Supplemental Digital Content is Available in the Text.
Key Words: tenofovir alafenamide, emtricitabine, chronic kidney disease, bone mineral density, HIV
Abstract
Background:
Tenofovir alafenamide (TAF) is a novel tenofovir prodrug with improved renal and bone safety compared with TDF-containing regimens. We report the 48 week safety and efficacy of a once-daily single tablet regimen of elvitegravir 150 mg (E), cobicistat 150 mg (C), emtricitabine 200 mg (F), and TAF 10 mg (E/C/F/TAF) in HIV-1-infected patients with mild to moderate renal impairment.
Methods:
We enrolled virologically suppressed HIV-1-infected subjects with estimated creatinine clearance (CrCl) 30–69 mL/min in a single-arm, open-label study to switch regimens to E/C/F/TAF. The primary endpoint was the change from baseline in glomerular filtration rate estimated using various formulae. This study is registered with ClinicalTrials.gov, number NCT01818596.
Findings:
We enrolled and treated 242 patients with mean age 58 years, 18% Black, 39% hypertension, 14% diabetes. Through week 48, no significant change in estimated CrCl was observed. Two patients (0.8%) discontinued study drug for decreased creatinine clearance, neither had evidence of renal tubulopathy and both had uncontrolled hypertension. Subjects had significant improvements in proteinuria, albuminuria, and tubular proteinuria (P < 0.001 for all). Hip and spine bone mineral density significantly increased from baseline to week 48 (mean percent change +1.47 and +2.29, respectively, P < 0.05). Ninety-two percent (222 patients) maintained HIV-1 RNA <50 copies per milliliter at week 48.
Interpretation:
Switch to E/C/F/TAF was associated with minimal change in GFR. Proteinuria, albuminuria and bone mineral density significantly improved. These data support the efficacy and safety of once daily E/C/F/TAF in HIV+ patients with mild or moderate renal impairment without dose adjustment.
INTRODUCTION
Renal disease is an important contributor to morbidity and mortality in HIV-infected patients.1–4 The prevalence of chronic kidney disease (CKD) among people with HIV-1 infection in the United States is 16% and is expected to rise as this population ages.5–8 The use of the currently approved nucleo(s)tides is cautioned in patients with increased risk of CKD and cardiovascular disease, given the potential for tenofovir disoproxil fumarate (TDF) nephrotoxicity and concern for cardiovascular risk associated with abacavir (ABC). For patients who cannot take either TDF or ABC, guidelines consider the use of nucleos(t)ide-sparing regimens as optional because of reduced virologic activity. Simple HIV regimens with proven efficacy that do not increase risk of prevalent comorbidities, such as bone-related comorbidity, decreased renal function, and cardiovascular risk, are needed for improved adherence and patient outcomes.
Tenofovir alafenamide (TAF) is a novel tenofovir (TFV) prodrug with pharmacokinetic (PK) properties distinct from TDF. The renal and bone toxicities seen with 300 mg TDF are associated with high circulating plasma levels of TFV,9–13 and TAF has 90% lower plasma TFV than TDF in the TAF-containing formulations currently in development. Because of TAF's reduced dose and a longer plasma half-life, plasma exposure of TFV is 90% lower with TAF than with TDF, which is hypothesized to reduce the risk of renal and bone toxicities.
Findings from 2 randomized, double-blind phase 3 trials of TAF vs TDF, both coformulated with elvitegravir, cobicistat, and emtricitabine (FTC), demonstrated noninferior efficacy of TAF vs TDF, with a significantly reduced effect of TAF compared with TDF on estimated glomerular filtration rate (eGFR), proteinuria, albuminuria, and bone mineral density (BMD).14 To assess whether E/C/F/TAF is safe and reduces the renal and bone effects associated with TDF-containing regimens in patients with mild to moderate renal impairment, a single-arm, open-label phase 3 clinical trial was conducted, with a protocol-specified focus on renal and bone safety.
METHODS
Study Design and Patients
GS-US-292-0112 is a single-arm, open-label phase 3 study performed at 70 outpatient centers in the United States, Thailand, United Kingdom, Australia, Spain, France, Dominican Republic, Mexico, and the Netherlands. The study was performed in accordance with the Declaration of Helsinki and approved by central or site-specific review boards or ethics committees. Each patient gave written informed consent. HIV-1-infected adults (aged ≥18 years) were enrolled in cohort 1 (switch) if they had no known history of resistance to elvitegravir, TDF, or FTC; plasma HIV-1 RNA concentrations at undetectable levels for at least 6 months preceding the screening visit, and had HIV-1 RNA <50 copies per milliliter at screening, eGFRCG (CG, Cockroft-Gault) 30–69 mL/min, CD4+ cell count of ≥50 cells per microliter, and stable renal function (a serum creatinine at least once within 3 months of screening that is not ≥25% different from the serum creatinine at screening), and cause of underlying CKD stable without change in medical management for 3 months before baseline. Patients with positive hepatitis B surface antigen or hepatitis C antibody or a new AIDS-defining illness within 30 days of screening were excluded. Six additional patients who had no previous antiretroviral treatment were included in cohort 2 [antiretroviral (ARV) naive], but because of the small sample size, these data are summarized separately (see Appendix, Supplemental Digital Content, http://links.lww.com/QAI/A772).
