Abstract
Introduction and Background:
Diagnostic tests for tuberculosis (TB) using sputum have suboptimal sensitivity among HIV-positive persons. We assessed health care worker adherence to TB diagnostic algorithms after negative sputum test results.
Methods:
The XTEND (Xpert for TB—Evaluating a New Diagnostic) trial compared outcomes among people tested for TB in primary care clinics using Xpert MTB/RIF vs. smear microscopy as the initial test. We analyzed data from XTEND participants who were HIV positive or HIV status unknown, whose initial sputum Xpert MTB/RIF or microscopy result was negative. If chest radiography, sputum culture, or hospital referral took place, the algorithm for TB diagnosis was considered followed. Analysis of intervention (Xpert MTB/RIF) effect on algorithm adherence used methods for cluster-randomized trials with small number of clusters.
Results:
Among 4037 XTEND participants with initial negative test results, 2155 (53%) reported being or testing HIV positive and 540 (14%) had unknown HIV status. Among 2155 HIV-positive participants [684 (32%) male, mean age 37 years (range, 18–79 years)], there was evidence of algorithm adherence among 515 (24%). Adherence was less likely among persons tested initially with Xpert MTB/RIF vs. smear [14% (142/1031) vs. 32% (364/1122), adjusted risk ratio 0.34 (95% CI: 0.17 to 0.65)] and for participants with unknown vs. positive HIV status [59/540 (11%) vs. 507/2155 (24%)].
Conclusions:
We observed poorer adherence to TB diagnostic algorithms among HIV-positive persons tested initially with Xpert MTB/RIF vs. microscopy. Poor adherence to TB diagnostic algorithms and incomplete coverage of HIV testing represents a missed opportunity to diagnose TB and HIV, and may contribute to TB mortality.
Key Words: Tuberculosis, TB/HIV integration, TB diagnosis, Xpert MTB/RIF, mortality, XTEND
INTRODUCTION
The South African tuberculosis (TB) epidemic is one of the largest in the world, with an estimated incidence of 860 (776–980) cases per 100,000 general population1 in 2013. TB is the leading cause of death among HIV-positive South Africans.2–4 In this context, prompt diagnosis and early initiation of TB treatment may save lives. The diagnosis of TB has been hampered by the poor diagnostic sensitivity of smear microscopy, especially among HIV-positive people.5 On the basis of improved sensitivity compared with smear microscopy,6 the GeneXpert platform for the diagnosis of TB (Xpert MTB/RIF) was endorsed by the World Health Organization (WHO) as the initial test for TB diagnosis among HIV-positive people being investigated for TB,7 as a conditional recommendation, and subject to resource availability.
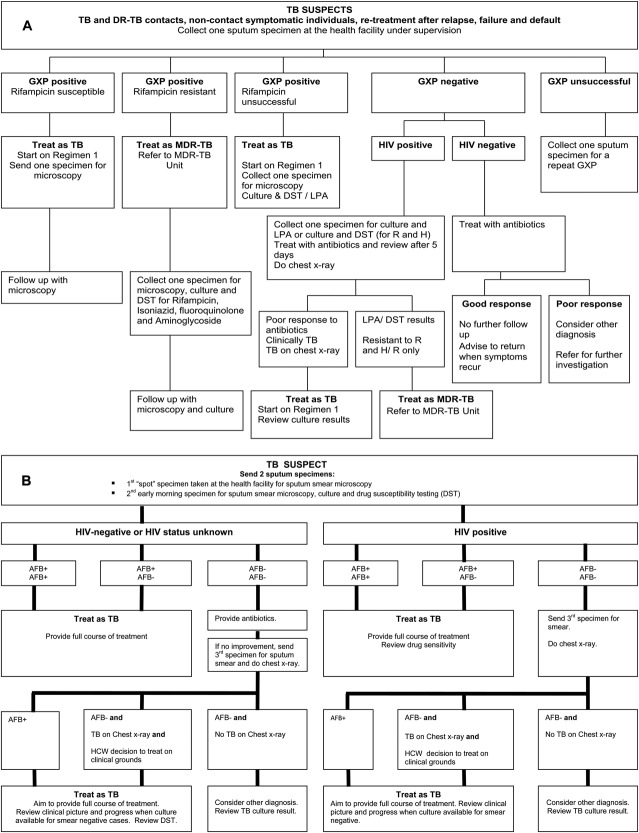
The South African National Health Laboratory Service (NHLS), which provides TB sputum testing for more than 4000 primary health clinics (PHC), rolled out Xpert MTB/RIF, replacing smear microscopy, as the initial test for TB between 2011 and 2013. The Xpert MTB/RIF roll-out was accompanied by a new diagnostic algorithm for TB diagnosis (Fig. 1A).8 As Xpert MTB/RIF is less sensitive than culture, it will miss cases of TB where the bacterial burden in sputum is lower than the threshold of detection of c.50–150 colony-forming units (cfu)/mL.9 The new algorithm therefore advises further investigations for HIV-positive people whose initial sputum Xpert MTB/RIF result is negative.10 The algorithm requires submission of a second sputum specimen for TB culture (followed by line probe assay or drug susceptibility testing), chest radiograph (CXR), and a trial of broad-spectrum antibiotics. These additional tests are appropriate among persons living with HIV who present with pulmonary symptoms, as the bacterial burden of TB in sputum is more likely to be low.11 Furthermore, persons living with HIV are more likely to have TB, and it is appropriate to investigate more intensively.12
FIGURE 1.
