Abstract
Individual differences in acute alcohol effects on cognitive control and subjective responses—and acute tolerance to these effects—are implicated in the risk for heavy drinking and alcohol-related harms. Few studies have examined these effects in drinkers under age 21. Additionally, studies of acute tolerance typically involve bolus oral alcohol administration, such that estimates of tolerance are confounded with blood alcohol concentration (BAC) limb. The current study examined cognitive control and subjective responses in young heavy drinkers (n = 88, M = 19.8 years old [SD = 0.8]) during a single-session alcohol clamp protocol. Participants completed an intravenous alcohol session comprising an ascending limb (0 to 80mg% in 20 minutes) and a BAC plateau (80mg% for 80 minutes). Serial assessments included a cued go/no-go task and measures of stimulation, sedation and craving. Relevant individual difference factors (ADHD symptoms and sensation seeking) were examined as moderators. Multi-level modeling demonstrated that response inhibition worsened following initial rise in BAC and showed increasing impairment during the BAC plateau. ADHD symptoms and sensation seeking moderated this effect. Significant within-person associations between stimulation and craving were evident on the ascending limb only. Participants with higher ADHD symptoms reported steeper increases in stimulation during the ascending limb. These findings provide initial information about subjective and behavioral responses during pseudo-constant BAC, and potential moderators of these outcomes, in late adolescence. Additional studies with placebo-controlled designs are necessary to confirm these findings.
Keywords: Adolescent, alcohol sensitivity, attention deficit hyperactivity disorder, impulsivity, intravenous alcohol
Individual differences in behavioral and subjective responses to alcohol are implicated in the risk for alcohol use disorders (AUD) and alcohol-related harms (King, de Wit, McNamara, & Cao, 2011; Morean & Corbin, 2010; Quinn & Fromme, 2011). Of the wide range of outcomes studied in alcohol challenge studies to date (Zoethout, Delgado, Ippel, Dahan, & van Gerven, 2011), cognitive control processes (Field, Wiers, Christiansen, Fillmore, & Verster, 2010) and subjective effects of alcohol (Morean & Corbin, 2010; Ray, Mackillop, & Monti, 2010) have particular relevance for current theoretical models. Acute alcohol effects on executive function figure prominently in cognitive theories of AUD (Field, et al., 2010; Wiers et al., 2007). In particular, acute impairment of cognitive control (including inhibitory control, or the ability to inhibit a prepotent response) is relevant for understanding liability for AUD and intoxicated risk behaviors (Amlung, Morris, & McCarthy, 2014; Weafer & Fillmore, 2008). A separate literature on subjective responses to alcohol has demonstrated that greater perceived stimulant effects and lower sedative effects during intoxication correlate with increased risk for AUD, although theoretical models differ as to the relative emphasis on stimulant vs. sedative effects and the specificity of these effects to blood alcohol concentration (BAC) limb (King, et al., 2011; Morean & Corbin, 2010, Newlin & Thomson, 1990; Schuckit & Smith, 2000).
An important caveat of this literature is that the vast majority of alcohol administration studies are restricted to adult drinkers ages 21 years and older (Miranda et al., 2014; Morean & Corbin, 2010). Although ethical considerations preclude alcohol administration research with young adolescents (for one exception see Behar et al., 1983), the resultant knowledge gaps in the human literature on alcohol responses are noteworthy. For instance, findings from animal models are persuasive in demonstrating variation in acute alcohol responses across development, raising implications for understanding age differences in the risk for alcohol-related risks in humans (Spear & Varlinskaya, 2010). Relative to adults, adolescent animals typically show diminished sensitivity to acute and post-consumptive aversive effects of alcohol, coupled with greater sensitivity to its social-facilitative effects (for review see Spear, 2011; Spear & Varlinskaya, 2005; 2010). These findings, presumed to reflect ontogenetic differences in structural and functional brain maturation, are relevant for understanding high rates of alcohol consumption in human adolescence (Spear & Varlinskaya, 2010), which is generally defined as the second decade of life (Cicchetti & Toth, 1996; Lerner & Steinberg, 2004). In particular, late adolescence (e.g., age 16-20, Brown et al., 2008) is associated with escalations in heavy episodic drinking, with AUD onset peaking around 18-20 years (Brown, et al., 2008; Li, Hewitt, & Grant, 2004). Human laboratory data on subjective alcohol effects in this age range are scarce. Notably, the first study to examine adolescents’ subjective responses to alcohol in the natural environment found unique patterns of subjective effects in adolescent versus adults (Miranda et al., 2014).