Procedures
Eligible patients received coformulated 150 mg elvitegravir, 150 mg cobicistat, 200 mg FTC, and 10 mg TAF (E/C/F/TAF) once daily administered with food. Post-baseline study visits occurred at weeks 1, 2, 4, 8, 12, 16, 24, 36, and 48, after which patients continued treatment with visits every 12 weeks until week 96.
Safety was assessed by physical examinations, laboratory tests, 12-lead electrocardiogram, and recording of adverse events. The PK of the components of E/C/F/TAF were evaluated through an intensive PK substudy performed on a subset of patients at sites with capability at week 4 or 8, which included plasma sampling for elvitegravir, cobcicistat, FTC, TAF, and TFV. Bioanalytical analyses of drug concentrations in plasma were done by QPS (Newark, DE).
Laboratory tests included hematological analysis, serum chemistry tests, fasting lipid parameters, CD4+ cell counts; and measures of renal function [eGFR, urine protein to creatinine ratio (UPCR), urine albumin to creatinine ratio (UACR), retinol-binding protein to creatinine ratio, β2-microglobulin to creatinine ratio, fractional excretion of uric acid, and fractional excretion of phosphate; Covance Laboratories, Indianapolis, IN]; HIV-1 RNA concentration (COBAS TaqMan HIV-1 Test, v2.0; Roche Diagnostics, Rotkreuz, Switzerland); and, in a convenience sample at sites with capability, measured GFR (mGFR) as assessed by iohexol clearance.
Virologic failure was defined as having HIV-1 RNA ≥50 copies per milliliter, confirmatory on a second sample drawn within 3–6 weeks. Confirmatory samples with HIV-1 RNA ≥400 copies per milliliter were sent for HIV-1 genotype/phenotype analysis (PhenoSense GT for Protease and Reverse Transcriptase genes and GenSeq Integrase and Phenosense Integrase for the Integrase gene; Monogram Biosciences, South San Francisco, CA).
In all patients, dual energy x-ray absorptiometry scans of the lumbar spine and hip were done at baseline, week 24, and week 48 to measure percent change in BMD. The scans were processed by BioClinica (Newton, PA).
The primary endpoint was the change from baseline at week 24 in eGFRCG, eGFRCKD-EPI-CysC based on Cystatin C, and eGFRCKD-EPI-creat based on serum creatinine.15 The secondary endpoints included change from baseline at weeks 48 and 96 in eGFR estimations, mGFR, change from baseline in renal and bone biomarkers, and hip and spine BMDs, at weeks 24, 48, and 96, the incidence of adverse events and graded laboratory abnormalities, and the proportion of patients maintaining virologic control at weeks 24, 48, and 96 (HIV-1 RNA <50 copies per milliliter, snapshot analysis algorithm).
The preliminary results were reviewed by an independent data monitoring committee when 50 patients had completed week 12. The primary endpoint analysis was done after all enrolled patients had completed their week 24 study visit or had prematurely discontinued study drug.
Statistical Analyses
For the primary endpoint (changes from baseline in eGFRCG, eGFRCKD−EPI-cysC, and eGFRCKD-EPI-creat) and for other secondary renal, bone, and metabolic endpoints, changes from baseline were summarized by visit using descriptive statistics and the median change from baseline was analyzed by 2-sided Wilcoxon signed rank test. In addition, change in mGFR was assessed using a parametric analysis of variance model, and comparisons of geometric least square mean ratios between week 24 and baseline and week 2, 4, or 8 and baseline, with 90% confidence intervals (CIs), were used with a prespecified lack of alteration boundary of 80%–125%. Subgroup analyses were conducted for all the above renal, bone, and metabolic endpoints for patients taking TDF-containing regimen before switch and for patients with baseline eGFRCG <50 mL/min.
Adverse events were coded with the Medical Dictionary for Regulatory Activities (version 17.0).
Number and percentage of patients with virologic success (achieved HIV-1 RNA <50 copies per milliliter), virologic failure, and reasons for no virologic data at week 48 as defined by the U.S. Food and Drug Administration snapshot algorithm were summarized. The 95% CIs for virologic success rate were constructed using the exact method.