A, The algorithm for the diagnosis of tuberculosis used by health care workers in facilities where GeneXpert testing is available (used throughout the roll-out for Xpert MTB/RIF, from 2011 to 2014, but only published in the “South African National Guidelines for TB diagnosis and management” in 2014). B, The algorithm for the diagnosis of tuberculosis used by health care workers in facilities where GeneXpert testing is not available (South African National Guidelines for TB diagnosis and management, 2009).
In an evaluation of Xpert MTB/RIF vs. smear microscopy for the diagnosis of TB (the XTEND trial, described below),13 we demonstrated no reduction in mortality among persons tested initially with Xpert MTB/RIF.13 In this secondary analysis of XTEND trial data, we assessed adherence to the TB diagnostic algorithm among HIV-positive adults and adults with unknown HIV status who had negative sputum tests for TB (either smear microscopy or XPERT MTB/RIF). In addition, we compared adherence to the algorithm by study arm and identified patient factors that were associated with having had further investigations.
METHODS
XTEND Trial—Study Design and Procedures
The XTEND (Xpert for TB—Evaluating a New Diagnostic) study was a pragmatic, 2-arm, parallel, cluster-randomized trial evaluating the effectiveness of Xpert MTB/RIF implementation in South Africa (Current Controlled Trials: ISRCTN68905568; South African Clinical Trial Register: DOH-27-1011-3849).14 During Xpert MTB/RIF roll-out, 20 laboratories in 4 provinces were identified to receive Xpert MTB/RIF machines. Forty PHCs served by but not colocated with these participating laboratories were identified for participation in XTEND. Twenty clusters, each comprising a NHLS laboratory and 2 PHC facilities, were allocated using stratified randomization to Xpert MTB/RIF (immediate implementation) or microscopy (with Xpert MTB/RIF implementation deferred) arms.13 Participant enrollment in the Xpert arm was initiated a median of 3.6 (range, 1–7.4) months after the laboratory started routine use of Xpert MTB/RIF.13 XTEND study staff enrolled a systematic sample drawn from persons undergoing sputum testing at the request of clinic staff and obtained baseline data including personal identifiers, locator information, demographic and clinical data relevant to TB and mortality risk. Study staff (who were masked to the results of sputum investigations and who also had no influence on clinical investigations done by clinic staff) kept contact with participants until 6 months after enrollment, when study staff reviewed patient's clinic records for sputum results, evidence of additional investigations including sputum culture, chest radiograph, or broad-spectrum antibiotic prescription, TB treatment start dates, or documented referral to hospital. For logistical reasons, we did not review participants' hospital records for evidence of relevant investigations. Six months after enrollment, study staff interviewed participants telephonically, or by home visit, to ascertain whether they had started TB treatment, had an HIV test or CD4 count, started antiretroviral therapy, or had been admitted to hospital. After completion of follow-up, investigators also obtained XTEND participant sputum results from the NHLS laboratory information system. XTEND study methods are described more completely elsewhere.13
Study Setting—Baseline Characteristics of Participating Clinics and Implementation of Xpert MTB/RIF in XTEND Clinics
Among 40 PHCs identified for participation in XTEND, 17 facilities (43%) were situated in a predominantly rural area (mean population density within 2.5 km2 radius <150 persons/km2). During the 3 months before start of enrollment into XTEND, participating clinics recorded a median of 4787 [interquartile range (IQR), 2353–6345] patient visits and conducted a median of 116 (IQR, 59–195) TB tests per month. The median number of patients per clinic who received TB treatment each month was 33 (IQR, 20–66). Nine clinics (23%) had radiography facilities on site, while those facilities without referred patients to the nearest hospital for chest radiography, a mean of 7 km away. During the national roll-out of Xpert MTB/RIF, TB control program staff and TB nurses for PHC facilities in the districts that were earmarked for implementation received training on the use of Xpert MTB/RIF and the algorithm for TB diagnosis (Fig. 1A). Training was conducted by University Research Corporation of South Africa (for clinical staff) and the NHLS (for laboratory staff). TB control program staff arranged on-site training for nurses from the larger facilities, whereas workshops attended by clinic TB nurses were held at central locations, and nurses were asked to return to their facility and train the clinical staff who had not attended the session. The algorithm in Figure 1A was made available as a wall chart and distributed to implementing facilities. Laboratories not earmarked to receive Xpert MTB/RIF, including those randomized to delayed Xpert MTB/RIF, continued to use smear microscopy as the initial test for TB, and the clinics continued to use the algorithm advised by the 2009 South African TB guidelines (Fig. 1B).15 At all participating clinics, clinic staff identified persons who required investigation for TB according to local practice, and XTEND study staff did not change this. In both arms, when initial sputum test results were negative for TB, further investigations (sputum mycobacterial culture, chest radiography, and therapeutic trial of antibiotics) or referral to hospital was made at the discretion of the PHC health care worker. In clinics using Xpert MTB/RIF, staff had recently been trained in the use of the new algorithm for TB diagnosis, as described above, whereas in clinics using smear microscopy, no additional training was given as part of XTEND trial procedures.
