Abstract
Neurochemical differences in the hypothalamic-pituitary axis between individuals and between ages may contribute to differential susceptibility to cocaine abuse. This study measured peptide levels in the pituitary gland (Pit) and lateral hypothalamus (LH) in adolescent (age 30 days) and adult (age 65 days) mice from four standard inbred strains, FVB/NJ, DBA/2J, C57BL/6J, and BALB/cByJ, which have previously been characterized for acute locomotor responses to cocaine. Individual peptide profiles were analyzed using mass spectrometric profiling and principal component analysis (PCA). Sequences of assigned peptides were verified by tandem mass spectrometry. PCA classified all strains according to their distinct peptide profiles in Pit samples from adolescent mice, but not adults. Select proopiomelanocortin (POMC)-derived peptides were significantly higher in adolescent BALB/cByJ and DBA/2J mice than in FVB/NJ or C57BL/6J mice. A subset of peptides in the LH, but not in the Pit, was altered by cocaine in adolescents. A 15 mg/kg dose of cocaine induced greater peptide alterations than a 30 mg/kg dose, particularly in FVB/NJ animals, with larger differences in adolescents than adults. Neuropeptides in the LH affected by acute cocaine administration included POMC-, myelin basic protein-, and glutamate transporter-derived peptides. The observed peptide differences could contribute to differential behavioral sensitivity to cocaine among strains and ages.
Keywords: adolescent, cocaine, label-free quantitation, MALDI MS, peptidomics, principal component analysis
Introduction
Recent studies of commonly abused drugs have focused on elucidating neurobiological differences between adolescent and adult responses to drug exposure. This is because drug use typically starts during adolescence (Chen & Kandel 1995, Degenhardt et al. 2008, Nelson et al. 1995) when the brain is still developing (Giedd et al. 1999), and adolescents respond differently to drugs as compared to adults in ways that appear to increase their risk for dependence (Schramm-Sapyta et al. 2009, Zakharova et al. 2009, Collins & Izenwasser 2002, Laviola et al. 1999). Cocaine use often begins in adolescence and can lead to long-lasting impacts on quality of life (Lawrence et al. 2008, Weiss et al. 2001, Koob & Volkow 2010). Cocaine also is one of the best-characterized drugs of abuse in terms of its neurological impact in animal models. Mouse models in particular have provided useful information due to the wide array of stable genotypes available for the study of the neurobiological features hypothesized to contribute to behavioral changes induced by drug exposure.
We recently documented significant differences in acute locomotor responses to cocaine in adolescent and adult mice across four common inbred mouse strains (Zombeck et al. 2010b). Acute locomotor responses are relevant as they provide a straightforward way to measure behavioral sensitivity to cocaine, and because these responses are correlated with other drug abuse-related behavioral measures such as conditioned place preference and self-administration (Allen et al. 2007, Romanova et al. 2010, Mandt et al. 2008). The underlying neurological differences between the strains and ages that explain the differences in acute locomotor responses to cocaine are not known. This study probes endogenous peptides in the pituitary gland (Pit) and lateral hypothalamus (LH) because peptides have been broadly implicated in mediating cocaine responses (Romanova et al. 2010, Rao et al. 2013, Borgland et al. 2006, Haass-Koffler & Bartlett 2012, Abul-Husn & Devi 2006, Geng et al. 2006, Kreek 1996). Using the same mice that were used in our earlier report of strain and age differences in acute locomotor responses to cocaine (Zombeck et al. 2010b), we explore whether peptide levels in the Pit and LH vary between genetically defined strains and animal age, as well as in response to acute cocaine exposure. These neuroendocrine regions were chosen for analysis as they each have a well-documented role in cocaine-responsive pathway modulation (Zhou et al. 2012, Aston-Jones et al. 2010, Zhou et al. 2003), are accessible, and are morphologically distinguished from neighboring brain regions or tissues.
Label-free matrix-assisted laser desorption ionization (MALDI)-time of flight (TOF) mass spectrometry (MS) was the method selected for executing this study. Due to its low sample volume requirements and excellent detection limits, MALDI MS is able to provide relative levels of peptides from rodent brain tissue samples that have not been pooled across multiple animals and/or brain regions (Romanova et al. 2013, Miura et al. 2010, Uys et al. 2010, Li & Sweedler 2008, Romanova et al. 2014). Comparative MALDI MS-based screening of peptides, lipids, and metabolites is actively being explored as a potential method for a nearly universal high throughput, cost-effective, sensitive, and discriminative diagnostic tool for various cancers (Li et al. 2013, Qiu et al. 2009, Shevchenko et al. 2010, Shin et al. 2007) and other pathological conditions (Hachani et al. 2011, Ollero et al. 2011, Perez et al. 2014, Rico Santana et al. 2014), and even cognitive dysfunction (Zhang et al. 2012).
Given the high complexity of the peptide complement in brain tissue extracts, multivariate statistical methods that measure relationships between many variables are useful for comparisons of spectral features. By comparing ions in the MALDI MS spectra from individual mice via principal component analysis (PCA), the dimensionality of the MS data can be reduced to reveal unique, trait-dependent trends in the detected peak patterns. PCA allows the extraction of functionally relevant information from the MALDI MS spectra, especially when multiple peaks have similar trends. When used with one-step extraction (Romanova et al. 2008), MALDI MS-based analyses are rapid and require minimal sample handling steps, thereby reducing bias due to sample handling and treatment. Compared with other “–omics” approaches, MALDI MS measures the final players in the biochemical mediation of cocaine responses, and offers potential insights into previously observed phenotypic differences between strains and ages (Yin et al. 2011). We hypothesized that cocaine exposure would lead to differences in the peptide profiles of the Pit and LH as measured by MALDI MS, and that those differences would vary in mice of different strains and ages. We also predicted that different strains and ages would have variable cocaine dose-sensitivity in their peptide responses.
Methods
Chemicals
All chemicals were purchased from Sigma Aldrich, St. Louis, MO, USA.
Animals and husbandry
A total of 96 male mice were used from 4 different standard inbred stains: C57BL/6J (C57BL), FVB/NJ (FVB), BALB/cByJ (BALB), and DBA/2J (DBA) (Jackson Laboratory, Bar Harbor, ME, USA). All 4 strains are listed as Tier 1 priority in the Mouse Phenome Database (Grubb et al. 2014) and were chosen because they are highly genetically divergent, each representing different branches of the phylogenetic tree for inbred mouse strains, as previously described (Rhodes et al. 2007). All mice were housed on a 12:12 reverse light/dark cycle (lights off at 7 AM and on at 7 PM) with the room temperature maintained at 21±1 °C. Mice had ad libitum access to food and water at all times.
The mice arrived in two different age groups: postnatal day 21 and 56. Mice were initially housed in groups of 3–4 for 5 days before being individually housed for an additional 4 days. Hence, adolescent mice were tested at postnatal day 30 and adults at day 65. These are commonly accepted ages to study adolescent and adult rodents (Spear 2000, Spear & Brake 1983).