This study was conducted according to the protocol without significant deviations and is registered with ClinicalTrials.gov, number NCT01818596.
RESULTS
Study Population
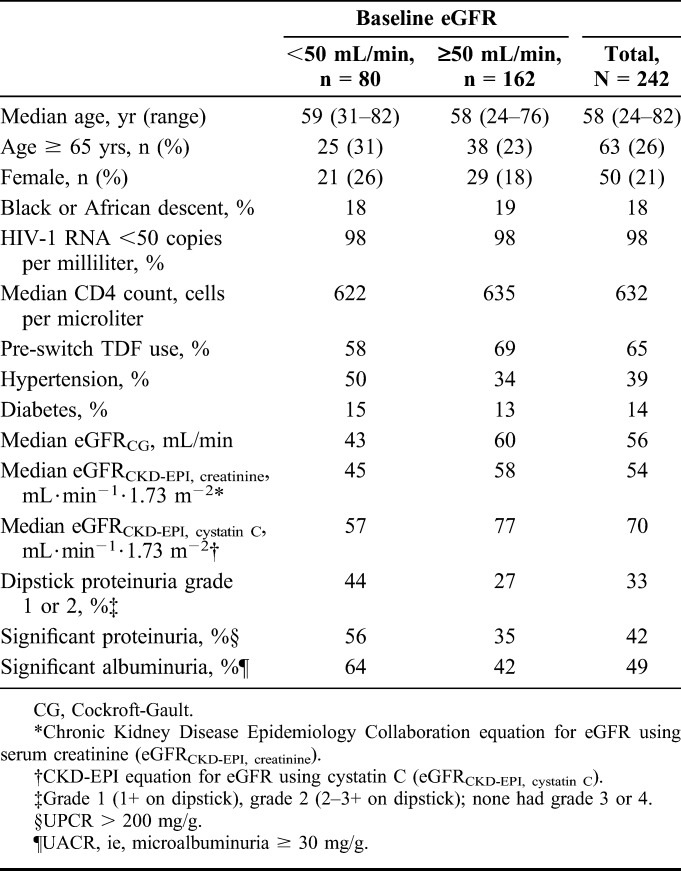
Two hundred forty-two patients with stable eGFRCG 30–69 mL/min (including 80 patients with baseline eGFRCG <50 mL/min) were enrolled and switched from baseline antiretroviral treatment regimens to receive E/C/F/TAF. The median age was 58 years (range = 24–82 years), including 63 patients ≥65 year; 21% of patients were female, 18% of patients identified themselves as Black, and median CD4 cell count was 632 cells per microliter (Table 1). The median eGFRCG at baseline was 56 mL/min, 42% of patients had significant proteinuria (UPCR > 200 mg/g), 49% had significant albuminuria (UACR ≥30 mg/g), 39% had hypertension, and 14% were diabetic. ARV regimens before switch were variable, with 65% taking a TDF-containing regimen, 22% taking an ABC-containing regimen, and 5% taking a nucleos(t)ide-free regimen (see Figure S1, Supplemental Digital Content, http://links.lww.com/QAI/A772). Median (Q1, Q3) study drug exposure was 48.1 (43.1, 60.1) weeks.
TABLE 1.
Baseline Characteristics
Renal Safety
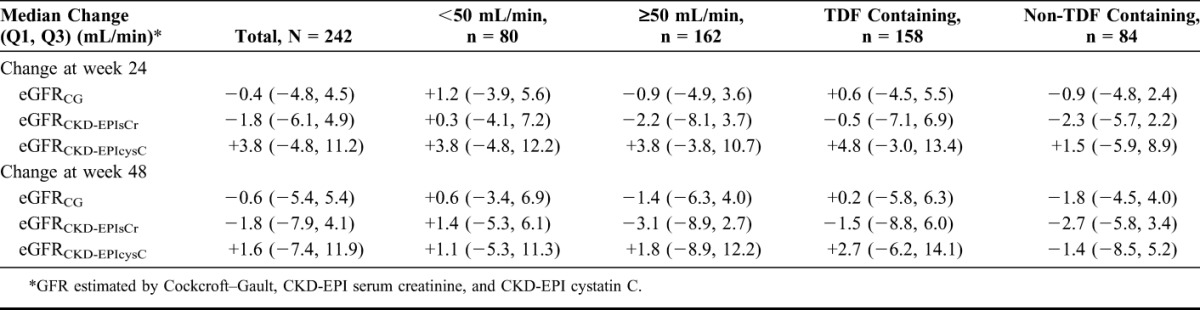
Through 48 weeks, no clinically appreciable change from baseline in estimated creatinine clearance was observed, with direction and magnitude varying by filtration marker and equation. Results were similar for patients whether baseline eGFR was <50 or ≥50 mL/min or whether they switched from a TDF-based regimen (Table 2).