Criteria for Inclusion in the Secondary Data Analysis
To evaluate adherence to the algorithm for the diagnosis of TB among persons with known HIV infection, a secondary analysis of XTEND data was conducted. Participants in the main XTEND study who were HIV positive or HIV status unknown and whose sputum tests at enrollment were Xpert MTB/RIF or smear microscopy negative were included in the data set.
Case Definitions
Participants were defined as “index test negative” if, at, or within 4 days of enrollment, both sputum specimens for smear microscopy or the one sputum specimen for Xpert MTB/RIF taken, were negative. Participants were identified as being HIV positive if there was evidence in the clinical record (any of a positive HIV test, prescription of antiretroviral therapy, or evidence of other HIV care), or participants reported being HIV positive or on antiretroviral agents at enrollment interview. The TB diagnostic algorithm was regarded as having been followed if there was evidence in the clinic records at the enrollment PHC or laboratory record during the 6-month follow-up period of any of (1) chest radiograph, (2) sputum for mycobacterial culture, and (3) referral to hospital for further investigations. Although we collected data on antibiotic prescription within 2 months of enrollment, the provision of antibiotics was not included in the definition of adherence to algorithm, although it is part of the algorithm, as record review could not distinguish “therapeutic trial” of antibiotics from prescription for another purpose.16 Referral to hospital was regarded as “adhering to the algorithm” as this is described in the NDoH10 and WHO16 algorithms when persons investigated for TB have respiratory distress or require investigations for extrapulmonary TB. Furthermore, referral is a reasonable alternative if further investigations are not possible at the PHC.
Statistical Analysis
Statistical analysis was conducted using STATA (version 12; StataCorp LP, College Station, TX). Analysis of the intervention (implementation of Xpert MTB/RIF) effect on algorithm adherence (defined above) was based on methods appropriate to the trial design with a small number of clusters17 and restricted to HIV-positive patients. Briefly, the analysis was conducted at the cluster level giving clusters equal weight and using log transformation of the cluster-level risk of the outcome. The risk ratio was calculated as the ratio of geometric means for Xpert MTB/RIF vs. microscopy arms, taking into account the stratified randomization. An approximate standard error of the log (risk ratio) was obtained based on the residual mean square from a 2-way analysis of variance of the cluster-level log risk on randomization stratum, study arm, and the interaction between stratum and study arm.17 This was used to calculate 95% CI for the risk ratio and associated P value. An adjusted risk ratio was calculated using a 2-stage approach, adjusting for baseline imbalance by study arm.17