On day 4, starting at 8 AM (1 hour after lights off), all mice received a single intraperitoneal (ip) injection of 0.9% saline, and then were immediately returned to their home cage to acclimate to the injection. Exactly 1 h after the saline injection, starting at 9 AM (2 h after lights off), mice were administered another injection of either saline or cocaine (15 or 30 mg/kg), and then immediately returned to their cages. Animals were euthanized exactly 1 h later (details below). Doses were chosen based on previous studies that have shown reliable behavioral differences between age groups in C57BL males (Zombeck et al. 2009, Zombeck et al. 2010b, Zombeck et al. 2010a). The behavioral data from these same animals has already been published (Zombeck et al. 2010b). All procedures were approved by the University of Illinois Institutional Animal Care and Use Committee and adhered to both the NIH and ARRIVE guidelines. The Beckman Institute Animal Facility where the mice were housed is accredited by AAALAC International.
Tissue sampling and peptide extraction
Animals were anesthetized with 150 mg/kg sodium pentobarbital (ip), then perfused transcardially with ice-cold physiological saline solution to remove the blood. Brains were immediately removed and snap frozen in cold isopentane. Fresh pituitaries were collected by scooping them out of the skull following brain removal, and transferring directly into the extraction solution. Frozen brains were sectioned in a cryostat with 1 mm steps, and tissue was collected using a 1 mm biopsy punch from the region corresponding to the LH. Tissue samples from each animal were immersed in a ≥10× volume of aqueous DHB solution (DHB: 2, 5-dihydroxybenzoic acid, 20 mg/ml) promptly after dissection and incubated at +4 °C for 48 h to facilitate peptide extraction, as described elsewhere (Romanova et al. 2008).
MALDI-TOF MS analysis
Sampling for peptide profiling was performed as previously described (Romanova et al. 2008, Romanova et al. 2012, Romanova et al. 2010). Briefly, 0.7 μl of the DHB-treated tissue extracts were co-spotted 1:1 (v/v) with fresh concentrated DHB matrix (50 mg/ml 50% acetone), applied in triplicate onto a target sample plate, and analyzed using a Bruker ultrafleXtreme mass spectrometer with Smartbeam technology (Bruker Daltonics, Billerica, MA, USA) operated at 500 Hz in positive reflectron mode. Sample measurement was randomized, and spectra in the m/z 700–5500 range were collected automatically using the AutoXecute™ software (Bruker Daltonics) with real-time fuzzy control logic, optimized for peptides. During acquisition, quadratic calibration constants were adjusted every 15–25 spots on a square area. Laser intensity was optimized separately for LH and Pit samples and maintained at a constant level during analysis. The number of laser shots was set to 1600 for Pit, 4000 for LH, and accumulated in 200- or 100-shot steps, respectively; laser movement was set to random walk over the entire sample area. Peak evaluation was based on a minimum resolution of 5000 and medium signal intensity for LH, and 7000 resolution/high signal intensity for Pit, with the intensity being defined as high or medium by the Bruker software as the ratio of base peak height to the number of fired laser shots; 20 failed judgments within the sample area were required before moving to the next sample. At the end of the automatic run, failed spots were re-tested individually using the same instrument parameters.
Statistical analysis of peptide profiles
Comparisons of the peptide profiles were performed on the MS data in their original format using PCA and individual peak statistics functions (Student t-test or one-way ANOVA for normally distributed peak signal intensities determined by the Anderson-Darling normality test) in ClinProTools 2.2 (Bruker Daltonics). The Benjamini & Hochberg p-value adjustment procedure was automatically applied to correct for the multiple testing hypothesis problem commonly associated with the MS data. A total of 24 parameters (24 = 4 strains × 2 ages × 3 treatments), were paired in a number of different ways for numerous PCA iterations. Only distinct results are reported here. Upon loading files into ClinProTools and prior to PCA calculations, spectra were normalized to total ion count with a built-in algorithm. The automatic data pretreatment was completed using the following settings in ClinProTools: convex hull baseline correction with 0.5 flatness, mass filter m/z 800–4500, Savitsky Golay smoothing over m/z 1.0 with four cycles, a data reduction factor of 2, null spectra exclusion, and spectra grouping from technical replicates into an average sample spectrum. Peak picking for PCA calculation (unlimited for LH and restricted to the 100 most intense peaks for Pit) was done on the group average spectrum with a signal-to-noise threshold of 6 and relative intensity of 1%. The number of peaks was restricted in the Pit samples due to overall higher signal intensity and the greater number of peaks detected from the Pit samples compared to the LH samples. Additionally, unsupervised hierarchical clustering was performed on PCA-transformed data (95% variance explained) using Euclidean distance and average linkage methods.
Peptide identification by liquid chromatography (LC)-MS
The workflow for LC-MS was the same as previously described (Romanova et al. 2012). After all tissue samples were processed individually for peptide extraction, and a small portion of each sample was used for high throughput semi-quantitative screening of peptides by MALDI-TOF MS, the larger leftover portions were combined according to strain and processed for peptide structural characterization using LC-tandem MS (MS/MS). The one-step extraction protocol (Romanova et al. 2008) used here is not directly compatible with LC due to excess DHB. Therefore, we initially removed the bulk of the DHB by solid phase extraction, as described previously (Romanova et al. 2010), and then used multistage high performance LC separation guided by off-line MALDI-TOF MS peptide scanning to choose peptide-containing fractions for further analysis.
We used two different LC-MS systems for our identification efforts. The first was a Dionex U3000 RSLC system with a capillary flow selector (Thermo Scientific, Sunnyvale, CA, USA) and an Acclaim PepMap RSLC column (Thermo Scientific, 300 μm × 150 mm, 2μm, 100Å) interfaced to a Bruker HCT Ultra PTM Discovery mass spectrometer via an electrospray ionization source (Bruker Daltonics). MS and MS/MS measurements were performed in the data dependent acquisition mode with dynamic exclusion (top 4 ions, 2 spectra each, released after 1 min), a full scan mass range of m/z 300–2000, and a collision induced dissociation scan at 35% energy. The second LC-MS/MS system was a Dionex U3000 split flow nanoLC (Thermo Scientific) with a sample preconcentration setup that included an Acclaim PepMap100 Nano-Trap column (200 μm × 2 cm, 5 μm, 300 Å) and an Acclaim PepMap RSLC column (75 μm × 150 mm, 2μm, 100Å) interfaced to a maXis 4G ultra high resolution-QTOF mass spectrometer (Bruker Daltonics) via a Bruker on-line nanoESI source.
Fragmentation spectra obtained on both LC-MS platforms were charge-deconvoluted and exported as Mascot Generic Files (mgf) for identification via the International Protein Index (IPI: mouse 3.21) database search using Peaks Studio 5.3 (Bioinformatics Solutions Inc., Waterloo, ON, Canada). Search parameters included a precursor mass tolerance of 0.5 Da for ion trap data and 0.1 Da for QTOF data; a fragment ion mass tolerance of 0.5 Da and 0.1 Da, respectively; a maximum of three PTMs per peptide; and variable PTMs included amidation, oxidation, acetylation, pyroGlu formation from E or Q, and disulfide bonds. The database was downloaded from ftp://ftp.ebi.ac.uk/pub/databases/IPI/current/ (Kersey et al. 2004). Peptides identified in the peptidomics experiments were back-referenced to the peptide peaks detected in the MALDI-TOF MS measurements based on their monoisotopic mass and accuracy of detection.
