed in the linear range. For these experiments, CHO cells transfected with the human A3 adenosine receptor cDNA were used as a positive control.14
Detection of Rho-GDI
Samples, containing about 20 μg protein each, were then loaded on 11% sodiumdodecylsulphate (SDS) polyacrilamide gels and blotted onto nitrocellulose filters. Filters were then incubated with rabbit polyclonal antibody anti-Rho-GDI (1:4,000, Santa Cruz Biotechnology), followed by a secondary antirabbit antibody (peroxidase conjugated, 1:4000), and reactive proteins visualized and quantified as described above. In selected experiments, changes of Rho-GDI were also confirmed by immunoprecipitation. In this case, samples, containing about 30 μg protein, prepared as described above, were incubated with anti-Rho-GDI polyclonal antibody overnight at 4°C (1μg/sample). Rho-GDI immunoprecipitates were then incubated with protein-A-agarose (Santa Cruz Biotechnology, one hour, room temperature) and the immune complexes were centrifuged. The supernatants were then collected and added of sample buffer (187.5 mM Tris-HCl, 6% SDS, 30% glycerol, 15% B-mercaptoethanol, 0.003% bromophenol blue), whereas the precipitates were washed with PBS and resuspended in water and sample buffer. Samples from both supernatants and precipitates were loaded on 11% sodium-dodecylsulphate polyacrylamide gels and processed as described above.
RESULTS
The presence of the A3 adenosine receptor in human astrocytoma ADF cells has been confirmed by inmmunoblotting analysis with a specific antibody raised against the human A3 receptor, and by using CHO cells expressing this receptor subtype to high levels as a positive control.14 In both CHO and ADF cells, a specific immunoreactive protein band with an apparent molecular weight of 36 kDa corresponding to that of the A3 receptor1 could be detected (see Figure 2).
FIGURE 2.

Detection of the A3 adenosine receptor (A3AR) by immunoblotting analysis in human astrocytoma ADF cells. After cell lysis, the A3 receptor was detected by immunoprecipitation with a specific antibody and Protein A-sepharose, followed by protein resolution on 11% SDS polyacrilamide gels as described in Materials and Methods. CHO cells transfected with the human A3 receptor cDNA were used as a positive control.14 Under such conditions, the A3 receptor can be detected in both ADF and CHO cells as a specific immunoreactive protein band with an apparent molecular weight of 36 kDa (arrow).
As expected, exposure of ADF cells to the A3 receptor agonist Cl-IB-MECA (100 nM for 48 h) resulted in marked cytoskeletal rearrangement, as demonstrated by the appearance of thick F-actin positive stress fibers (compare Figure 3 b with control, Figure 3 a). Inhibition of Rho, as a result of exposure of cells to the C3B toxin, provoked retraction of the cell body and breakdown of the actin cytoskeleton (Fig. 3 c). This effect is not associated with a permanent disruption of the actin cytoskeleton, since C3B toxin-treated cells still respond to agents (e.g., cytotoxic necrotizing factor-127) that stimulate actin polymerization. Hence, C3B toxin is widely used to assess the involvement of Rho in cellular function.23 Addition of Cl-IB-MECA to C3B toxin treated astrocytoma cells could not reproduce the typical actin changes normally induced by the A3 agonist (compare FIGS. 3 d and 3 b), suggesting that integrity of Rho is needed to mediate such effects. To evaluate whether Cl-IB-MECA can affect the expression of Rho-GDI, we performed Western blot analysis with a specific anti-Rho-GDI antibody. As expected,28 Rho-GDI was detected as a specific protein band with a molecular weight of 28 kDa (see Figure 4 A). A 48-h exposure of cells to 100 nM Cl-IB-MECA resulted in a marked reduction of this protein band with respect to control cells (Fig. 4 A). A specific role for the A3 receptor in this effect was demonstrated by the ability of the selective A3 antagonist MRS1191 to fully prevent Cl-IB-MECA-induced reduction of the 28-kDa protein band (Fig. 4 A).
FIGURE 3.
Inhibition of Rho prevented Cl-IB-MECA-induced reinforcement of the actin cytoskeleton. In comparison to control cells (a), exposure of ADF cells to 100 nM Cl-IB-MECA for 48 h (b) resulted in marked formation of thick actin stress fibers. Exposure of ADF cells to C3B toxin (c) provoked the retraction of the cell body and the break-down of the actin cytoskeleton. Pretreatment of cells with C3B toxin for three hours before exposure to Cl-IB-MECA (d) fully prevented the actin changes induced by the A3 agonist.
FIGURE 4.