Analysis of patient factors associated with adherence to the algorithm for TB diagnosis was performed combining data across study arm from HIV-positive patients and using logistic regression with random effects to account for between-clinic variation. Factors with evidence of an association with the outcome (P value <0.05) in the univariable analysis were included in the multivariable model and retained in the model if the P value <0.05. Adjusted odd ratios (aORs) and 95% CIs are reported. A priori, sex and age group were included in the fully adjusted model.
RESULTS
Participant Characteristics
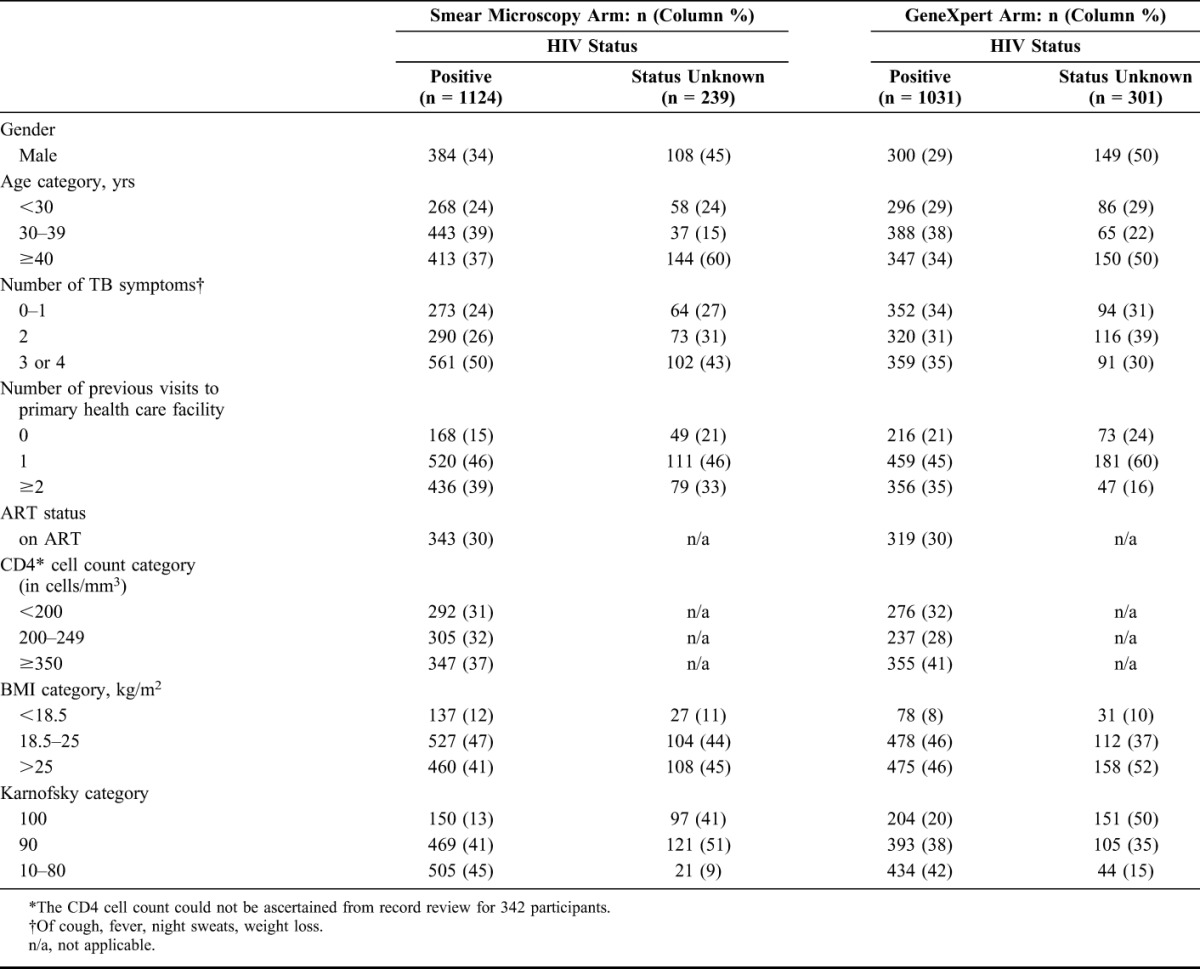
Of the 4656 XTEND participants, 4411 had an index test result available; of whom, 4037 (92%) had a negative test result. Among these 4037 participants, 2155 (53%) were HIV positive, and 540 (13%) had unknown HIV status, and were included in this analysis. Demographic and medical characteristics of these 2695 participants stratified by study arm and HIV status are shown in Table 1. Participants with HIV positive or unknown serostatus (n = 2695) had a mean age of 38 years (range, 18–94 years) and 941 (35%) participants were male. Forty-one percent (1113/2695) had at least 3 TB symptoms and 2189 (81%) had attended a health care facility at least once before enrollment to address their TB symptoms. Ten percent (273/2695) of participants had a body mass index (BMI) less than 18.5. Of 2155 who were HIV positive at enrollment, 662 (31%) were on antiretroviral therapy, and of the 944 with CD4 result available, 31% (292) had a CD4 count less than 200 cells per cubic millimeter. As in the main XTEND cohort,13 those in the smear microscopy arm tended to have more TB symptoms, a lower BMI, and lower Karnofsky score than those in the Xpert MTB/RIF arm.
TABLE 1.
Characteristics of XTEND Participants With Negative Result on the Index Sputum Test Who Were HIV Positive, or Who had Unknown HIV Status, by Study Arm and HIV Status (n = 2695)
Investigations for Tuberculosis
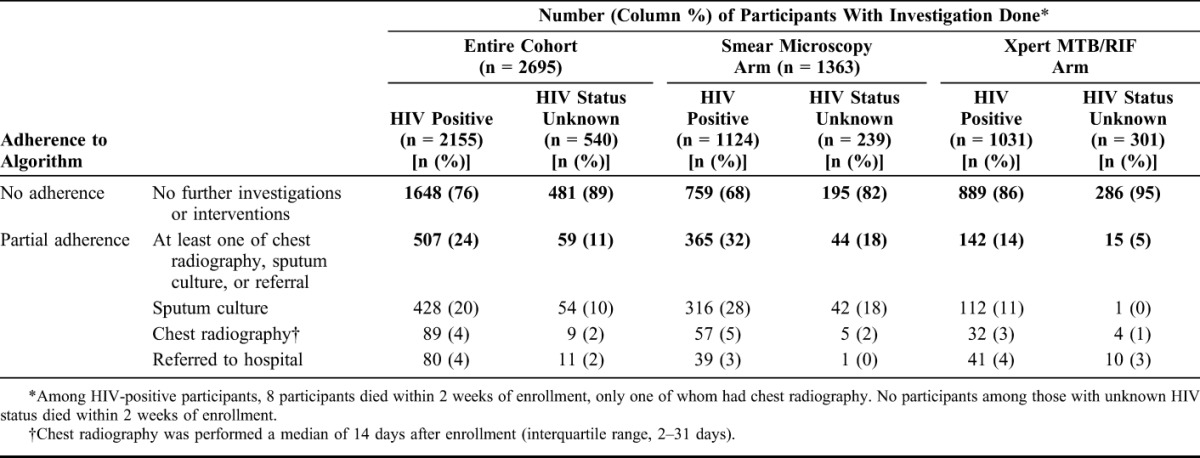
Among 2155 HIV-positive participants, during the 6 months after a negative index test result, evidence of further investigations or interventions (such as referral to hospital) was found in clinic records for 24% (507) of participants (Table 2). Evidence for sputum culture was found for 427 (20%) participants, and chest radiography was documented in 4% (89/2153) participants' files, performed a median of 14 days after enrollment (IQR, 2–31 days). Eight participants (7 and one in the Xpert MTB/RIF and microscopy arms, respectively) died within 2 weeks of enrollment, of whom only one participant had evidence of further investigations. Among 540 participants with unknown HIV status, no evidence of further investigations was found in clinic records for 89% (481), 10% (54) had a sputum culture, 2% (9) had chest radiography, and 2% (11) were referred to hospital. No participants with unknown HIV status died within 2 weeks of enrollment.