Results
Mouse strain differences under baseline (saline) conditions
Initially, we investigated peptide profiles from the extracts of the individual LH and Pit samples from control animals in each age and strain group to determine whether inbred strains have common detectable peptide complements at different life stages. A variety of known and putative signaling peptides were detected. In Pit samples, masses matching peptides encoded by proopiomelanocortin (POMC) and arginine vasopressin (AVP) precursors were detected in all samples (Fig. 1). Detection of multiple known peptides resulting from proteolytic processing of the POMC prohormone adds confidence to our assignments, similar to the peptide fingerprinting of the trypsin-digested protein used for protein identification (Cottrell 1994). Peptide assignments were confirmed by a follow-up peptidomics approach on pooled samples, separately for the Pit and LH (Tables S1–S4).
Fig. 1.
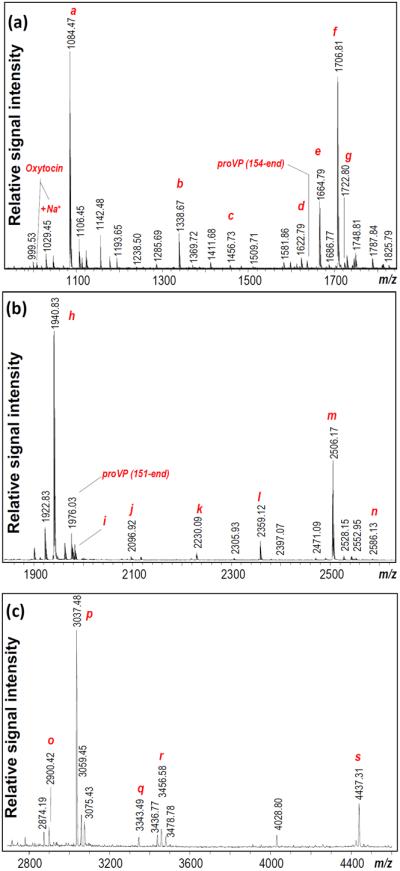
Pituitary peptide profiles obtained using direct MALDI-TOF MS. Panels (a–c) show peptides detected in different mass regions. Known peptides are labeled based on mass match to the LC-MS sequencing data: a–AVP; b–γ-MSH; c–di-Ac-α-MSH (3–13); d–α-MSH; e–Ac-α-MSH; f–di-Ac-α-MSH; h–J-peptide; i–Ac-J-peptide; j–J-peptide + N-terminal Arg; k–CLIP (1–20); l–CLIP (1–21); m–CLIP, n–phosphorylated CLIP; o–Ac-β-endorphin (1–26); p–Ac-β-endorphin (1–27), q–ACTH (1–28); r–γ-LPH (10–38); s–γ-LPH. Abbreviations: proVP, provasopressin-neurophysin 2 copeptin; AVP, arginine vasopressin; ACTH, adrenocorticotropic hormone; MSH, melanocyte-stimulating hormone; Ac, acetylated; CLIP, corticotropin-like intermediate peptide; and LPH, lipotropin. Labeled m/z correspond to monoisotopic masses of protonated molecular ions.
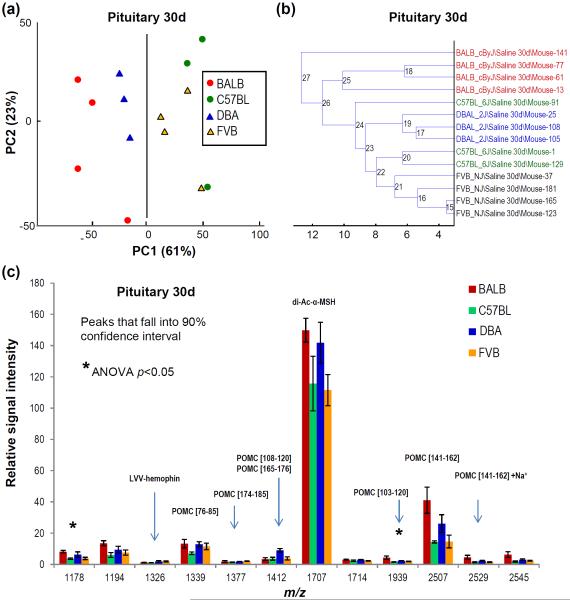
The Pit profiles of adolescent mice were distinguishable by strain (Fig. 2a), with >60% of the variation accounted for by PC1 and >20% by PC2. As shown in Fig. 2c, among 54 peaks detected by MALDI-TOF MS, a subset of peaks matched to peptides sequenced in pooled samples fitted statistically in the 90% confidence interval, with two peptides having p ≤ 0.05, thus demonstrating differential levels among mouse strains. The majority of differentially detected peptides were POMC-derived peptides. One interesting finding was the differently cleaved corticotropin-like intermediate peptide (CLIP) residues [141-162] flanked by basic residues on both termini. Traditionally, CLIP is thought to be cleaved at residues [142-162], and we did detect and confirm the sequence of this form in the Pit. However, only the alternate [141-162] form contributed to strain classification. Spectra classification by unsupervised hierarchical clustering was 100% correct for the BALB, DBA, and FVB strains (Fig. 2b). The data indicated that even small a population of adolescent mice (n = 16) can be classified by overall peptide profiles in the Pit using the multivariate statistical approach. Pit extracts from adult mice had more variability and hence could not be classified correctly using PCA or univariate sorting approaches. Recognition of adolescent and adult mice according to strain by peptide profiles in the LH was not achieved.
Fig. 2.
Genotypes of adolescent mice can be distinguished according to their overall peptide profiles in pituitaries: (a) PCA score plot for the first two principal components (PCs) with the variance described by each PC; (b) unsupervised hierarchical clustering of MALDI-TOF MS spectra; (c) relative intensities of select peaks in Pit profiles that fall into the 90% confidence interval; known peptides are labeled. Mouse strains are shape and color coded; each data point represents one animal; DBA #153 and C57BL #49 mice were excluded due to sampling; m/z corresponds to maximum height at half width of the deisotoped centroid peak from the group mean spectrum.
Further, as shown in Fig. 3, using PCA pairwise comparisons of strains across ages revealed that DBA mice can be distinguished from FVB mice, regardless of age (Fig. 3a) due to statistically higher levels of select POMC peptides in the Pit (Fig. 3c). The peptide peak that matches masses of both the amidated POMC [108–120] and acetylated POMC [165–176] (Table S2) is higher in adolescence (2.3 fold) and adulthood (2.8 fold) than in FVB mice, and POMC peptide [165–182] is higher at 1.5 and 1.8 fold, respectively. Likewise, FVB mice contrast from BALB mice, regardless of age (Fig. 3b) due to significantly lower levels of the unknown peptides m/z 1178, 1194, and 2545, and higher levels of m/z 2924, 2940, and 2986. The LH peptide profiles were not significantly distinct in either age group or when compared across age (data not shown).