TABLE 2.
Estimated GFR: Change From Baseline to Weeks 24 and 48
In the iohexol substudy (n = 32), actual GFR was not affected over 24 weeks of treatment (see Figure S2a and 2b, Supplemental Digital Content, http://links.lww.com/QAI/A772). The geometric mean ratio (90% CI) between week 24 and baseline was 103% (97%, 109%). No difference in mGFR between week 24 and baseline was observed between patients with baseline eGFRCG <50 vs ≥50 mL/min or between those taking TDF vs non–TDF-containing regimens before switching to E/C/F/TAF.
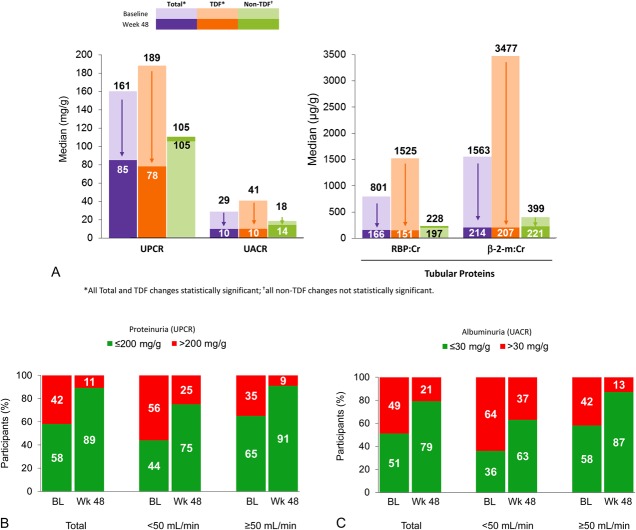
Significant improvements in total proteinuria, albuminuria, and tubular proteinuria (urine retinol-binding protein/creatinine ratio and β2-microglobulin/creatinine ratio) were observed (Fig. 1A). Improvements in renal parameters were particularly marked in patients switching to E/C/F/TAF from a TDF-containing regimen, whereas renal function in patients switching from non-TDF regimens did not have significant changes. The prevalence of significant proteinuria (UPCR > 200 mg/g) and albuminuria (UACR ≥ 30 mg/g) decreased from 42% to 11% and from 49% to 21%, respectively (Figs. 1B, C). These changes occurred at week 1 and remained through 48 weeks. Significant improvements in other measures of renal tubular function, including fractional excretion of uric acid levels [median (Q1, Q3) change from baseline to week 48, −1.5 (−3.6, 0.0); P < 0.001], were observed, whereas there was no significant change in fractional excretion of phosphate [median (Q1, Q3) change from baseline to week 48, 1.1 (−4.1, 6.1), P = 0.07] or serum phosphorus [median (Q1, Q3) change from baseline to week 48, 0.0 (−0.4, 0.4); P = 0.50].
FIGURE 1.
A, Proteinuria: change from baseline to week 48. B, Significant proteinuria: baseline to week 48. C, Significant albuminuria: baseline to week 48.
Eighty patients with baseline GFR <50 mL/min switched to E/C/F/TAF. These patients were slightly older and had a higher proportion of subjects with hypertension (see Table S1, Supplemental Digital Content, http://links.lww.com/QAI/A772). As noted above, these patients had no significant change from baseline estimated creatinine clearance by any measure, and other measures of renal function (proteinuria, albuminuria, and tubular proteinuria) improved significantly from baseline to week 48. An exploratory analysis of patients with the lowest (bottom 5%) eGFRCG and highest (top 5%) tubular proteinuria demonstrated improvements for all measures (data not shown).
Bone Mineral Density
BMD significantly increased after switch to E/C/F/TAF for patients on a TDF-containing regimen pre-switch and remained stable after switch to E/C/F/TAF for patients on non–TDF-containing regimen pre-switch. Mean percent changes from baseline to week 48 in hip and spine BMDs significantly increased (+1.47% and +2.29%, respectively), and more patients had significant (≥3%) gains in hip or spine BMD than those who had significant loss (Figs. 2A, B).
FIGURE 2.