TABLE 2.
Further Investigations and Interventions at Enrollment Clinic Among Participants in the XTEND Study During the 6-Month Follow-up Period After Enrollment, Stratified by Study Arm and HIV Status (n = 2695)
Among HIV-positive participants, the algorithm was followed in 14% (142/1031) of participants in the Xpert MTB/RIF arm, compared with 32% (365/1124) of persons in the smear microscopy arm, yielding an unadjusted risk ratio of 0.32 (95% CI: 0.17 to 0.62), which was similar after adjustment for gender, age, and exposures of TB symptoms and BMI (adjusted risk ratio, 0.34; 95% CI: 0.17 to 0.65).
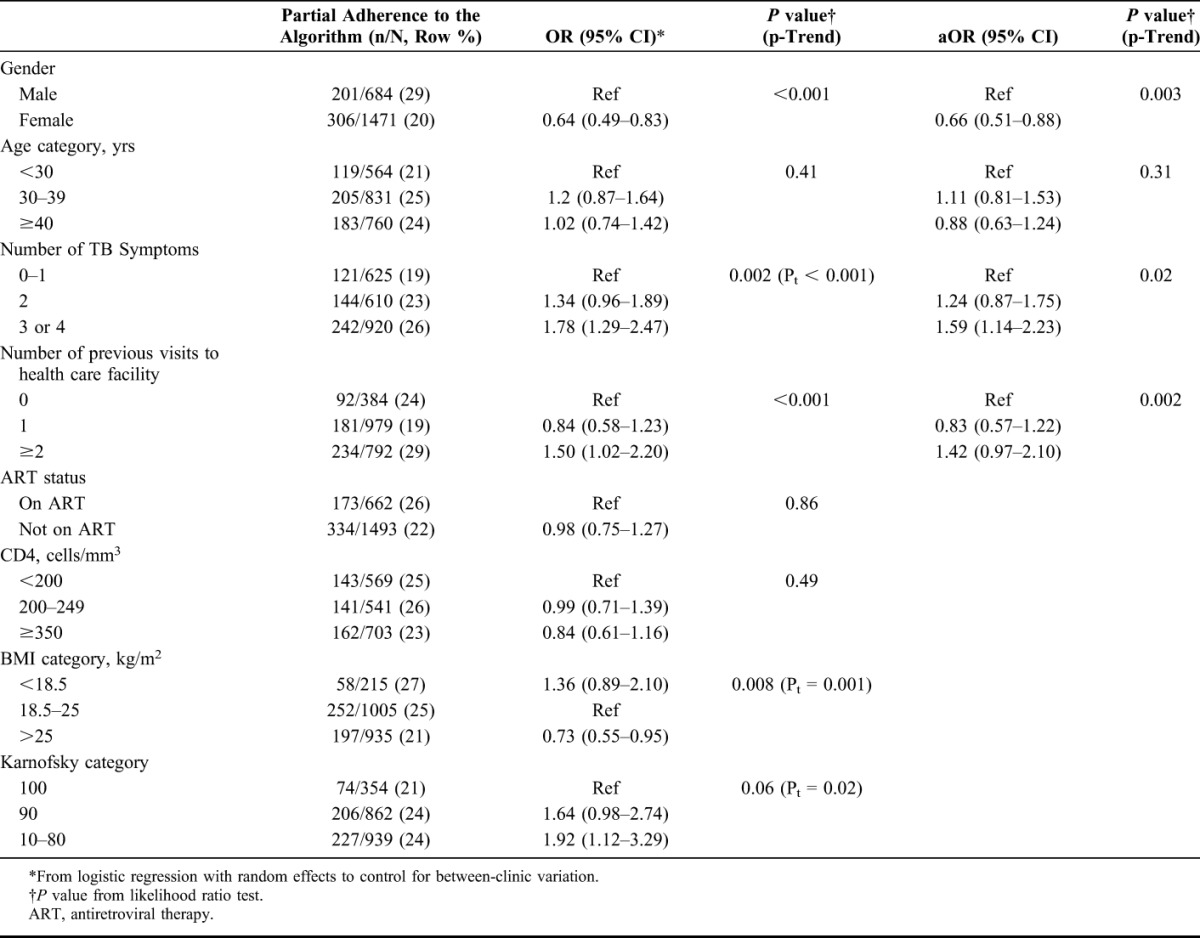
Combining data across study arms, patient-level factors associated with a higher odds of adherence to the algorithm among persons with known HIV status were male gender; 2 or more previous clinic visits at the time of enrollment versus none; and more severe illness at enrollment, as manifested by having more TB symptoms, a lower BMI, lower Karnofsky score, and CD4 count below 350 cells per cubic millimeter (Table 3). In a multivariable model, having 3 or more TB symptoms (aOR, 1.59; 95% CI: 1.14 to 2.23) and 2 or more previous visits to clinic (aOR, 1.42; 95% CI: 0.97 to 2.10) remained associated with increased odds of having had further investigations. Being female (aOR, 0.66; 95% CI: 0.51 to 0.88) was associated with decreased odds of having had further investigations. Participants with unknown HIV status were less likely to be investigated further [59/540 (11%) vs. 507/2155 (24%)] compared with participants with known HIV-positive status.