Fig. 3.
The DBA and BALB strains can be distinguished from the FVB strain by their overall peptide profiles in Pit, independent of age. (a,b) PCA plots for the first three PCs are shown, along with the % of variance described by each PC; DBA mouse #153 was excluded due to sampling. (c,d) Intensities of peptides detected in Pit at significantly different levels between strains; p-value is for the Student's t-test; m/z corresponds to maximum height at half width of the deisotoped centroid peak from the group mean spectrum as calculated by ClinProTools.
Effect of acute cocaine on peptide dynamics in LH
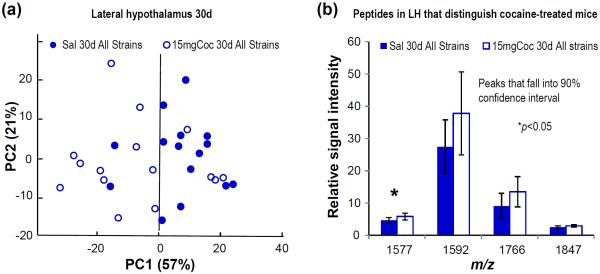
Exposing mice to acute cocaine treatment resulted in measurable alterations of peptide levels in LH extracts, but not Pit. Speculating that the neurochemical response to cocaine is mediated by a fundamental mechanism that may be strong enough to override baseline phenotypic differences, we combined the LH spectra from all saline-treated adolescent mice and compared it against the combined spectra from cocaine-treated mice, in a dose-dependent manner, to see if these overall spectra show specific peptides that differentiate between control and treated adolescents. By PCA, from 226 detected peaks, a number of peptides contributed to the distinction (Fig. 4a) between the saline- and cocaine-treated groups, with the peptides contributing to these differences being peptides from myelin basic protein (MBP), claudin-11, excitatory amino acid transporter, and pyruvate dehydrogenase (Fig. 4b).
Fig. 4.
(a) PCA plot showing that the effect of acute cocaine exposure on peptide profiles in the LH is similar in adolescent mice from all four strains. Data points are color coded according to treatment. (b) Intensities of peptides that allow distinction of peptide profiles between control and cocaine-treated (15mg/kg) adolescent mice; m/z 1577, excitatory amino acid transporter 1; m/z 1592, MBP (m/z corresponds to maximum height at half width of the deisotoped centroid peak from the group mean spectrum as calculated by ClinProTools).
Effects of age and acute cocaine administration on peptide profiles in FVB mice
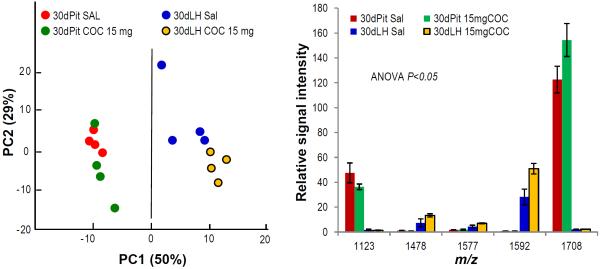
Of the four tested strains, male FVB mice showed exceptionally low locomotor responses to acute cocaine treatment, and no age differences or dose dependence in our behavioral study (Zombeck et al. 2010b). However, PCA analysis of peptide detection levels in the Pit and LH indicates dramatic dose-dependent changes among adolescent FVB mice that clearly differentiate cocaine-treated mice from those that received control injections (Fig. 5). The LH spectra, in particular, are well separated between the control and 15mg/kg cocaine treatment groups by PC1 and PC2 (Fig. 5a), with the greatest difference being due to an increase in the level of peptides from MBP (1.8 fold), excitatory amino acid transporter (1.6 fold), and unknown peptide m/z 1478 (1.8 fold). It is interesting that the low dose cocaine effect was not found among adult FVB mice, where classification of low and high cocaine dose samples was 50% for either group, and 75% for the control saline samples (data not shown). Contrary to behavioral observations, these findings suggest that age differences in brain neuropeptide dynamics coincide with acute cocaine treatment in FVB mice. Cocaine-induced changes in the Pit were less prominent than in the LH, but levels of di-acetylated α–melanocyte-stimulating hormone from POMC increased 1.3 fold and unidentified peptide m/z 1123 decreased significantly (Fig. 5b).
Fig. 5.
Cocaine induces peptide changes in the Pit and LH of adolescent FVB mice. (a) PCA plot for the first two PCs showing segregation of spectra by sample type and treatment according to peptide profile features. (b) Relative intensities of peptides detected at significantly different levels as determined by ANOVA; known peptides are: m/z 1577, excitatory amino acid transporter 1; m/z 1592, MBP; m/z 1708, di-Ac-α- melanocyte-stimulating hormone (m/z corresponds to maximum height at half width of the deisotoped centroid peak from the group mean spectrum as calculated by ClinProTools); remaining m/z are not identified.
Diversity of peptides detected in the Pit and LH
The mouse Pit and LH have been well investigated using peptidomics approaches, and lists of peptides from known prohormones have been published (Nilsson et al. 2012, Sasaki et al. 2010, Perroud et al. 2009, Romanova et al. 2008, Che et al. 2006). We performed our own sequencing to aid in the interpretation of the MALDI and PCA data because many of the detected masses were either not previously reported or could not be reliably assigned to a protein by the peptide mass fingerprinting approach alone. We also wanted to provide consistent results across the strains used here, as several of them have been more extensively studied in the past than others. The peptides we identified in the mouse Pit and LH are presented in the Supporting Information (Tables S1–S4).
Discussion
Phenotypic assessment of inbred mouse strains has recently become of interest in light of variable drug-induced behaviors among numerous strains used in addiction studies (Wiltshire et al. 2015, Zombeck et al. 2010b, Thomsen & Caine 2011, Eisener-Dorman et al. 2011). With the exception of a few biochemical investigations, (Brodkin et al. 1998, McCarthy et al. 2004, Adkins et al. 2013), the focus had been placed on genetic and behavioral differences between strains. We sought to determine biochemical differences in the hypothalamic-pituitary axis relevant to drug responsiveness in the strains we studied. We show that just as in rats (Romanova et al. 2010), acute cocaine induces detectable changes in the peptide profiles of mice, and that some changes are common to all strains, whereas others are unique to particular strains. Neuropeptides and hormones play important roles in modulating neural circuits that control behavior. Therefore, an understanding of how genetic differences between strains affect peptide concentrations in tissues could provide an important missing link between the genetics and the complex behavioral phenotypes. The genotypes of standard inbred strains are reproducible, hence, genetic (e.g., DNA sequence, polymorphisms), and phenotypic data are cumulative. In addition to many gross morphological, physiological, and behavioral traits, data are also available for gene expression profiles in particular tissues in response to various treatments (Grubb et al. 2014). This information can be used to identify causal connections between structural DNA variants (i.e., alleles) and phenotypic traits (Grupe et al. 2001). Notably missing from the data, however, is peptide information from various tissues in response to treatments that connect the gene expression differences with behavioral patterns. This work offers a robust way to perform peptidomic analysis for comparisons between strains.