Individual differences in acute tolerance to alcohol are also relevant for understanding developmental aspects of alcohol sensitivity (Spear & Varlinskaya, 2005) and the risk for alcohol-related harms (Fillmore, Marczinski, & Bowman, 2005; Martin & Moss, 1993; Radlow, 1994). Acute tolerance (or the “Mellanby effect”) refers to short-term, compensatory adaptations that occur in a single session of drug exposure, independent of changes in BAC (Martin & Moss, 1993; Morzorati, Ramchandani, Flury, Li, & O'Connor, 2002). Following initial animal research (Mellanby, 1919), acute tolerance has been noted across a range of response domains in human studies (Cromer, Cromer, Maruff, & Snyder, 2010; Morzorati, et al., 2002; Schweizer & Vogel-Sprott, 2008). One important finding is that measures of subjective intoxication, simple reaction time, and motor coordination often show within-session recovery, whereas impairments on cognitive measures of inhibitory control often show slower or no recovery (e.g., Fillmore, et al., 2005; Fillmore & Weafer, 2012; Miller & Fillmore, 2014; Ostling & Fillmore, 2010; Schweizer & Vogel-Sprott, 2008), which raises potential clinical implications. Specifically, ongoing impairments in the ability to inhibit behavior—coupled with recovery in subjective intoxication and the ability to activate behavior—could lead to increased risks, particularly in the later stages of a drinking episode (Amlung, et al., 2014; Weafer & Fillmore, 2012). Studying patterns of acute tolerance is therefore relevant for understanding intoxicated risk behavior, including ability to terminate a heavy drinking episode once initiated (Marczinski, Combs, & Fillmore, 2007; Schweizer & Vogel-Sprott, 2008).
Efforts to study individual variation in subjective and behavioral responses to alcohol have focused largely on family history and heavy drinking status as predictors (Newlin & Renton, 2010; Quinn & Fromme, 2011). However, traits related to behavioral undercontrol have also been associated with differences in acute alcohol effects. Higher scores on measures of sensation seeking and impulsivity have been linked to greater self-reported stimulant effects of alcohol (e.g., Erblich & Earleywine, 2003; Fillmore, Ostling, Martin, & Kelly, 2009; Leeman et al., 2014; Scott & Corbin, 2014), and poorer response inhibition during intoxication (Fillmore et al. 2009). Similarly, attention deficit-hyperactivity disorder (ADHD) has been implicated in acute alcohol responses and the risk for AUD (Shirley & Sirocco, 2014). Relative to controls, participants with ADHD showed greater alcohol-induced impairment of response inhibition (Weafer, Fillmore, & Milich, 2009) and motor coordination (Roberts, Milich, & Fillmore, 2013). With respect to acute tolerance, those with ADHD showed slower recovery of motor coordination relative to controls (despite similar tolerance to subjective effects) on the descending limb (Roberts, et al., 2013). Notably, both ADHD and personality correlates of behavioral undercontrol denote liability for externalizing spectrum disorders (Iacono, Malone, & McGue, 2008), suggesting that externalizing traits are relevant for studying individual differences in alcohol response. Few studies have examined externalizing traits as moderators of acute tolerance effects (Fillmore, et al., 2009; Roberts, et al., 2013).
An important limitation of most acute tolerance research is the reliance on bolus oral alcohol administration procedures. In this context, tolerance is inferred by comparing performance at equivalent BAC levels on the ascending and descending limbs following a single oral dose (although other methods have been reported; Kaplan, Sellers, Hamilton, Naranjo, & Dorian, 1985; Martin & Moss, 1993; Morzorati, et al., 2002). A consequence of this approach is that assessments of acute tolerance assessments are confounded with BAC limb (Martin & Moss, 1993). Interpreting these results can be difficult, especially given substantial evidence for limb effects on subjective and behavioral responses. An additional complication is the differential rate of BAC change across limb after oral ingestion (the rate of change being faster on the ascending limb). For instance, differences in BAC slope on the ascending limb were found to predict the extent of behavioral impairment during intoxication, suggesting that acute tolerance may be slowed or diminished in the context of faster BAC increase (Fillmore & Vogel-Sprott, 1998).