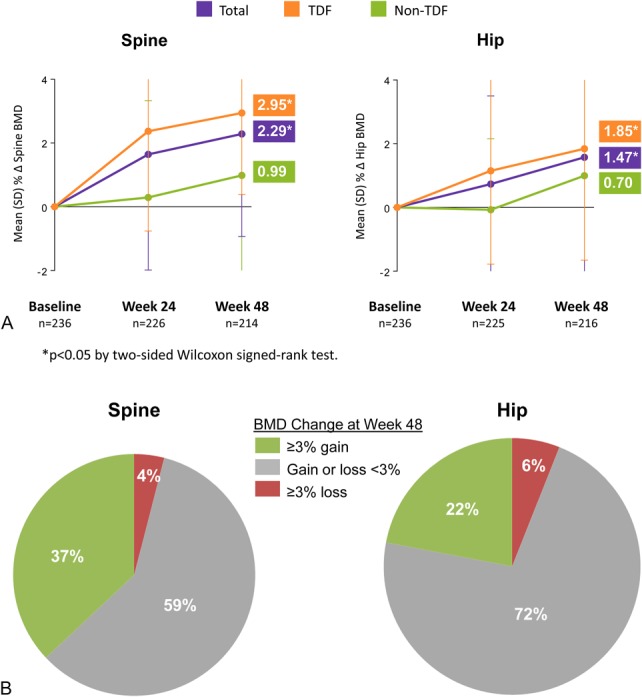
A, BMD: mean change from baseline to week 48. B, Proportions of patients with BMD changes.
Metabolic Changes
Fasting lipid levels decreased in patients who used non–TDF-containing regimens before switching to E/C/F/TAF, whereas levels increased in those using TDF-containing regimens before switching to E/C/F/TAF (see Figure S3, Supplemental Digital Content, http://links.lww.com/QAI/A772). However, there was no significant difference in the total:high-density lipoprotein (HDL) cholesterol ratio between those receiving either TDF or non-TDF regimen before switch because there were concordant changes for both the total cholesterol and the HDL cholesterol fraction.
Adverse Events
E/C/F/TAF was well tolerated, with most adverse events reported as mild or moderate in severity (see Table S2a, Supplemental Digital Content, http://links.lww.com/QAI/A772). Adverse events leading to study drug discontinuation were uncommon, occurring in 3% of patients (n = 8). Two patients (0.8%) discontinued study drug for decreased GFR by eGFRCG and eGFRCKD-EPI, cystatin C. One patient (baseline eGFRCG = 49 mL/min) who had uncontrolled hypertension, an episode of vomiting and dehydration, concomitant ramipril and valsartan, and discontinued study drug after 3 months of therapy was assessed by the investigator to have worsening renal insufficiency possibly related to the study drug. This patient had significant improvement in UPCR (1609–178 mg/g) and no glycosuria. Another patient (baseline eGFRCG = 36 mL/min) was considered to have progression of hypertension-related CKD unrelated to the study drug. Neither of these subjects, or any other study participant, had laboratory evidence of proximal renal tubulopathy or Fanconi syndrome. There were 6 fractures, all related to mechanical trauma and considered by the investigator to be unrelated to the study drugs. The most common adverse events were 11% diarrhea, 9% upper respiratory infection, 9% arthralgia, 8% bronchitis, 8% osteopenia, 8% nausea, 7% headache, 7% pain in extremity, 7% back pain, 6% dizziness, 6% fatigue, 6% renal cyst, and 6% cough. Adverse events, grades, and frequencies were similar in patients with baseline eGFR <50 vs ≥50 mL/min (see Table S2b, Supplemental Digital Content, http://links.lww.com/QAI/A772). Because FTC was administered at 200 mg daily, a comparison of FTC adverse drug reactions was performed by baseline eGFRCG, and no significant difference between groups was observed (see Table S2c, Supplemental Digital Content, http://links.lww.com/QAI/A772).
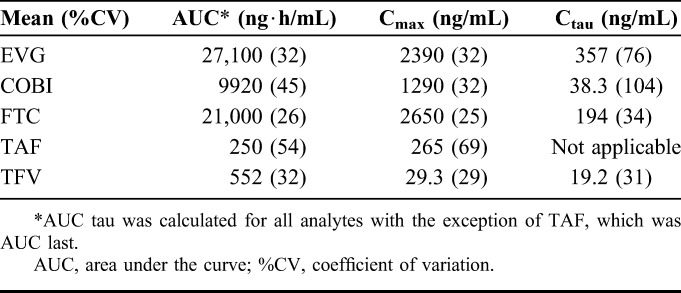
Pharmacokinetics
Plasma PK parameters of the components of E/C/F/TAF in a subset of patients who participated in the intensive PK substudy (n = 30) are presented in Table 3. The elvitegravir (EVG) and cobicistat (COBI) PK parameters were in the range of historical data after administration of E/C/F/TAF in HIV-infected patients without renal impairment.16 EVG and COBI exposure were comparable across eGFR groups of eGFRCG <50 or ≥50 mL/min, consistent with the nonrenal elimination pathway of these agents. As expected, because of FTC being primarily renally eliminated, FTC PK was higher in patients in this study vs historical E/C/F/TAF data in nonrenally impaired patients. FTC exposure was higher in patients with eGFR <50 vs ≥50 mL/min, consistent with the FTC PK parameters reported for EMTRIVA in patients with mild or moderate renal impairment.17 The TAF PK parameters were consistent with historical data in nonrenally impaired patients. Additionally, the TAF metabolite TFV, which is primarily renally eliminated, was higher in patients in this study vs historical TAF data in nonrenally impaired patients but was well below the TFV exposures from TDF-containing regimens.18
TABLE 3.