Differences in peptide profiles are more prominent in adolescent mice than adults
A surprising finding was that the peptide profiles of adolescents were easier to classify based on strain and cocaine dose than adults. Prominent peptide differences among genotypes found in adolescent mice allowed strain classification according to overall baseline peptide profiles in the Pit, as well as according to peptide profile alterations in the LH upon cocaine treatment. Moreover, in one of the strains, FVB, multiple peptides from POMC in the LH of adolescent mice provided the basis for dose-dependent differentiation between control and cocaine-treated mice. It proved more challenging, however, to discriminate between the genotypes in adult mice, where only the DBA and FVB strains, compared against each other, could be distinguished by overall peptide profiles in the Pit and LH. One possible explanation is that adolescent mice of different strains develop at different rates and thus, peptide profiles in their brains and hypothalamic-pituitary axis are distinct at a certain age during development, but converge to the same endpoint as adults. An alternative possibility is that as the mice age, they develop more idiosyncratic differences in peptide biochemistry related to the accrual of subtle individual-specific experiences. With time, these subtle differences may accumulate, causing individuals within a genotype to diverge, and effectively increasing the individual within-strain variation for the trait.
Mouse strain differences in Pit peptide profiles
Previous work using radioimmunoassay analysis showed that the contents of β-endorphin and adrenocorticotropic hormone (ACTH) in the Pit vary among inbred strains of mice. For example, DBA and BALB mice β-endorphin values are 2.5-fold lower, and ACTH values are 1.5-fold lower, than in C57BL mice (Crabbe et al. 1981). Based on this information, one might expect other peptides encoded by POMC prohormone to be differentially expressed among strains. Our data do show significant differences in levels for specific POMC-related peptides, although differences among adolescent mice are more prominent than adult mice under baseline conditions. One possible explanation is that the circadian dynamics of the hypothalamic-pituitary axis show individual, age, and strain differences, but are hard to account for during tissue sampling when working with multiple genetically diverse strains. Another possible explanation is that our small sample size represented in each group/condition (n = 4) was not sufficient to overcome individual variation in peptide signal intensity among mice. The data demonstrate that a straightforward relationship between a specific peptide level and a genotype may not be obvious, and that a multivariate analytical approach such as PCA is better suited for discriminating complex traits because it is simultaneously controlling for the effects of each of the predictors (peptides) on the entire profile. In the future, it would be interesting to see if linear discriminant analysis would be helpful in overcoming the variability within each compared group and hence, document the peaks that allow for classification of mouse strains by neuroendocrine peptide profiles. Although differences in peptide profiles were observed in the Pit, strain-specific differences from the LH were not observed. This may be because the LH has lower levels of peptides and so it is easier to observe subtle changes in peptide levels in the Pit due to genetic diversity between strains.
Consistent peptide responses to acute cocaine in the LH of adolescents across 4 genotypes
Acute cocaine dosage appears to disrupt multiple signaling pathways in the LH of adolescent mice in a similar fashion, irrespective of strain. In our study, animals were euthanized exactly 1 hour following an injection of saline or 15 or 30 mg/kg cocaine. The peptide response to cocaine is almost certainly a dynamic process, with certain peptides increasing in concentration and then falling in a tissue-specific manner at different time courses following exposure to the drug. Therefore, the peptide response to cocaine characterized in our study must be considered a snapshot that captured only changes that occurred within the first hour following acute cocaine exposure.
The 1 h period was chosen because this matches, at least approximately, the duration of the psychoactive effects of cocaine as measured, for example, by changes in locomotor activity. One hour is enough time for some genes to become expressed and translated, but perhaps not others. Peptides that manifest changes are derived from glutamate transporter 1, MBP, claudin-11, and pyruvate dehydrogenase. Previously, we identified via MALDI that MBP isoform 9 changed in cocaine-treated rats as compared to controls (Romanova et al. 2010, Romanova et al. 2012, Uys et al. 2010). Alteration in myelin regulation was identified in postmortem samples of the human nucleus accumbens in cocaine users when compared to controls using Affymetrix microarray technology to measure gene expression on a global scale (Albertson et al. 2004). Endorphin, enkephalin, and corticotropin (derivatives of POMC) appear to play important roles in an animal's response to cocaine exposure, as well as in reward and aversion behaviors associated with cocaine. Previous studies have identified changes in POMC derivatives in response to acute cocaine administration (Marquez et al. 2008, Roth-Deri et al. 2004, Roth-Deri et al. 2003, Olive et al. 2001, Forman & Estilow 1988, DuMars et al. 1988, Crespo et al. 2003, Zhou et al. 2003, Zhou et al. 2002, Zhou et al. 1996, Sarnyai et al. 1993, Sarnyai et al. 1992).
Changes in the abundance of peptides in our study may reflect altered gene expression, translation from already expressed genes, degradation of peptides within cells, or release of peptides into extracellular spaces followed by rapid degradation. Although this work only represents a snapshot of the peptides in several defined brain locations, given the high throughput capability of the peptidomic analysis using MALDI MS, it is feasible to characterize a time-course for peptide responses in specific brain regions of interest using a series of animals in the future.
Correlations with locomotor behavioral sensitivity to cocaine
The animals in this study were also measured for acute locomotor responses to cocaine during the 1 hour period after injection, and before euthanasia for tissue extractions. In our prior report of the behavior of these animals (Zombeck et al. 2010b), the main results were greater locomotor responses to cocaine in C57BL, followed by DBA, BALB, then FVB, and much greater locomotor responses to the 30 mg/kg dose than the 15 mg/kg dose across strains. BALB and C57BL adolescents were less sensitive than adults to the acute locomotor activating effects of cocaine. In DBA and FVB, adolescents and adults displayed similar sensitivity to cocaine. When combined with the results from the present study, these data suggest that locomotor responses to acute cocaine are inversely related to changes in peptide profiles in the LH. This conclusion comes from the following three pieces of evidence. First, adolescents displayed a stronger peptide signal from cocaine than adults, but a weaker locomotor response. Second, the low dose of cocaine elicited a greater peptide response than the high dose, but a weaker locomotor response. Third, the FVB displayed the least sensitivity to the acute locomotor activating effects of cocaine of all 4 strains, yet displayed the most robust peptide response in the LH to cocaine. One interpretation of the inverse relationship is that changes in peptide profiles in the LH in response to cocaine represent plasticity or tolerance-related mechanisms that enable the animal to compensate for the chemical perturbation and maintain homeostasis, despite the dramatic change in neurochemistry caused by the acute cocaine treatment. This interpretation posits that a larger peptide change represents greater plasticity, resulting in increased tolerance and a less overt behavioral response.
