Dear Editor,
In arthritis studies, it is pivotal to use evaluation techniques where the different components of the joint can be easily identified. It is, however, often difficult to distinguish between cartilage degradation and bone erosion in routine histological staining techniques, such as the hematoxylin and eosin (H&E). This confers a risk of misinterpretation of histological data, as the mechanisms that lead to cartilage damage and bone loss are fundamentally different (McInnes et al. 2007). There are alternative staining methods that provide good differentiation between bone and cartilage; for example, Safranin O staining for cartilage (Camplejohn et al. 1988) and van Gieson’s method for collagen and other connective tissues (Luna 1992). However, to achieve an optimal histological evaluation of the inflamed joint, the different staining methods have to be employed on consecutive slides. We have therefore established a novel staining method, the Bone-Inflammation-Cartilage (BIC) stain, in which Safranin O staining is combined with van Gieson’s staining to visualize and correctly assess bone erosions, the loss of articular cartilage, and inflammation in a single stain.
To establish a new staining protocol, we used joints from mice with septic arthritis: a joint infection characterized by pronounced bone destruction, loss of proteoglycan expression in the articular cartilage, and extensive synovial inflammation (Bremell et al. 1992). All animal studies were approved by the local Animal Ethics Committee (ref no 278-2011). Joints were fixed in 4% paraformaldehyde, decalcified using Parengy (HistoLab), and embedded in paraffin. Tissue sections from fore- and hind paws were cut and deparaffinized prior to staining. The results were analyzed using a Nikon eclipse E1000M microscope (Nikon; Tokyo, Japan) and recorded with a ProgRes C7 camera (Jenoptik; Jena, Germany). The degree of cartilage destruction was assessed using Safranin O (Sigma-Aldrich AB, Stockholm, Sweden), for which the staining intensity is directly proportional to the proteoglycan content in the cartilage (Camplejohn et al. 1988). The degree of bone destruction was detected by Weigert’s hematoxylin van Gieson’s procedure for the differential staining of collagen and other connective tissue (Luna, 1992). Routine H&E staining was performed using a standard staining protocol.
The BIC staining protocol was as follows: Paraffin sections were cut and deparaffinized to 96% ethanol. Sections were then stained with Weigert’s Iron hematoxylin solution for 10 min, soaked under running water for approximately 30 min for destaining, and then stained with Van Gieson’s stain for 15 min. The sections were gently rinsed in distilled water (to remove excess stain), and then counterstained in 0.01% Fast Green for 3 min. Sections were then briefly incubated in 1% acetic acid solution for 10–15 sec, and then stained with 0.1% Safranin O for 5 min. Sections were dehydrated with two changes of 95% ethanol and two changes of 100% ethanol (approximately 2 minutes per change). Sections were then cleared in xylene (three changes) and coverslipped.
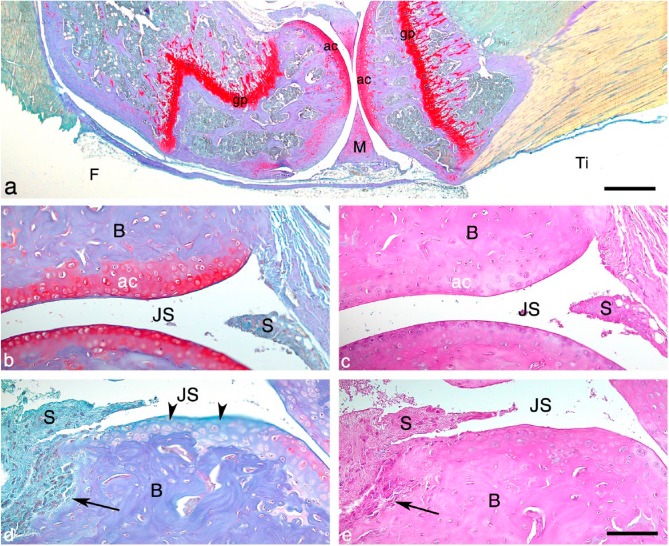
The new BIC staining method results in a specific staining of cartilage, bone and inflammation (Fig. 1a). The proteoglycan content in the articular cartilage and the growth plate stains brightly red, which is comparable with that seen when sections are stained with the Safranin O stain alone. The bone is stained purple or blue, and the inflammatory infiltrate and cells in the bone marrow are stained blue-green.