PK Parameters of E/C/F/TAF
Efficacy
At week 48, 222 patients (92%) maintained an HIV-1 RNA <50 copies per milliliter, 17 patients (7%) did not have virologic data available, and 3 patients (1%) were considered to have virologic failure. Of these 3 patients, one patient had HIV-1 RNA <50 copies per milliliter on E/C/F/TAF before switching to a new regimen, the second patient with HIV-1 RNA <400 copies per milliliter on E/C/F/TAF demonstrated nucleoside reverse transcriptase inhibitor and protease inhibitor resistance identical to a pre-study historical genotype, and a third patient took additional ARVs (rilpivirine/FTC/TDF) through day 67 (protocol violation) but was maintained on E/C/F/TAF alone with HIV-1 RNA <50 copies per milliliter after that was noted through week 48. The mean increase from baseline in CD4 cell counts through week 48 (observed data) was +13 (SD = 170.1) cells per cubic millimeter.
DISCUSSION
Through 48 weeks, patients with mild to moderate renal impairment (eGFR = 30–69 mL/min) who switched to TAF (E/C/F/TAF) had no appreciable change in estimated or actual GFR, whereas proteinuria, albuminuria, proximal renal tubular function, and BMD significantly improved. Of particular note, the subset of patients with eGFR <50 mL/min, who currently require dose adjustment for both TDF and FTC, had stable eGFR and significant improvements in tubular function through 48 weeks after switching to once-daily E/C/F/TAF without dose-limiting adverse events. In these patients with eGFR <50 mL/min, adverse events were similar in grade and frequency to patients with eGFR ≥50 mL/min, indicating no untoward effects from higher FTC exposure. E/C/F/TAF was well tolerated, and discontinuations for drug-related adverse events were uncommon. No cases of proximal tubulopathy occurred. Although this is a single-arm study, our data suggest that E/C/F/TAF may be used safely in HIV-infected patients with mild to moderate renal impairment without the need for dose adjustment.
The improvements in renal and bone effects were primarily experienced by patients in this study who switched from TDF-containing regimens and likely represent a reduction in the off-target renal and bone effects of plasma TFV concentrations, which are 90% lower with TAF. Some prospective studies have shown that patients receiving TDF-based therapies have significantly greater loss of kidney function and higher risk of acute renal failure.19 TFV exposure has been independently associated with proteinuria, eGFR decline, and the development eGFR <60 mL/min in older US veterans with elevated risk of renal disease.20 Risk factors for TDF-related nephrotoxicity include older age, coadministration with ritonavir-boosted protease inhibitors (which further increase TFV plasma levels), and comorbidities associated with renal disease.21
In this study, TDF-associated nephrotoxicity was assessed using proteinuria (UPCR) and albuminuria (UACR)—these tests have validated thresholds associated with elevated risk for adverse clinical outcomes22–24 and recommended guidelines for both HIV-infected25 and HIV-uninfected26,27 patients. Markers specific for proximal renal tubular dysfunction were also assessed (urine retinol-binding protein to creatinine ratio and β2-microglobulin to creatinine ratio), as recommended for monitoring TDF nephrotoxicity in current Infectious Diseases Society of America Guidelines.25 In this study, subjects with preexisting renal impairment who switched from TDF-containing regimens to E/C/F/TAF had normalization of proteinuria, albuminuria, and tubular proteinuria and subjects who switched from non–TDF-containing regimens to E/C/F/TAF had stable proteinuria, albuminuria, and tubular proteinuria. Many patients had resolution of significant proteinuria and albuminuria after switching to E/C/F/TAF. Reversal of these effects within the first 2 weeks after switching to E/C/F/TAF suggests that TFV's effect on kidney function, as measured by these markers, is reversible. TFV is not a direct inhibitor of any creatinine transporter (ie, OAT2, OCT2, OCT3, MATE1, and MATE2K). The combined clinical results suggest a global effect of the higher circulating levels of TFV associated with TDF administration on proximal tubule function. Although the exact mechanism is unknown, a recent study has shown TFV inhibition of sodium phosphate transporters that are involved in phosphate reabsorption in the proximal tubule that may cause a perturbation in proximal tubular ion flow, consistent with this global effect.28
Treatment with TDF has consistently been associated with less increase in lipids compared with other regimens in treatment-naive patients. Interestingly, this study showed increases in total and low-density lipoprotein cholesterol and triglycerides in patients switching from TDF-containing regimens. These changes are likely related to the significant reductions in plasma TFV concentrations. TDF seems to have independent effects to lower cholesterol fractions; so, the increases observed with switching from TDF to TAF are consistent with the phenomenon. Of note, decreases in total, low-density lipoprotein, and HDL cholesterol and triglycerides in patients switching from non–TDF-containing regimens were observed.