Reduction of Rho-GDI in cultures exposed to Cl-IB-MECA. Cells were exposed to 100 nM Cl-IB-MECA for 48 h in the absence or presence of MRS1191, as indicated. For the Rho-GDI immunoblotting analysis shown in A, cells were lysed, homogenized, proteins separated by SDS-PAGE, and Rho-GDI identified by immunoblot analysis as described in Materials and Methods. For the Rho-GDI immunoprecipitation experiments shown in B, cell homogenates were incubated with the anti-Rho-GDI antibody overnight, and immune complexes separated by centrifugation as described in Materials and Methods. Thus, SDS-PAGE and immunoblot analysis were performed on both supernatants and precipitates. *p < 0.05 with respect to control, **p < 0.05 with respect to control, and Cl-IB-MECA alone, one way ANOVA (Fisher test).
These data have also been confirmed by an immunoprecipation technique. Cell homogenates have been incubated with the anti-Rho-GDI antibody, and the immunocomplex quantified in precipitates. The results indicated in Figure 3 B show that, under the experimental conditions used (see Materials and Methods), immunoprecipation was complete, with no detectable residual immunoreactivity for Rho-GDI in the supernatants of both control and treated cells. A notable reduction of the amount of Rho-GDI was also detected with this methodology after exposure of cells to Cl-IB-MECA (Fig. 4 B), hence confirming the results described in Figure 4 A.
DISCUSSION
The adenosine A3 receptor has been reported to be expressed at low levels in brain-derived tissues.1,8 For this reason, we deemed it important to confirm its presence in the human astrocytoma cells used in this study. Immunoblotting analysis performed with a specific antibody raised against the human A3 receptor confirmed that these cells do indeed express the receptor protein to significant levels, hence validating this experimental model for studying the functional effects mediated by this receptor in cells of the astroglial lineage. The present data also demonstrate for the first time that activation of the adenosine A3 receptor results in signaling to the small G-protein Rho, possibly through the phospholipase C pathway.24 Moreover, they suggest that the previously reported reinforcement of the actin cytoskeleton on exposure to selective A3 agonists12,13 is mediated by an interference with the activation/inactivation cycle of Rho-proteins. This conclusion is based on the following evidence: (1) inhibition of Rho with the specific C3B toxin completely abolishes the actin changes induced by the A3 selective agonist Cl-IB-MECA; and (2) exposure of cells to Cl-IB-MECA under experimental conditions that are associated with cell protection (i.e., 100 nM for 48 h) resulted in a reduction of Rho-GDI, as demonstrated by both standard Western blot analysis and by immunoprecipitation. We do not know at present whether the detected reduction of Rho-GDI is a result of diminished protein synthesis, of increased protein turn over, and/or induction of conformational changes that decrease its ability to bind to both Rho (Fig. 1) and to the anti-Rho-GDI antibody. Experiments specifically aimed at quantifying the expression of Rho-GDI (i.e., reverse transcriptase polymerase chain reaction) will enable us to shed light on the mechanisms underlying this effect. However, this result indirectly suggests a potentiation of Rho mediated effects. A lower availability of the inhibitory protein necessary to keep Rho at a basal, unstimulated state may in fact result in a higher percentage of GDS bound Rho, which in turn would lead to increased Rho activation and stimulation of actin polymerization (Fig. 1). To confirm this hypothesis, we plan to used the immunoprecipitation technique to quantify GDI-bound Rho proteins in immunoprecipitates after incubation of cell homogenates with the anti-Rho-GDI antibody. If stimulation of the A3 receptor increases the percentage of Rho activation, smaller amounts of Rho proteins are expected to coimmunoprecipate with Rho-GDI in A3 agonist-treated cells. We also plan to identify the Rho protein(s) involved in this effect by using antibodies that specifically recognize the various members of this family (i.e., Rho-A, Rho-B, Rho-C, rac1/2, cdc42, etc).21 Of course, we cannot rule out the possibility that activation of the A3 receptor leads to a direct stimulation of GEFs, which would directly potentiate Rho (Fig. 1). In this case, the detected reduction of Rho-GDI represents a compensatory mechanism aimed at counteracting the excessive activation of the system.
Our data also imply that Rho activation via a subthreshold stimulation of the A3 receptor (as that attained by nanomolar agonist concentrations) is associated with cell protection; that is, inhibition of apoptosis.12 It is not clear at present whether induction of cell death upon a robust activation of this receptor (as that attained with micromolar agonist concentrations) also occurs through modulation of Rho proteins. By using other experimental paradigms,29 it has been demonstrated that prolonged inhibition of Rho is indeed associated with induction of apoptosis. It has been hypothesized that the dual effects induced by the A3 receptor may depend on either the state of receptor activation and/or induction of receptor desensitization by high agonist concentrations.19,20 We suggest that, depending upon the agonist concentrations, the degree of receptor activation may regulate the balance between Rho activation/inactivation, which in turn regulates the susceptibility to cell death.