Figure 1.
The Bone-Inflammation-Cartilage (BIC) stain. (a) Histological section of a knee joint from a mouse with a case of mild septic arthritis, stained with the BIC stain. The proteoglycan content in the articular cartilage and growth plate stains red and the bone stains purple/blue. Scale, 500 μm. BIC- (b and d) and H&E (c and e)-stained sections of ankle joints from mice with septic arthritis. (b, c) Histology of a joint from a mouse with a mild case of arthritis. (d, e) Histology of a joint from a mouse with a severe case of arthritis, where synovitis, bone erosions and loss of articular cartilage are present. Note where the synovial inflammatory tissue invades the subchondral bone (arrows in d and e). The arrowheads in (d) indicate the loss of proteoglycan content in the articular cartilage (not visualized in H&E-stained section, d). Abbreviations: ac, articular cartilage; gp, growth plate; F, femur; M, meniscus; Ti, tibia; B, bone; JS, joint space; S, synovial membrane. Scacle, 100 μm.
We compared the differences between BIC and classical H&E staining on cartilage and bone pathology by staining serial joint sections from mice with septic arthritis. As expected, cartilage is better visualized in sections stained with BIC (Fig. 1b, stained brightly red) as compared with H&E (Fig. 1c). Articular cartilage damage is more obvious using the BIC stain (Fig. 1d, arrowhead), with the red staining intensity diminishing as the proteoglycans disappear. Proteoglycan loss in the articular cartilage is not detected using the H&E stain (Fig. 2e). Additionally, bone erosions are more prominent and readily detected when evaluating sections stained with BIC compared with those stained with H&E (Fig. 1d and 1e, arrows).
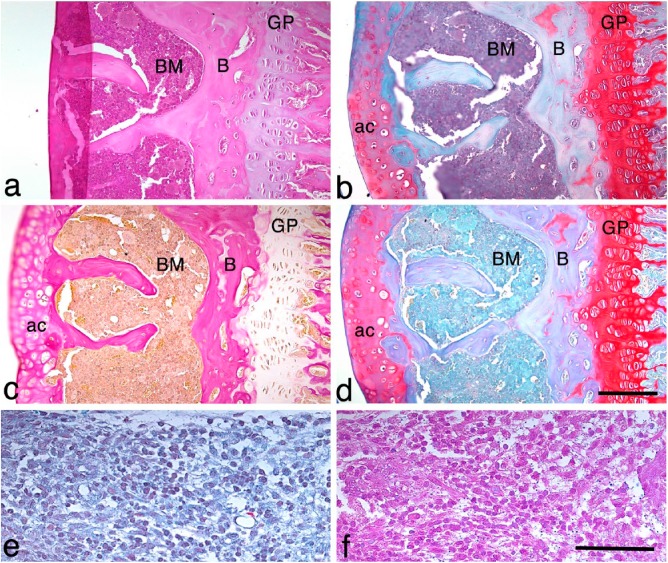
Figure 2.
Comparisons of the single stains of H&E, van Gieson’s and Safranin O with the BIC stain. Serial sections of a knee joint from a mouse with a mild case of septic arthritis stained with (a) H&E stain, (b) BIC stain, (c) van Gieson’s stain and (d) Safranin O stain. Abbreviations: ac, articular cartilage; B, bone; BM, bone marrow; GP, growth plate. Scale, 120 μm. (e, f) An area of synovitis showing the inflammatory cell infiltrate using BIC (e) and H&E (f) staining. Scale, 100 μm.