Conclusions
Proteins and peptides are widely considered critical molecules that affect most aspects of brain function and behavior (Kandel et al. 2012, Strand 1999). Genomics approaches are often used as proxies for measuring proteins and peptides, and it is assumed that protein levels and gene expression levels are correlated when often, in practice, they are not (Vogel & Marcotte 2012). Peptidomics is more complex than genomics because one gene transcript can produce many peptides or proteins, and each product can be heavily altered by posttranslational modifications. Our study demonstrates the feasibility of using MALDI MS to reliably measure peptidome dynamics in a specific brain region and an endocrine gland of genetically defined, standard inbred mouse strains at different ages and in response to cocaine. With larger studies, both in terms of number of animals and strains, it may be possible to combine peptide data with gene expression data and other phenotypic information that has been accumulated for inbred strains. Together, this information may be able to explain more of the variation in acute locomotor responses to cocaine (or any other phenotype of interest that has been measured in the same panel of inbred strains). We anticipate that future studies will be able to leverage the methods described herein to further advance our understanding of the neurochemical changes associated with individual behavioral variation.
Supplementary Material
Acknowledgements
The project described was supported by Award Nos. RO1 MH083807 and RO1 DA027487 to J.S.R., and Award No. P30 DA018310 to JVS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Abbreviations used
- ACTH
adrenocorticotropic hormone
- AVP
arginine vasopressin
- CLIP
corticotropin-like intermediate peptide
- DHB
2, 5-dihydroxybenzoic acid
- ip
intraperitoneal
- LC
liquid chromatography
- LH
lateral hypothalamus
- MALDI
matrix-assisted laser desorption ionization
- MS
mass spectrometry
- MS/MS
tandem mass spectrometry
- MBP
myelin basic protein
- PCA
principal component analysis
- Pit
pituitary gland
- POMC
proopiomelanocortin
- TOF
time-of-flight
Footnotes
Supporting information Additional supporting information may be found in the online version of this article at the publisher's web site.
conflict of interest disclosure The authors have no conflicts of interest to declare.
References
- Abul-Husn NS, Devi LA. Neuroproteomics of the synapse and drug addiction. J. Pharmacol. Exp. Ther. 2006;318:461–468. doi: 10.1124/jpet.105.091520. [DOI] [PubMed] [Google Scholar]
- Adkins DE, McClay JL, Vunck SA, et al. Behavioral metabolomics analysis identifies novel neurochemical signatures in methamphetamine sensitization. Genes Brain Behav. 2013;12:780–791. doi: 10.1111/gbb.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertson DN, Pruetz B, Schmidt CJ, Kuhn DM, Kapatos G, Bannon MJ. Gene expression profile of the nucleus accumbens of human cocaine abusers: evidence for dysregulation of myelin. J. Neurochem. 2004;88:1211–1219. doi: 10.1046/j.1471-4159.2003.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RM, Everett CV, Nelson AM, Gulley JM, Zahniser NR. Low and high locomotor responsiveness to cocaine predicts intravenous cocaine conditioned place preference in male Sprague-Dawley rats. Pharmacol. Biochem. Behav. 2007;86:37–44. doi: 10.1016/j.pbb.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, Richardson KA. Lateral hypothalamic orexin/hypocretin neurons: A role in reward-seeking and addiction. Brain Res. 2010;1314:74–90. doi: 10.1016/j.brainres.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Brodkin ES, Carlezon WA, Jr., Haile CN, Kosten TA, Heninger GR, Nestler EJ. Genetic analysis of behavioral, neuroendocrine, and biochemical parameters in inbred rodents: initial studies in Lewis and Fischer 344 rats and in A/J and C57BL/6J mice. Brain Res. 1998;805:55–68. doi: 10.1016/s0006-8993(98)00663-5. [DOI] [PubMed] [Google Scholar]
- Che FY, Vathy I, Fricker LD. Quantitative peptidomics in mice: effect of cocaine treatment. J. Mol. Neurosci. 2006;28:265–275. doi: 10.1385/JMN:28:3:265. [DOI] [PubMed] [Google Scholar]
- Chen K, Kandel DB. The natural history of drug use from adolescence to the mid-thirties in a general population sample. Am. J. Public Health. 1995;85:41–47. doi: 10.2105/ajph.85.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SL, Izenwasser S. Cocaine differentially alters behavior and neurochemistry in periadolescent versus adult rats. Brain Res. Dev. Brain Res. 2002;138:27–34. doi: 10.1016/s0165-3806(02)00471-6. [DOI] [PubMed] [Google Scholar]
- Cottrell JS. Protein identification by peptide mass fingerprinting. Pept. Res. 1994;7:115–124. [PubMed] [Google Scholar]
- Crabbe JC, Jr., Allen RG, Gaudette ND, Young ER, Kosobud A, Stack J. Strain differences in pituitary beta-endorphin and ACTH content in inbred mice. Brain Res. 1981;219:219–223. doi: 10.1016/0006-8993(81)90286-9. [DOI] [PubMed] [Google Scholar]
- Crespo JA, Manzanares J, Oliva JM, Corchero J, Garcia-Lecumberri C, Ambrosio E. Extinction of cocaine self-administration produces alterations in corticotropin releasing factor gene expression in the paraventricular nucleus of the hypothalamus. Brain Res. Mol. Brain Res. 2003;117:160–167. doi: 10.1016/s0169-328x(03)00316-4. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Chiu WT, Sampson N, et al. Toward a global view of alcohol, tobacco, cannabis, and cocaine use: findings from the WHO World Mental Health Surveys. PLoS Med. 2008;5:e141. doi: 10.1371/journal.pmed.0050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuMars LA, Rodger LD, Kalivas PW. Behavioral cross-sensitization between cocaine and enkephalin in the A10 dopamine region. Behav. Brain Res. 1988;27:87–91. doi: 10.1016/0166-4328(88)90111-8. [DOI] [PubMed] [Google Scholar]
- Eisener-Dorman AF, Grabowski-Boase L, Tarantino LM. Cocaine locomotor activation, sensitization and place preference in six inbred strains of mice. Behav. Brain Funct. 2011;7:29. doi: 10.1186/1744-9081-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman LJ, Estilow S. Cocaine influences beta-endorphin levels and release. Life Sci. 1988;43:309–315. doi: 10.1016/0024-3205(88)90108-7. [DOI] [PubMed] [Google Scholar]
- Geng T, Seitz PK, Thomas ML, Xu B, Soman KV, Kurosky A, Luxon BA, Cunningham KA. Use of surface enhanced laser desorption/ionization-time of flight mass spectrometry (SELDI-TOF MS) to study protein expression in a rat model of cocaine withdrawal. J. Neurosci. Methods. 2006;158:1–12. doi: 10.1016/j.jneumeth.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Grubb SC, Bult CJ, Bogue MA. Mouse phenome database. Nucleic Acids Res. 2014;42:D825–834. doi: 10.1093/nar/gkt1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe A, Germer S, Usuka J, Aud D, Belknap JK, Klein RF, Ahluwalia MK, Higuchi R, Peltz G. In silico mapping of complex disease-related traits in mice. Science. 2001;292:1915–1918. doi: 10.1126/science.1058889. [DOI] [PubMed] [Google Scholar]