Ensuring control over BAC level, limb and rate of change is difficult in oral alcohol paradigms due to significant between-subjects variation in pharmacokinetics, resulting in high variability in the BAC time course. Alcohol clamp paradigms (O'Connor, Morzorati, Christian, & Li, 1998; Ramchandani, Bolane, Li, & O'Connor, 1999) use intravenous alcohol administration to circumvent these sources of variability, allowing much improved control over BAC profiles. Importantly, clamping (i.e., imposing pseudo-constant BAC) allows assessment of acute tolerance as a function of time and cumulative exposure, absent directional changes in BAC, providing a strong platform for characterizing acute tolerance effects (Morzorati, et al., 2002; Ramchandani et al., 1999). Another important consideration is that pharmacokinetic profiles in bolus dosing paradigms can differ substantially from those observed in typical heavy drinking scenarios, which will often involve persistent elevations in BAC. From this standpoint, studying alcohol responses while BAC remains elevated could help to clarify psychopharmacological effects that might emerge in natural settings (Rose et al., 2010).
The current study investigated cognitive control and subjective responses in the context of an alcohol clamp paradigm. The first aim was to characterize within-person changes and acute tolerance on these outcomes over two discrete periods of alcohol exposure (ascending limb and pseudo-constant BAC). A second aim was to examine relevant individual difference factors by evaluating externalizing traits (ADHD symptoms and sensation seeking) as moderators. A final aim was to examine within-person associations between subjective effects and craving across these intervals. By focusing on a younger sample (mean age < 20 years) relative to most previous work, this study also represents a step toward characterizing cognitive and subjective responses to alcohol during a relatively narrow period during late adolescence (Brown, et al., 2008).
Method
Participants
The sample consisted of 88 participants (mean age: 19.81 years, SD = 0.81, 46 women). Community recruitment consisted primarily of Internet advertisements that targeted social drinkers for participation in laboratory research on the behavioral effects of alcohol. Participants were ages 19 (the legal drinking age in Ontario, Canada), 20, or 21 years old, with recruitment efforts focusing predominantly on participants 19 or 20 years of age (76% of the sample). Additional eligibility criteria included: at least one heavy drinking episode (4+ drinks for women/5+ drinks for men) in the past month, no current psychiatric medications or diagnoses requiring treatment, no recent illicit drug use except cannabis, no current mediation use or medical conditions for which alcohol would be contraindicated, and no history of difficulty with intravenous protocols (e.g., fainting, nausea). Participants were also required to have a Brief Michigan Alcohol Screening Test (MAST; Pokorny, Miller, & Kaplan, 1972) score <10, with no history of treatment for alcohol use or current attempts to reduce drinking, and a Fagerstrom Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991) score <6. Participant characteristics, separated by sex, are shown in Table 1.
Table 1.
Participant characteristics separated by sex.
| Women (n=46) | Men (n=42) | ||||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | χ2 | df | p | |
| Race | |||||||
| White | 29 | 63 | 27 | 64 | 0.02a | 1 | .90 |
| Non-White | 17 | 37 | 15 | 36 | |||
| Black/African | 3 | 7 | 3 | 7 | |||
| Asian | 2 | 4 | 6 | 14 | |||
| East Indian | 3 | 7 | 2 | 5 | |||
| Multiracial/Other | 9 | 20 | 4 | 10 | |||
| Current Cigarette Smoker | |||||||
| No | 34 | 74 | 32 | 76 | 0.06 | 1 | .81 |
| Yes | 12 | 26 | 10 | 24 | |||
| Cannabis Use – Past 90 Days | |||||||
| No | 13 | 28 | 10 | 24 | 0.23 | 1 | .64 |
| Yes | 33 | 72 | 32 | 76 | |||
| M | SD | M | SD | t | df | p | |
|---|---|---|---|---|---|---|---|
| Age | 19.89 | 0.88 | 19.71 | 0.74 | 1.02 | 86 | .31 |
| Past 90 Day Drinking Frequency | 21.54 | 10.12 | 21.00 | 12.91 | 0.22 | 86 | .83 |
| Drinks Per Drinking Day | 4.83 | 1.95 | 5.68 | 2.04 | −2.01* | 86 | .05 |
| Heavy Episodic Drinking Frequency | 15.00 | 11.08 | 11.55 | 10.06 | 1.53 | 86 | .13 |
| AUDIT Score | 11.09 | 6.15 | 10.05 | 3.90 | 0.94 | 86 | .35 |
| ADHD Symptom Count | 5.30 | 3.44 | 5.62 | 3.79 | −0.41 | 86 | .68 |
| Sensation Seeking | 6.65 | 2.41 | 8.14 | 2.50 | −2.84** | 86 | .01 |
Note.