TABLE 3.
Patient factors at Enrollment Associated With Health Care Workers' adherence to the Algorithm for the Diagnosis of Tuberculosis Amongst HIV-positive Persons With Negative Initial Tests for Tuberculosis (n = 2155)
Among participating clinics, and for those who were sputum test negative, there was large between-clinic variation in ascertainment of participant HIV status and adherence to algorithms. The percentage of participants at each clinic in whom HIV status was not ascertained ranged from 1% to 41%. Evidence of partial adherence to the algorithm, including chest radiography, TB culture, or referral for further investigations ranged from 0% to 86% at participating clinics. Among participants with negative index sputum tests and known HIV-positive status, 7 clinics performed sputum culture in over 50% of persons, while 21 clinics requested sputum culture on less than 5% of persons. Chest radiography was performed only occasionally at any clinic, with 14 clinics having no evidence of chest radiography among HIV-positive persons with negative index sputum tests.
DISCUSSION
In this evaluation of routine primary health care practice among persons being investigated for TB in the context of a pragmatic trial, we observed poor adherence to TB diagnostic algorithms when initial sputum tests were negative, and fewer investigations (sputum TB cultures or chest radiography) among persons with negative Xpert MTB/RIF compared with persons with negative smears. Coverage of HIV testing was also suboptimal. Failure to adhere to this algorithm represents a missed opportunity for TB diagnosis and HIV testing, and may have contributed to the high mortality rate in this cohort.13
Although it is well established that adherence to the 2007 (pre-Xpert) WHO algorithm for the diagnosis of smear-negative TB (ie, the addition of sputum culture, chest radiography, and a trial of antibiotics) increases algorithm sensitivity, shortens time to TB treatment initiation, and reduces mortality,18–22 there are no studies explicitly evaluating the effectiveness of the current South African algorithm (incorporating Xpert) for TB diagnosis. However, 2 South African studies illustrate the potential for additional investigations, specifically chest radiography, to facilitate TB diagnosis. In a prospective evaluation of persons undergoing a single Xpert MTB/RIF test for TB diagnosis in a single PHC, of 116 persons started on TB treatment after initial Xpert MTB/RIF, 66 (57%) were Xpert MTB/RIF negative, of whom 44 had chest radiography or clinical evidence suggestive of TB.23 Subsequently 22 (50%) of these 44 persons had a positive sputum culture.23 In the TB-NEAT study among participants tested initially with Xpert MTB/RIF, only half of those starting TB treatment did so on the basis of a positive Xpert MTB/RIF.24 In the XTEND study population, in which 89% of persons with negative initial sputum results had no further investigations for TB, the omission of additional investigations may have led to poor outcomes.
The XTEND study identified that persons with unknown HIV status had a greater mortality risk when compared to persons with HIV-positive status and those on antiretroviral therapy.13 Our analysis reveals that persons with unknown HIV status are also less likely to receive further investigations for TB when initial sputum tests are negative. We and others demonstrate that persons living in areas with high HIV seroprevalence whom health care workers identify as requiring TB investigations have an HIV prevalence more than 50%25,26 and should therefore receive priority for HIV testing, staging, and rapid initiation of antiretroviral therapy. Failure to ascertain HIV status when initial sputum tests are negative for TB may lead to inappropriate underinvestigation for TB.
There are few reported studies that assess adherence to TB diagnostic guidelines in Africa. Among patients diagnosed in hospital, Loveday (South Africa)27 and Tafuma (Botswana)28 reported poor adherence to TB diagnostic algorithms, with a dependence on chest radiography and little use of sputum microscopy or culture. In an urban and rural setting in Uganda, Alamo et al18 reported poor adherence under operational conditions to the WHO 2007 algorithm for smear-negative TB. In the study of Alamo, reasons for nonadherence included failure by patients to attend for follow-up and operational constraints, such as absence of a doctor to interpret the chest radiography, or supply chain problems with radiographic reagents. In Ethiopia,29 physicians cited the absence of appropriate diagnostic modalities, ambiguity in TB guidelines, lack of faith in laboratory results, including smear microscopy, as reasons for failure to adhere to TB diagnostic guidelines. Patient difficulty in making multiple visits to health facilities was cited as a reason why doctors included radiography along with smear microscopy at patient's initial visit.29 In Ethiopia, poor documentation and lack of transparency regarding which investigations had been done hampered adherence to guidelines when patients were required to visit multiple service providers.29
Review of diagnostic algorithms may identify simpler or more effective strategies to diagnose TB. For example, in certain provinces in South Africa, where XTEND was not conducted but Xpert MTB/RIF is implemented, 2 sputum samples are sent simultaneously to the laboratory, which retains the second specimen for further testing if necessary. Efforts to strengthen adherence to TB diagnostic pathways could include mentoring and on-site supervision.