- Haass-Koffler CL, Bartlett SE. Stress and addiction: contribution of the corticotropin releasing factor (CRF) system in neuroplasticity. Front. Mol. Neurosci. 2012;5:91. doi: 10.3389/fnmol.2012.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachani J, Duban-Deweer S, Pottiez G, Renom G, Flahaut C, Perini JM. MALDI-TOF MS profiling as the first-tier screen for sickle cell disease in neonates: matching throughput to objectives. Proteomics Clin. Appl. 2011;5:405–414. doi: 10.1002/prca.201000093. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM, Siegelbaum SA, Hudspeth AJ. Principles of Neural Science. Fifth Edition McGraw-Hill Professional; New York: 2012. p. 1760. [Google Scholar]
- Kersey PJ, Duarte J, Williams A, Karavidopoulou Y, Birney E, Apweiler R. The International Protein Index: an integrated database for proteomics experiments. Proteomics. 2004;4:1985–1988. doi: 10.1002/pmic.200300721. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ. Cocaine, dopamine and the endogenous opioid system. J. Addict. Dis. 1996;15:73–96. doi: 10.1300/J069v15n04_05. [DOI] [PubMed] [Google Scholar]
- Laviola G, Adriani W, Terranova ML, Gerra G. Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animal models. Neurosci. Biobehav. Rev. 1999;23:993–1010. doi: 10.1016/s0149-7634(99)00032-9. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Beart PM, Kalivas PW. Neuropharmacology of addiction--setting the scene. Br. J. Pharmacol. 2008;154:259–260. doi: 10.1038/bjp.2008.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Sweedler JV. Peptides in the brain: mass spectrometry-based measurement approaches and challenges. Annu. Rev. Anal. Chem. (Palo Alto Calif.) 2008;1:451–483. doi: 10.1146/annurev.anchem.1.031207.113053. [DOI] [PubMed] [Google Scholar]
- Li P, Yang J, Ma QY, Wu Z, Huang C, Li XQ, Wang Z. Biomarkers screening between preoperative and postoperative patients in pancreatic cancer. Asian Pac. J. Cancer Prev. 2013;14:4161–4165. doi: 10.7314/apjcp.2013.14.7.4161. [DOI] [PubMed] [Google Scholar]
- Mandt BH, Schenk S, Zahniser NR, Allen RM. Individual differences in cocaine-induced locomotor activity in male Sprague-Dawley rats and their acquisition of and motivation to self-administer cocaine. Psychopharmacology (Berl.) 2008;201:195–202. doi: 10.1007/s00213-008-1265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez P, Baliram R, Dabaja I, Gajawada N, Lutfy K. The role of beta-endorphin in the acute motor stimulatory and rewarding actions of cocaine in mice. Psychopharmacology (Berl.) 2008;197:443–448. doi: 10.1007/s00213-007-1053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy LE, Mannelli P, Niculescu M, Gingrich K, Unterwald EM, Ehrlich ME. The distribution of cocaine in mice differs by age and strain. Neurotoxicol. Teratol. 2004;26:839–848. doi: 10.1016/j.ntt.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Miura D, Fujimura Y, Tachibana H, Wariishi H. Highly sensitive matrix-assisted laser desorption ionization-mass spectrometry for high-throughput metabolic profiling. Anal. Chem. 2010;82:498–504. doi: 10.1021/ac901083a. [DOI] [PubMed] [Google Scholar]
- Nelson DE, Giovino GA, Shopland DR, Mowery PD, Mills SL, Eriksen MP. Trends in cigarette smoking among US adolescents, 1974 through 1991. Am. J. Public Health. 1995;85:34–40. doi: 10.2105/ajph.85.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson A, Stroth N, Zhang X, Qi H, Falth M, Skold K, Hoyer D, Andren PE, Svenningsson P. Neuropeptidomics of mouse hypothalamus after imipramine treatment reveal somatostatin as a potential mediator of antidepressant effects. Neuropharmacology. 2012;62:347–357. doi: 10.1016/j.neuropharm.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. Stimulation of endorphin neurotransmission in the nucleus accumbens by ethanol, cocaine, and amphetamine. J Neurosci. 2001;21:RC184. doi: 10.1523/JNEUROSCI.21-23-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollero M, Guerrera IC, Astarita G, Piomelli D, Edelman A. New lipidomic approaches in cystic fibrosis. Methods Mol. Biol. 2011;742:265–278. doi: 10.1007/978-1-61779-120-8_16. [DOI] [PubMed] [Google Scholar]
- Perez V, Ibernon M, Lopez D, Pastor MC, Navarro M, Navarro-Munoz M, Bonet J, Romero R. Urinary peptide profiling to differentiate between minimal change disease and focal segmental glomerulosclerosis. PLoS One. 2014;9:e87731. doi: 10.1371/journal.pone.0087731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perroud B, Alvarado RJ, Espinal GM, Morado AR, Phinney BS, Warden CH. In vivo multiplex quantitative analysis of 3 forms of alpha melanocyte stimulating hormone in pituitary of prolyl endopeptidase deficient mice. Mol. Brain. 2009;2:14. doi: 10.1186/1756-6606-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu F, Liu HY, Dong ZN, Feng YJ, Zhang XJ, Tian YP. Searching for potential ovarian cancer biomarkers with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Am. J. Biomed. Sci. 2009;1:80–90. doi: 10.5099/aj090100080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao Y, Mineur YS, Gan G, et al. Repeated in vivo exposure of cocaine induces long-lasting synaptic plasticity in hypocretin/orexin-producing neurons in the lateral hypothalamus in mice. J. Physiol. 2013;591:1951–1966. doi: 10.1113/jphysiol.2012.246983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T, Jr., Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Rico Santana N, Zapico Muniz E, Cocho D, Bravo Y, Delgado Mederos R, Marti-Fabregas J. Analysis of peptidome profiling of serum from patients with early onset symptoms of ischemic stroke. J. Stroke Cerebrovasc. Dis. 2014;23:235–240. doi: 10.1016/j.jstrokecerebrovasdis.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Romanova EV, Aerts JT, Croushore CA, Sweedler JV. Small-volume analysis of cell-cell signaling molecules in the brain. Neuropsychopharmacology. 2014;39:50–64. doi: 10.1038/npp.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanova EV, Dowd SE, Sweedler JV. Quantitation of endogenous peptides using mass spectrometry based methods. Curr. Opin. Chem. Biol. 2013;17:801–808. doi: 10.1016/j.cbpa.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanova EV, Lee JE, Kelleher NL, Sweedler JV, Gulley JM. Mass spectrometry screening reveals peptides modulated differentially in the medial prefrontal cortex of rats with disparate initial sensitivity to cocaine. AAPS J. 2010;12:443–454. doi: 10.1208/s12248-010-9204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanova EV, Lee JE, Kelleher NL, Sweedler JV, Gulley JM. Comparative peptidomics analysis of neural adaptations in rats repeatedly exposed to amphetamine. J. Neurochem. 2012;123:276–287. doi: 10.1111/j.1471-4159.2012.07912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanova EV, Rubakhin SS, Sweedler JV. One-step sampling, extraction, and storage protocol for peptidomics using dihydroxybenzoic acid. Anal. Chem. 2008;80:3379–3386. doi: 10.1021/ac7026047. [DOI] [PubMed] [Google Scholar]