We further compared the BIC stain with all three single staining methods of H&E, van Gieson’s and Safranin O using serial joint sections from the same mouse. This revealed that the characteristics of each single stain are present in the BIC stain (Fig. 2a–2d). The cartilage staining in the BIC-stained sections (Fig. 2b) is very similar to the cartilage staining using Safranin O (Fig. 2d). Bone and inflammatory infiltrates in the subchondral bone are as clearly visible in the BIC-stained sections as in those stained with van Gieson’s stain (Fig. 2b, 2c). The nuclear staining using BIC (Fig. 2e) is very similar to the nuclear staining using H&E (Fig. 2f). Thus, the BIC stain can be used in the same manner as the H&E stain to identify the different cell types present in inflammatory infiltrates in the synovial membrane.
In summary, our new histological staining method, BIC stain, clearly discriminates the destruction of bone, the loss of cartilage, and synovial inflammation in one single slide. In cases where an assessment of joint damage is desired, this novel stain provides a superior option to the principal histological H&E stain. The distinction between cartilage and bone is better visualized using the BIC stain as compared with that achieved with H&E staining. The loss of cartilage is easy to demonstrate with the BIC stain, as it is proportional to loss of proteoglycan content in the cartilage. Proteoglycan content is not detected with the H&E stain. In addition, bone erosions are easily detectible in BIC-stained sections. The nuclear staining that differentiates among various cell types in the H&E stain is equally good using the BIC stain.
Imaging techniques, such as magnetic resonance imaging and micro-computed tomography, are frequently used to study arthritis development. These techniques are valuable tools that can detect joint inflammation (synovial volume), bone and cartilage destruction as well as systemic effects to bone in vivo (Proulx et al., 2007). However, imaging techniques are unable to substitute for the use of histological methods, as histology identifies the cellular and molecular factors that contribute to the pathogenesis of the disease, and validate and compliment imaging findings.
The BIC stain is comparable to the use of the single stains of Safranin O, van Gieson’s and H&E but offers the possibility to visualize bone destruction, loss of articular cartilage and inflammation in a single slide. We consider the BIC stain as a new valuable tool that optimizes the use of research material and reduces the number of experimental animals. Thus, the BIC stain allows a better understanding of the pathological processes in the joint at the same time as it saves time and resources.
Footnotes
Declaration of Competing Interests: The authors declare no potential competing interests with respect to the research, authorship, and/or publication of this article
Author Contributions: BB prepared and evaluated all slides and contributed to writing the manuscript. JM evaluated and validated the BIC-protocol, prepared the photos, and contributed to writing the manuscript. IG designed the study, evaluated all slides and wrote the paper, together with BB and JM.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: IG receives support from Amlöv”s foundation and Gustav V 80 years foundation.
References
- Bremell T, Abdelnour A, Tarkowski A. (1992). Histopathological and serological progression of experimental Staphylococcus aureus arthritis. Infect Immun 60:2976-2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camplejohn K L, Allard S A. (1988). Limitations of safranin “O” staining in proteoglycan-depleted cartilage demonstrated with monoclonal antibodies. Histochemistry 89:185-188. [DOI] [PubMed] [Google Scholar]
- Luna L G. (1992). Histopathologic methods and color atlas of special stains and tissue artifacts. Maryland: American Histolabs, Inc. [Google Scholar]
- McInnes I B, Schett G. (2007). Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol 7:429-442. [DOI] [PubMed] [Google Scholar]
- Proulx S T, Kwok E, You Z, Papuga M O, Beck C A, Shealy D J, Schwarz E M. (2007). Longitudinal assessment of synovial, lymph node, and bone volumes in inflammatory arthritis in mice by in vivo magnetic resonance imaging and microfocal computed tomography. Arthritis Rheum 56:4024-4037. [DOI] [PMC free article] [PubMed] [Google Scholar]