In general, inadequate adherence to guidelines across the spectrum of clinical disciplines, providers, and country income strata is a recognized problem that contributes to poor health outcomes.30–32 Reasons for inadequate adherence include guideline complexity, omission of the target user group in drawing up guidelines, weaker implementation strategies, lack of awareness of or familiarity with guidelines among the users, and a negative attitude or limited support from peers or superiors.31 A study in Uganda32 reported duplicated content, conflicting guidelines, and difficulties in guideline dissemination. Importantly, a mismatch between guidelines and conditions on the ground made implementation difficult.32 Even when guidelines were available, a “culture of not reading guidelines” was cited by managers as one of the difficulties faced by implementers. Poor supervision by superiors, who face logistical obstacles and who are burdened with administrative responsibilities, contributes to difficulties in guideline implementation. In our analysis, reasons for poor adherence are difficult to ascertain as the parent study was not designed to address this question. However, the high burden of persons requiring additional TB investigations and operational constraints when chest radiography is not available at site with logistical issues regarding patient transport may be among the reasons. Furthermore in our study, the relatively poorer adherence in the arm using Xpert MTB/RIF, despite recent training in the newer algorithm, may indicate that nurses place more confidence in a negative Xpert MTB/RIF result than is warranted. The fact that, across both arms, nurses tended to conduct further investigations on participants who were more ill (as evidenced by increasing number of TB symptoms, BMI, and Karnofsky score) is reassuring.
The strengths of our analysis include the pragmatic design of the parent study, allowing examination of current practice, large sample size including multiple clinics across rural and urban sites, multiple information sources to determine algorithm adherence (patient interview, case note abstraction and NHLS laboratory information system), and the opportunity to compare adherence to the algorithm during the roll-out of Xpert MTB/RIF. To our knowledge, it is also the first such evaluation of adherence to the new South African TB diagnostic algorithm in an operational setting. Limitations of the pragmatic study design are the absence of a gold standard for TB diagnosis, thus we are unable to quantify the effect of poor algorithm adherence in terms of numbers of cases of TB missed. Although we collected data on provision of antibiotics, data were limited and did not permit us to distinguish between antibiotics given as part of the TB diagnostic algorithm from those given for other purposes such as co-trimoxazole prophylaxis or to treat bacterial infections. In addition, we did not cross-check algorithm adherence with participant hospital records, nor were we able to examine health care worker rationale behind clinical judgments taken when patients returned for sputum results. We are also not able to differentiate patient vs. health system factors contributing to nonadherence.
In this study among a large cohort of persons undergoing TB investigations, drawn from 40 PHCs across 4 South African provinces, our analysis provides evidence that under routine clinic conditions at least 60% of HIV-positive persons with negative index sputum test results have no evidence of further investigation for TB. Persons tested initially with Xpert MTB/RIF were less likely to receive additional investigations. Furthermore, we observed that persons with negative initial TB tests and unknown HIV status were even less likely to receive additional investigations. In our South African context, national mortality risk among persons diagnosed with TB exceeds 8%, and postmortem studies indicated high rates of undiagnosed TB among deaths in community.33 We recommend targeted efforts to improve the uptake of HIV testing among all persons undergoing investigations for TB. TB diagnostic pathways need to be reviewed and strengthened to ensure that a negative sputum test result is not the end of the diagnostic road, particularly for those at highest risk of poor outcomes.
Footnotes
The authors have no funding or conflicts of interest to disclose
G.C. and K.F. are co-senior authors.
REFERENCES
- 1.WHO. Global TB Report. Geneva, Switzerland; 2014. Available at: http://www.who.int/tb/publications/global_report/en/. WHO/HTM/TB/2014.08. Accessed March 12, 2015. [Google Scholar]
- 2.Bradshaw D, Pillay-Van Wyk V, Laubscher R, et al. Cause of Death Statistics for South Africa: Challenges and Possibilities for Improvement. Cape Town, South Africa: Burden of Disease Research Unit, Medical Research Council; 2010. Available at: www.mrc.ac.za/bod/cause_death_statsSA.pdf. Accessed March 12, 2015. [Google Scholar]
- 3.Wong EB, Omar T, Setlhako GJ, et al. Causes of death on antiretroviral therapy: a post-mortem study from South Africa. PLoS One. 2012;7:e47542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mortality and causes of death in South Africa, 2013. Findings from Death Notification. Pretoria: Statistics South Africa; 2014. Available at: http://beta2.statssa.gov.za/publications/P03093/P030932013.pdf. Accessed March 12, 2015. [Google Scholar]
- 5.Lawn SD, Mwaba P, Bates M, et al. Advances in tuberculosis diagnostics: the Xpert MTB/RIF assay and future prospects for a point-of-care test. Lancet Infect Dis. 2013;13:349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO. Automated Real-time Nucleic Acid Amplification Technology for Rapid and Simultaneous Detection of Tuberculosis and Rifampicin Resistance: Xpert MTB/RIF System for the Diagnosis of Pulmonary and Extra-pulmonary TB in Adults and Children. Policy update. Geneva: WHO; 2013. Available at: http://whqlibdoc.who.int/publications/2011/9789241501545_eng.pdf. Accessed March 12, 2015. [PubMed] [Google Scholar]