Test for sex differences performed on dichotomous race variable (i.e., white vs. non-white). AUDIT=Alcohol Use Disorder Identification Test.
Procedures
Participants completed a telephone screening to determine basic eligibility criteria, followed by an in-person visit that included informed consent, completion of self-report measures via computer, and a 90-day Timeline Follow Back (TLFB) assessment conducted by a trained interviewer. Data on individual difference variables were collected at this visit. A medical screen was conducted to confirm eligibility for the intravenous alcohol infusion, as verified by the study physician. Participants who remained eligible were invited to complete subsequent alcohol administration sessions as part of a prospective study. The data presented here are derived from participants’ first laboratory session, one aim of which was to ensure tolerability of intravenous alcohol administration before proceeding further in the study.
Participants were asked to abstain from eating, consuming caffeine, or using nicotine during the four hours prior to their appointment, and to refrain from alcohol or psychoactive drugs for at least 24-hours prior to the session. All infusion sessions took place in a hospital setting under medical supervision. Upon arrival participants were verified as negative for recreational drugs (excluding cannabis) via urine toxicology screen, and female participants completed a pregnancy screening. Participants also provided a breath alcohol reading (all tests were negative) and consumed a standard low calorie meal. Participants were then escorted to a private room and seated in a recliner chair, where the study nurse placed an indwelling catheter. Immediately prior to the start of the infusion, participants completed baseline subjective and cognitive assessments (see Measures). Alcohol administration consisted of an infusion of 6% (v/v) ethanol in normal saline using an alcohol clamp paradigm with physiologically-based pharmacokinetic (PBPK) modeling (O'Connor, et al., 1998; Ramchandani, et al., 1999). The model stipulated a linear ascent in BAC from 0 to 80mg% in exactly 20 minutes, after which BAC was held pseudo-constant (clamped) at 80mg% for another 80 minutes.
The experimental timeline is depicted in Figure 1. A cued go/no-go task (described below) was administered at baseline (G1) and at two time points during the BAC plateau period: at approximately 40 minutes (G2, M = 40.35, SD = 3.81 minutes after the start of the infusion) and 90 minutes (G3, M=89.46, SD=3.26). Subjective questionnaires were administered baseline (S1) and at 5 time points during the infusion: twice during the ascending limb (S2, M = 9.03, SD=0.97; S3, M = 20.46, SD = 1.04), and three times during the plateau: S4 (M = 56.44, SD = 2.80), S5 (M = 72.87, SD = 2.87), and S6 (M = 96.89, SD = 3.92). Serial breath alcohol concentration (BrAC) readings were obtained every 4 minutes on the ascending limb and regularly thereafter, including prior to each go/no-go task subjective questionnaire assessment. Readings were entered back into the PBPK model, allowing online adjustment of the infusion profile. Shortly after 100 minutes the nurse removed the catheter. Participants were escorted to a private room for a monitored recovery period and remained in the lab until BAC fell below 30mg%, at which point they were provided with compensation and public transportation tokens.
Figure 1.
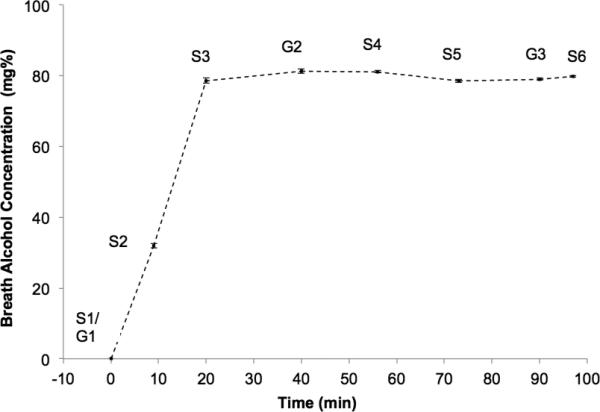
Observed breath alcohol concentrations (BrACs) prior to each administration of the go/no-go task and subjective questionnaires during the alcohol infusion. G= go/no-go time point; S=Subjective questionnaire time point. Standard errors shown in error bars.