The elucidation of the molecular mechanisms forming the basis of the protective effects induced by A3 agonists may have important pathophysiological implications. Besides their established role in cardioprotection30 and their putative contribution to neuronal recovery in brain ischemic penumbra,19,20 these effects may also play a role in the development of brain preconditioning, a phenomenon according to which a brief ischemic attack can protect the brain from a subsequent, and lethal, stronger ischemic insult.31 The demonstration that such protection is mediated by modulation of Rho proteins activity may disclose further biological targets for the identification of novel antiischemic and neuroprotective strategies.
ACKNOWLEDGMENTS
This work was supported by the Italian National Research Council (CNR) Contributo di Ricerca No. 98.01047.CT04 to MPA and by the Ministero dell’Universita’ e della Ricerca Scientifica e Tecnologica (MURST), Cofinanziamento di ricerche di interesse nazionale 1999 to FC on “Recettori purinergici e neuroprotezione”. The authors are grateful to Dr. Charly Klotz (University of Wurzburg, Germany) for kindly providing CHO cells tranfected with the human A3 adenosine receptor.
REFERENCES
- 1.FREDHOLM BB, ABBRACCHIO MP, BURNSTOCK G, et al. Nomenclature and classification of purinoceptors. Pharm. Rev. 1994;46:143–156. [PMC free article] [PubMed] [Google Scholar]
- 2.LINDEN J. Cloned adenosine A3 receptors: pharmacological properties, species-differences and receptor functions. Trends Pharmacol. Sci. 1994;15:298–306. doi: 10.1016/0165-6147(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 3.GALLO-RODRIGUEZ C, JI X-D, MELMAN N, et al. Structure–activity-relationships of N6-benzyladenosine-5′-uronamides as A3-selective adenosine agonists. J. Med. Chem. 1994;37:636–646. doi: 10.1021/jm00031a014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.KIM HO, JI XD, SIDDIQI SM, et al. 2-Substitution of N6-benzyladenosine-5′-uronamides enhances selectivity for A3-adenosine receptors. J. Med. Chem. 1994;37:3614–3621. doi: 10.1021/jm00047a018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.JACOBSON KA, PARK KS, JIANG JI, et al. Pharmacological characterization of novel A3 adenosine receptor-selective antagonists. Neuropharmacol. 1997;36:1157–1165. doi: 10.1016/s0028-3908(97)00104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.KIM YC, JI XD, JACOBSON KA. Derivatives of the triazoloquinazoline adenosine antagonist (CGS15943) are selective for the human A3 receptor subtype. J. Med. Chem. 1996;39:4142–4148. doi: 10.1021/jm960482i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.HANNON JP, PFANNKUCHE HJ, FOZARD JR. A role for mast cells in adenosine A3 receptor-mediated hypotension in the rat. Br. J. Pharmacol. 1995;115:945–952. doi: 10.1111/j.1476-5381.1995.tb15902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.JACOBSON KA, NIKODIJEVIC O, SHI D, et al. A role for central A3-adenosine receptors: mediation of behavioral depressant effects. FEBS Lett. 1993;336:57–60. doi: 10.1016/0014-5793(93)81608-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.VON LUBITZ DKJE, LIN RCS, POPIK P, et al. Adenosine A3 receptor stimulation and cerebral ischemia. Eur. J. Pharmacol. 1994;263:59–67. doi: 10.1016/0014-2999(94)90523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.STAMBAUGH C, JIANG JL, JACOBSON KA, LIANG BT. Novel cardioprotective function of adenosine A3 receptor during prolonged simulated ischemia. Am. J. Physiol. 1997;273:H501–H505. doi: 10.1152/ajpheart.1997.273.1.H501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.YAO Y, SEI Y, ABBRACCHIO MP, et al. Adenosine A3 receptor agonists protect HL-60 cells and U-937 cells from apoptosis induced by A3 antagonists. Biochem. Biophys. Res. Commun. 1997;232:317–322. doi: 10.1006/bbrc.1997.6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ABBRACCHIO MP, CERUTI S, BRAMBILLA R, et al. Adenosine A3 receptors and viability of astrocytes. Drug Dev. Res. 1998;45:379–386. doi: 10.1002/(sici)1098-2299(199811/12)45:3/4<379::aid-ddr38>3.0.co;2-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ABBRACCHIO MP, RAINALDI G, GIAMMARIOLI AM, et al. The A3 adenosine receptor mediates cell spreading, reorganization of actin cytoskeleton, and distribution of Bcl-xL. Studies in human astroglioma cells. Biochem. Biophys. Res. Commun. 1997;241:297–304. doi: 10.1006/bbrc.1997.7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.BRAMBILLA R, CATTABENI F, CERUTI S, et al. Activation of the A3 Adenosine Receptor Affects Cell Cycle Progression and Cell Growth. N-S Arch. Pharmacol. 2000;361:224–234. doi: 10.1007/s002109900186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.SEI Y, VON LUBITZ DKJE, ABBRACCHIO MP, et al. Adenosine A3 receptor agonist-induced neurotoxicity in rat cerebellar granule neurons. Drug Dev. Res. 1997;40:267–273. [Google Scholar]