- Roth-Deri I, Schindler CJ, Yadid G. A critical role for beta-endorphin in cocaine-seeking behavior. Neuroreport. 2004;15:519–521. doi: 10.1097/00001756-200403010-00027. [DOI] [PubMed] [Google Scholar]
- Roth-Deri I, Zangen A, Aleli M, et al. Effect of experimenter-delivered and self-administered cocaine on extracellular beta-endorphin levels in the nucleus accumbens. J. Neurochem. 2003;84:930–938. doi: 10.1046/j.1471-4159.2003.01584.x. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Biro E, Gardi J, Vecsernyes M, Julesz J, Telegdy G. Alterations of corticotropin-releasing factor-like immunoreactivity in different brain regions after acute cocaine administration in rats. Brain Res. 1993;616:315–319. doi: 10.1016/0006-8993(93)90224-b. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Biro E, Penke B, Telegdy G. The cocaine-induced elevation of plasma corticosterone is mediated by endogenous corticotropin-releasing factor (CRF) in rats. Brain Res. 1992;589:154–156. doi: 10.1016/0006-8993(92)91176-f. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Takahashi N, Satoh M, Yamasaki M, Minamino N. A peptidomics strategy for discovering endogenous bioactive peptides. J. Proteome Res. 2010;9:5047–5052. doi: 10.1021/pr1003455. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Walker QD, Caster JM, Levin ED, Kuhn CM. Are adolescents more vulnerable to drug addiction than adults? Evidence from animal models. Psychopharmacology (Berl.) 2009;206:1–21. doi: 10.1007/s00213-009-1585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchenko VE, Arnotskaya NE, Zaridze DG. Detection of lung cancer using plasma protein profiling by matrix-assisted laser desorption/ionization mass spectrometry. Eur. J. Mass Spectrom. 2010;16:539–549. doi: 10.1255/ejms.1080. [DOI] [PubMed] [Google Scholar]
- Shin S, Cazares L, Schneider H, Mitchell S, Laronga C, Semmes OJ, Perry RR, Drake RR. Serum biomarkers to differentiate benign and malignant mammographic lesions. J. Am. Coll. Surg. 2007;204:1065–1071. doi: 10.1016/j.jamcollsurg.2007.01.036. discussion 1071–1063. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev. Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Strand FL. Neuropeptides: Regulators of Physiological Processes: Cellular and molecular neuroscience. MIT Press; Cambridge, MA: 1999. [Google Scholar]
- Thomsen M, Caine SB. Psychomotor stimulant effects of cocaine in rats and 15 mouse strains. Exp. Clin. Psychopharmacol. 2011;19:321–341. doi: 10.1037/a0024798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uys JD, Grey AC, Wiggins A, Schwacke JH, Schey KL, Kalivas PW. Matrix-assisted laser desorption/ionization tissue profiling of secretoneurin in the nucleus accumbens shell from cocaine-sensitized rats. J. Mass Spectrom. 2010;45:97–103. doi: 10.1002/jms.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012;13:227–232. doi: 10.1038/nrg3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Ciccocioppo R, Parsons LH, et al. Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors. Ann. N. Y. Acad. Sci. 2001;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- Wiltshire T, Ervin R, Duan H, Bogue M, Zamboni W, Cook S, Chung W, Zou F, Tarantino L. Initial locomotor sensitivity to cocaine varies widely among inbred mouse strains. Genes Brain Behav. 2015;14:271–280. doi: 10.1111/gbb.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin P, Hou X, Romanova EV, Sweedler JV. Neuropeptidomics: mass spectrometry-based qualitative and quantitative analysis. Methods Mol. Biol. 2011;789:223–236. doi: 10.1007/978-1-61779-310-3_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharova E, Leoni G, Kichko I, Izenwasser S. Differential effects of methamphetamine and cocaine on conditioned place preference and locomotor activity in adult and adolescent male rats. Behav. Brain Res. 2009;198:45–50. doi: 10.1016/j.bbr.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Li SZ, Feng CS, et al. Serum proteomics of early postoperative cognitive dysfunction in elderly patients. Chin. Med. J. (Engl.) 2012;125:2455–2461. [PubMed] [Google Scholar]
- Zhou Y, Kruyer A, Ho A, Kreek MJ. Cocaine place conditioning increases proopiomelanocortin gene expression in rat hypothalamus. Neurosci. Lett. 2012;530:59–63. doi: 10.1016/j.neulet.2012.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Spangler R, LaForge KS, Maggos CE, Ho A, Kreek MJ. Corticotropin-releasing factor and type 1 corticotropin-releasing factor receptor messenger RNAs in rat brain and pituitary during “binge”-pattern cocaine administration and chronic withdrawal. J. Pharmacol. Exp. Ther. 1996;279:351–358. [PubMed] [Google Scholar]
- Zhou Y, Spangler R, Schlussman SD, Ho A, Kreek MJ. Alterations in hypothalamic-pituitary-adrenal axis activity and in levels of proopiomelanocortin and corticotropin-releasing hormone-receptor 1 mRNAs in the pituitary and hypothalamus of the rat during chronic `binge' cocaine and withdrawal. Brain Res. 2003;964:187–199. doi: 10.1016/s0006-8993(02)03929-x. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Spangler R, Schlussman SD, Yuferov VP, Sora I, Ho A, Uhl GR, Kreek MJ. Effects of acute “binge” cocaine on preprodynorphin, preproenkephalin, proopiomelanocortin, and corticotropin-releasing hormone receptor mRNA levels in the striatum and hypothalamic-pituitary-adrenal axis of mu-opioid receptor knockout mice. Synapse. 2002;45:220–229. doi: 10.1002/syn.10101. [DOI] [PubMed] [Google Scholar]
- Zombeck JA, Gupta T, Rhodes JS. Evaluation of a pharmacokinetic hypothesis for reduced locomotor stimulation from methamphetamine and cocaine in adolescent versus adult male C57BL/6J mice. Psychopharmacology (Berl.) 2009;201:589–599. doi: 10.1007/s00213-008-1327-0. [DOI] [PubMed] [Google Scholar]
- Zombeck JA, Lewicki AD, Patel K, Gupta T, Rhodes JS. Patterns of neural activity associated with differential acute locomotor stimulation to cocaine and methamphetamine in adolescent versus adult male C57BL/6J mice. Neuroscience. 2010a;165:1087–1099. doi: 10.1016/j.neuroscience.2009.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zombeck JA, Swearingen SP, Rhodes JS. Acute locomotor responses to cocaine in adolescents vs. adults from four divergent inbred mouse strains. Genes Brain Behav. 2010b;9:892–898. doi: 10.1111/j.1601-183X.2010.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.