Materials and Measures
Cued go/no-go task
A cued go/no-go task was used to measure inhibitory control and response activation, as defined below. This task has been used extensively in alcohol administration studies (e.g., Fillmore, et al., 2005; Miller & Fillmore, 2014). Participants are instructed to press a key when a go target is presented, and to withhold their response when a nogo target is presented. Each trial consists of a cue followed by a target. The cue is intended to establish a prepotent response set based on anticipated target type, with the ensuing target indicating the response required (go or no-go). Specifically, at the beginning of each trial, the cue (a rectangle oriented either vertically or horizontally) signaled that a go target (a green rectangle) or no-go target (a blue rectangle) was likely to follow. The preceding cue correctly predicted the following target stimulus on 80% of trials, thereby establishing the prepotent response set. Each run consisted of 125 trials, with each trial consisting of the following sequence of events: (a) fixation point (+) for 800 milliseconds (ms); (b) a blank white screen for 500 ms.; a cue (displayed for a variable interval of 100, 200, 300, 400, or 500 ms); (d) a target (Go or NoGo), which remained visible until the participant made a response or until 1000 ms had elapsed. The inter-trial interval was 700 ms. The primary outcomes of interest were a) the proportion of incongruent inhibition trials (i.e., NoGo trials preceded by a Go cue) for which participants failed to inhibit the response (i.e., response inhibition failures), and b) mean reaction time to Go targets preceded by Go cues (i.e., response activation). Trials on which participants’ response time was less than 100 ms were excluded (less than 2% of trials).
Subjective questionnaires
Subjective response to alcohol was measured using the Biphasic Alcohol Effects Scale (BAES; Martin, Earleywine, Musty, Perrine, & Swift, 1993), which contains 7 items assessing subjective stimulation (e.g., “energized,” “high”) and 7 items assessing subjective sedation (e.g., “drowsy,” “tired”). Participants responded on a visual analogue scale (0-100) to indicate to what extent they were currently experiencing each effect; mean responses for each subscale at each of the 6 time points were used for analyses. In order to facilitate model estimation, values were rescaled to a 0-10 scale by dividing each variable by a constant (i.e., 10). Adequate internal reliability for each scale was observed across all time points (Cronbach's alphas ranged from 0.83 to 0.90 for stimulation and 0.75 to 0.86 for sedation).
Alcohol urge questionnaire (AUQ)
Craving was assessed using the 8-item AUQ (Bohn, Krahn, & Staehler, 1995). Participants responded to each item using a 0-100 scale, and the mean of the items was used in the analyses. Values were rescaled to a 0-10 scale. Cronbach's alphas indicated adequate internal reliability across the 6 timepoints (.79 to .89).
Timeline Followback (TLFB)
Drinking variables were derived from the TLFB (Sobell & Sobell, 1992), a structured calendar assessment of recent substance use. Past 90 day drinking frequency was calculated as the total number of days on which any alcohol use was reported. Typical quantity of alcohol consumed was indexed based on average drinks per drinking day.
Alcohol Use Disorders Identification Test (AUDIT)
The AUDIT (Babor, Higgins-Biddle, Saunders, & Monteiro, 2001) is a 10-item measure assessing hazardous drinking. Items cover quantity/frequency (3 items), dependence (3 items) and alcohol consequences (4 items).
The World Health Organization Adult ADHD Self-Report Scale (ASRS)
The ASRS (Kessler et al., 2005) is a self-report measure comprised of 18 questions designed to operationalize the DSM-IV Criterion A symptoms for attention deficit hyperactivity disorder. Participants were required to indicate how often each of the symptoms occurred within the last 6 months, with responses ranging from 0 = never to 4 = very often. Based on previously validated clinical cutoffs (Kessler, et al., 2005), responses to each item were dichotomized to code whether the symptom was present (1) or absent (0). Consistent with scoring guidelines provided by Kessler et al. (2005), these dichotomized items were summed to produce a total ADHD symptom count for each participant. To maximize power, we analyzed this symptom count as a continuous variable rather than categorizing participants into diagnostic groups based on cut off values. Cronbach's alpha in this sample was 0.79.