Strengths of this study include the clinical relevance of the study population, which included a significant number of patients with moderately impaired renal function, older age, and comorbidity, and the rigorous evaluations performed during a full 48-week period of follow-up. Study sites were geographically diverse. One limitation of the present study is the single-arm noncomparative study design. However, 2 international phase 3 clinical trials comprising over 1700 patients suggested a favorable renal and bone safety profile of TAF vs TDF in a randomized double-blind design, where the TFV prodrug was the only variable. These studies showed that, compared with TDF, TAF-treated patients had significantly smaller reductions in eGFR, more favorable changes in proteinuria, albuminuria, and tubular proteinuria, and more favorable BMD.
In summary, patients with significant comorbidities and mild to moderate renal impairment (eGFR = 30–69 mL/min) who switched to E/C/F/TAF had no change in estimated or actual GFR, whereas proteinuria, proximal renal tubular function, and BMD significantly improved over 48 weeks. Of particular note, patients with eGFR <50 mL/min who currently require dose adjustment for both TDF and FTC had stable eGFR and significant improvements in tubular function through 48 weeks after switching to once-daily E/C/F/TAF without dose adjustment. In these patients with eGFR <50 mL/min, adverse events were similar in grade and frequency to patients with eGFR ≥50 mL/min, indicating no untoward effects from higher FTC exposure. No cases of proximal tubulopathy occurred. E/C/F/TAF was well tolerated and discontinuations for drug-related adverse events were rare. Our data support the safe use of single-tablet E/C/F/TAF in HIV+ patients with mild and moderate renal impairment without dose adjustment over the first year of use. Longer duration studies are needed to confirm these initial findings.
Supplementary Material
ACKNOWLEDGMENTS
The authors extend our thanks to the patients, their partners and families, and all principal investigators and their study staff for the GS-US-292-0112 study. The authors also thank the complete GS-US-292-0112 study team.
Footnotes
This clinical trial was funded by Gilead Sciences.
Portions of this study were presented at the 22nd Conference on Retroviruses and Opportunistic Infections, February 23–26, 2015; Seattle, WA, and at the eighth IAS Conference on Pathogenesis, Treatment, and Prevention, July 19–22, 2015; Vancouver, Canada.
A.P. reports receiving grants and speaker fees from Gilead, ViiV, BMS, Janssen, and Merck. J.R.A. reports receiving grants and speaker fees from Gilead, Viiv, BMS, Janssen, and Abbvie. S.K.G. reports receiving grants and speaker fees from Gilead, BMS, Janssen, and Merck. F.A.P. reports receiving grants and speaker fees from Gilead, Janssen, ViiV, Abbvie, and MSD. M.B. reports receiving grants and speaker fees from Gilead, ViiV, Abbvie, MSD, Lilly, Novartis, and Amgen. G.C. reports receiving grants and speaker fees from Gilead, Janssen, ViiV, and Merck. J.G., P.B., K.L., and M.R. report receiving grants from Gilead. P.C. reports receiving grants and speaker fees from Gilead and MSD. A.A. reports no conflicts to disclose. J.M.C., M.E.A., X.W., A.C., S.M., D.S., and M.W.F. are employees of Gilead Sciences.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
REFERENCES
- 1.Wyatt CM. The kidney in HIV infection: beyond HIV-associated nephropathy. Top Antivir Med. 2012;20:106–110. [PMC free article] [PubMed] [Google Scholar]
- 2.Choi AI, Li Y, Deeks SG, et al. Association between kidney function and albuminuria with cardiovascular events in HIV-infected persons. Circulation. 2010;121:651–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ibrahim F, Hamzah L, Jones R, et al. Baseline kidney function as predictor of mortality and kidney disease progression in HIV-positive patients. Am J Kidney Dis. 2012;60:539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC). Estimated HIV Incidence in the United States, 2007–2010. 2012. [Google Scholar]
- 5.Schwartz EJ, Szczech LA, Ross MJ, et al. Highly active antiretroviral therapy and the epidemic of HIV+ end-stage renal disease. J Am Soc Nephrol. 2005;16:2412–2420. [DOI] [PubMed] [Google Scholar]
- 6.Wyatt CM, Winston JA, Malvestutto CD, et al. Chronic kidney disease in HIV infection: an urban epidemic. AIDS. 2007;21:2101–2103. [DOI] [PubMed] [Google Scholar]