- 8.Keeton C. New diagnostic test changes tuberculosis landscape. Bull World Health Organ. 2013;91:163–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helb D, Jones M, Story E, et al. Rapid detection of Mycobacterium tuberculosis and rifampicin resistance by use of on-demand, near-patient technology. J Clin Microbiol. 2010;48:229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Department of Health SA. National Tuberculosis Management Guidelines. Tshwane, South Africa: South African National Department of Health; 2014. [Google Scholar]
- 11.Colebunders R, Bastian I. A review of the diagnosis and treatment of smear-negative pulmonary tuberculosis. Int J Tuberc Lung Dis. 2000;4:97–107. [PubMed] [Google Scholar]
- 12.Wood R, Lawn SD, Caldwell J, et al. Burden of new and recurrent tuberculosis in a major South African city stratified by age and HIV-status. PLoS One. 2011;6:e25098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Churchyard G, Stevens W, Mametja LD, et al. Xpert MTB/RIF replacing sputum microscopy as the initial diagnostic test for tuberculosis: a cluster-randomised trial embedded in South African roll-out. Lancet Glob Health. 2015;3:e450–7. [DOI] [PubMed] [Google Scholar]
- 14.Current Controlled Trials. Xpert for tuberculosis: evaluating a new diagnostic (XTEND). ISRCTN68905568 London: ISRCTN Registry. [Google Scholar]
- 15.National Department of Health SA. The diagnosis and management of tuberculosis Pretoria: Department of health, Republic of South Africa. Pretoria: South African National Department of Health; 2009. [Google Scholar]
- 16.WHO. Improving the diagnosis and treatment of smear-negative pulmonary and extrapulmonary tuberculosis among adults and adolescents; Recommendations for HIV-prevalent and resource-constrained settings. Geneva: WHO; 2007. [Google Scholar]
- 17.Hayes RJ, Moulton LH. Cluster Randomised Trials. Boca Raton, FL: Chapman & Hall; 2009. [Google Scholar]
- 18.Alamo ST, Kunutsor S, Walley J, et al. Performance of the new WHO diagnostic algorithm for smear-negative pulmonary tuberculosis in HIV prevalent settings: a multisite study in Uganda. Trop Med Int Health. 2012;17:884–895. [DOI] [PubMed] [Google Scholar]
- 19.Walley J, Kunutsor S, Evans M, et al. Validation in Uganda of the new WHO diagnostic algorithm for smear-negative pulmonary tuberculosis in HIV prevalent settings. J Acquir Immune Defic Syndr. 2011;57:e93–100. [DOI] [PubMed] [Google Scholar]
- 20.Holtz TH, Kabera G, Mthiyane T, et al. Use of a WHO-recommended algorithm to reduce mortality in seriously ill patients with HIV infection and smear-negative pulmonary tuberculosis in South Africa: an observational cohort study. Lancet Infect Dis. 2011;11:533–540. [DOI] [PubMed] [Google Scholar]
- 21.Abebe G, Deribew A, Apers L, et al. Evaluation of the 2007 WHO guideline to diagnose smear negative tuberculosis in an urban hospital in Ethiopia. BMC Infect Dis. 2013;13:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dimairo M, MacPherson P, Bandason T, et al. The risk and timing of tuberculosis diagnosed in smear-negative TB suspects: a 12 month cohort study in Harare, Zimbabwe. PLoS One. 2010;5:e11849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanrahan CF, Selibas K, Deery CB, et al. Time to treatment and patient outcomes among TB suspects screened by a single point-of-care xpert MTB/RIF at a primary care clinic in Johannesburg, South Africa. PLoS One. 2013;8:e65421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Theron G, Zijenah L, Chanda D, et al. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet. 2014;383:424–435. [DOI] [PubMed] [Google Scholar]
- 25.Fielding K, Ginindza S, McCarthy K, et al. High self-reported HIV positive status among clinic attendees suspected of TB enrolled in a cluster randomised trial of Xpert MTB/RIF, South Africa. Paper presented at: Abstract #PC-857–903. 44th Union World Conf Lung Health; 31st 2013; Paris.
- 26.Macpherson P, Dimairo M, Bandason T, et al. Risk factors for mortality in smear-negative tuberculosis suspects: a cohort study in Harare, Zimbabwe. Int J Tuberc Lung Dis. 2011;15:1390–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loveday M, Thomson L, Chopra M, et al. A health systems assessment of the KwaZulu-Natal tuberculosis programme in the context of increasing drug resistance. Int J Tuberc Lung Dis. 2008;12:1042–1047. [PubMed] [Google Scholar]
- 28.Tafuma TA, Burnett RJ, Huis in 't Veld D. National guidelines not always followed when diagnosing smear-negative pulmonary tuberculosis in patients with HIV in Botswana. PLoS One. 2014;9:e88654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mala G, Moser A, Dinant GJ, et al. Why tuberculosis service providers do not follow treatment guideline in Ethiopia: a qualitative study. J Eval Clin Pract. 2014;20:88–93. [DOI] [PubMed] [Google Scholar]
- 30.Gagliardi AR, Brouwers MC, Palda VA, et al. How can we improve guideline use? A conceptual framework of implementability. Implement Sci. 2011;6:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Francke AL, Smit MC, de Veer AJ, et al. Factors influencing the implementation of clinical guidelines for health care professionals: a systematic meta-review. BMC Med Inform Decis Mak. 2008;8:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nabyonga Orem J, Bataringaya Wavamunno J, Bakeera SK, et al. Do guidelines influence the implementation of health programs?—Uganda's experience. Implement Sci. 2012;7:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Omar T, Variava E, Lebina L, et al. Post mortem pulmonary pathology in adults dying at home: a study from North West Province. Cape Town, South Africa: IAP; 2012. [Google Scholar]