- 16.KOHNO Y, SEI Y, KOSHIBA M, et al. Induction of apoptosis in HL-60 human promyelocytic leukemia cells by selective adenosine A3 receptor agonists. Biochem. Biophys. Res. Commun. 1996;219:904–910. doi: 10.1006/bbrc.1996.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.BARBIERI D, ABBRACCHIO MP, SALVIOLI S, et al. Apoptosis by 2-chloro-2′-deoxy-adenosine and 2-chloro-adenosine in human peripheral blood mononuclear cells. Neurochem. Int. 1997;32:493–504. doi: 10.1016/s0197-0186(97)00129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.JACOBSON KA, HOFFMANN C, CATTABENI F, ABBRACCHIO MP. Adenosine-induced cell death: evidence for receptor-mediated signalling. Apoptosis. 1999;4:197–211. doi: 10.1023/a:1009666707307. [DOI] [PubMed] [Google Scholar]
- 19.VON LUBITZ DKJE, YE W, MCCLELLAN J, LIN RCS. Stimulation of adenosine A3 receptors in cerebral ischemia. Neuronal death, recovery, or both? Ann. N.Y. Acad. Sci. 1999;890:93–106. doi: 10.1111/j.1749-6632.1999.tb07984.x. [DOI] [PubMed] [Google Scholar]
- 20.ABBRACCHIO MP, CATTABENI F. Brain adenosine receptors as targets for therapeutic intervention in neurodegenerative diseases. Ann. N.Y. Acad. Sci. 1999;890:79–92. doi: 10.1111/j.1749-6632.1999.tb07983.x. [DOI] [PubMed] [Google Scholar]
- 21.HALL A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 22.BISHOP AL, HALL A. Rho GTPases and their effector proteins. Biochem. J. 2000;348:241–255. [PMC free article] [PubMed] [Google Scholar]
- 23.SEASHOLTZ TM, MAJUMDAR M, HELLER BROWN J. Rho as a mediator of G-protein-coupled receptor signaling. Mol. Pharmacol. 1999;55:949–956. doi: 10.1124/mol.55.6.949. [DOI] [PubMed] [Google Scholar]
- 24.ABBRACCHIO MP, BRAMBILLA R, CERUTI S, et al. G-protein-dependent activation of phospholipase C by adenosine A3 receptors in rat brain. Mol. Pharmacol. 1995;48:1038–1045. [PubMed] [Google Scholar]
- 25.MALORNI W, RAINALDI G, RIVABENE R, SANTINI MT. Different susceptibilities to cell death induced by t-butylhydroperoxide could depend upon cell histotype-associated growth features. Cell Biol. Toxicol. 1994;10:207–218. doi: 10.1007/BF00756761. [DOI] [PubMed] [Google Scholar]
- 26.AULLO P, GIRY M, POPOFF MR, et al. A chimeric toxin to study the role of the 21 kDa GTP binding protein rho in the control of actin microfilament assembly. EMBO J. 1993;12:921–931. doi: 10.1002/j.1460-2075.1993.tb05733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.FIORENTINI C, FABBRI A, FLATAU G, et al. Escherichia coli cytotoxic necrotizing factor 1 (CNF1), a toxin that activates the rho GTPase. J. Biol. Chem. 1997;272:19532–19537. doi: 10.1074/jbc.272.31.19532. [DOI] [PubMed] [Google Scholar]
- 28.HIRAO M, SATO N, KONDO T, et al. Regulation mechanism of ERM (Erzin/Radixin/Moesin) protein/plasma membrane association: possible involvement of phosphatidylinositol turnover and Rho-dependent signaling pathway. J. Cell Biol. 1996;135:37–51. doi: 10.1083/jcb.135.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.FIORENTINI C, FABBRI A, FALZANO L, et al. Clostridium difficile toxin B induces apoptosis in intestinal cultured cells. Infect. Immun. 1998;66:2660–2665. doi: 10.1128/iai.66.6.2660-2665.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LIANG BT, JACOBSON KA. A physiological role of the adenosine A3 receptor: sustained cardioprotection. Proc. Natl. Acad. Sci. U.S.A. 1998;95:6995–6999. doi: 10.1073/pnas.95.12.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.KITAGAWA K, MATSUMOTO M, TAGAYA M, et al. “Ischemic tolerance” phenomenon found in the brain. Brain Res. 1990;528:21–24. doi: 10.1016/0006-8993(90)90189-i. [DOI] [PubMed] [Google Scholar]