- 7.Linley L, Prejean J, Qian A, et al. Racial/ethnic disparities in HIV diagnoses among persons aged 50 years and older in 37 US States, 2005-2008. Am J Public Health. 2012;102:1527–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adekeye OA, Heiman HJ, Onyeabor OS, et al. The new invincibles: HIV screening among older adults in the U.S. PLoS One. 2012;7:e43618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee WA, He GX, Eisenberg E, et al. Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue. Antimicrob Agents Chemother. 2005;49:1898–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Rompay KK, Durand-Gasselin L, Brignolo LL, et al. Chronic administration of tenofovir to rhesus macaques from infancy through adulthood and pregnancy: summary of pharmacokinetics and biological and virological effects. Antimicrob Agents Chemother. 2008;52:3144–3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fux CA, Simcock M, Wolbers M, et al. Tenofovir use is associated with a reduction in calculated glomerular filtration rates in the Swiss HIV Cohort Study. Antivir Ther. 2007;12:1165–1173. [PubMed] [Google Scholar]
- 12.Goicoechea M, Liu S, Best B, et al. Greater tenofovir-associated renal function decline with protease inhibitor-based versus nonnucleoside reverse-transcriptase inhibitor-based therapy. J Infect Dis. 2008;197:102–108. [DOI] [PubMed] [Google Scholar]
- 13.Kiser JJ, Carten ML, Aquilante CL, et al. The effect of lopinavir/ritonavir on the renal clearance of tenofovir in HIV-infected patients. Clin Pharmacol Ther. 2008;83:265–272. [DOI] [PubMed] [Google Scholar]
- 14.Sax PE, Wohl D, Yin MT, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet. 2015;385:2606–2615. [DOI] [PubMed] [Google Scholar]
- 15.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilead Sciences. Stribild (elvitegravir, cobicistat, emtricitabine, tenofovir disoproxil fumarate) US prescribing information. Available at: http://www.gilead.com/pdf/stribild_pi.pdf. Accessed December 28, 2015.
- 17.Gilead Sciences. Emtriva (emtricitabine) US prescribing information. Available at: http://www.gilead.com/pdf/emtriva_pi.pdf. Accessed December 28, 2015.
- 18.Gilead Sciences. Atripla (efavirenz, emtricitabine, tenofovir disoproxil fumarate) US prescribing information. Available at: http://www.gilead.com/pdf/atripla_pi.pdf. Accessed December 28, 2015.
- 19.Cooper RD, Wiebe N, Smith N, et al. Systematic review and meta-analysis: renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin Infect Dis. 2010;51:496–505. [DOI] [PubMed] [Google Scholar]
- 20.Scherzer R, Estrella M, Li Y, et al. Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS. 2012;26:867–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morlat P, Vivot A, Vandenhende MA, et al. , the Groupe D'epidemiologie Clinique du Sida en Aquitaine (Gecsa). Role of traditional risk factors and antiretroviral drugs in the incidence of chronic kidney disease, ANRS CO3 Aquitaine cohort, France, 2004–2012. PLoS One. 2013;8:e66223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chronic Kidney Disease Prognosis Consortium, Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gansevoort RT, Matsushita K, van der Velde M, et al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 2011;80:93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79:1341–1352. [DOI] [PubMed] [Google Scholar]
- 25.Lucas GM, Ross MJ, Stock PG, et al. Clinical practice guideline for the management of chronic kidney disease in patients infected with HIV: 2014 update by the HIV medicine Association of the infectious diseases Society of America. Clin Infect Dis. 2014;59:e96–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.KDIGO Guideline Development Staff. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013;84:622–623. v-150. [DOI] [PubMed] [Google Scholar]
- 27.Eknoyan G, Hostetter T, Bakris GL, et al. Proteinuria and other markers of chronic kidney disease: a position statement of the national kidney foundation (NKF) and the national institute of diabetes and digestive and kidney diseases (NIDDK). Am J Kidney Dis. 2003;42:617–622. [DOI] [PubMed] [Google Scholar]
- 28.Billington S, Chung G, Brown C. Tenofovir abolishes Na+-dependent phosphate uptake in human proximal tubule cell monolayers. American Association of Pharmaceutical Scientists Annual Meeting and Exposition, 2014. Abstract R6328. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.