Sensation Seeking
Sensation seeking was assessed with the impulsive sensation seeking scale (ImpSS) of the Zuckerman-Kuhlman Personality Questionnaire (Zuckerman, 1993). The sensation seeking subscale was selected based on evidence that sensation seeking is associated with acute responses to alcohol and performance on the cued go/no-go task (M. Fillmore, et al., 2009). Participants indicated whether each trait descriptor was true or false for them. These items were summed to produce a total sensation seeking score (Cronbach's alpha = .73).
Data Analysis Plan
We first examined descriptive statistics and bivariate associations. All variables reasonably approximated univariate normal distributions (skewness ≤ 1.02 and kurtosis ≤ .70). One or two extreme outliers were observed on several of the variables; these outliers were recoded to one unit greater than the next most extreme value to reduce their influence (Tabachnick & Fidell, 2007). Five participants were excluded from the go/no-go task analyses because of a preponderance of missing data or extreme responses that suggested they did not follow task instructions. An additional four participants were missing go/no-go data at the final assessment point, but these participants were retained in analyses through the use of multilevel modeling (MLM). Thus, the sample size for the go/no-go models was n = 83. Also, 8 participants were missing data for at least one time point on the subjective questionnaires (no participant was missing more than half of the time points). We therefore retained all 88 participants in the subjective response analyses. Overall, missing data points for the repeated-measures analyses on go/no-go task and subjective response items were minimal (<1%).
We used MLM to examine the within-person changes in go/no-go task performance and subjective responses as a function of assessment time point during the alcohol infusion (within-subjects effect) as well as individual difference in ADHD symptoms and sensation seeking (between-subjects effect). MLM is well suited to examining the nature of within-person changes in repeated-measures data over time, as well as individual differences in these changes as a function of between-person predictors.
We first examined the main effects of time point on within-person changes in go/no-gotask performance (inhibition failures, response activation) and subjective variables (stimulation, sedation, and craving). Separate MLM models were specified for each outcome. Given that BAC was raised from 0 to 80mg% during the first 20 minutes of the session then maintained at 80mg% for the remainder of the session, we expected a discontinuous effect of time on our outcomes. For go/no-go task performance, we examined the within-person effect of time point using two orthogonal contrasts comparing baseline (G1) to the 40 minute time point (G2) and 40 min to 90 minutes (G2 vs. G3). Because we had more observations for the subjective response variables, in these analyses the effect of time point was modeled as a piecewise linear effect with the first 3 subjective assessments (baseline, 10 minutes, and 20 minutes; i.e., S1, S2, and S3) representing the linear effect of time on the ascending limb, and the subsequent 3 time points (56 minutes, 73 minutes, and 97 minutes; i.e., S4, S5, S6) representing the linear effect of time during the plateau period. S3 was specified as the “knot” joining the two linear time effects. Also, both random intercepts and slopes were specified for the time effects.1
We also examined within-person associations between subjective responses and craving using MLM. Craving scores at each of the 6 time points were entered as the dependent variable, and subjective stimulant and sedative effects were entered simultaneously as predictors. In order to isolate within-person changes, the subjective stimulation and sedation scores were person-centered (i.e., each value was centered around that participant's mean stimulation or sedation score) and mean stimulation and sedation scores across the 6 time points (grand-mean centered) also were entered in the model to control for between-person variation. To evaluate whether these within-person associations depended on limb of the BAC curve, a dummy coded variable (representing ascending limb vs. plateau) was entered into the model, along with its interactions with the person-centered stimulation and sedation scores. An unstructured covariance matrix for the random effects was specified, including estimates for the random intercept and random slopes for the effect of limb and the within-person changes in stimulation and sedation.
We next examined whether the within-person changes in the go/no-go and subjective response variables depended on individual differences in ADHD symptoms and sensation seeking. Interactions between the individual difference variables (which were standardized across participants) and within-person time point effects were entered into the models. Significant interactions suggest that the change in the outcome during the session depends on levels of the between-person variable (i.e., ADHD symptoms, sensation seeking). To simplify interpretation of the conditional effects of time, ADHD symptoms and sensation seeking were tested in separate models. To explore sex differences, a parallel analysis examined sex as a moderator of changes during the session on all outcomes. Significant interactions were probed using simple slopes analysis to describe the within-person changes in the outcome at high and low levels of ADHD or sensation seeking (i.e., 1 SD above and below the mean). This was accomplished by re-centering the between-person moderator and re-running the model to obtain conditional slopes for the effects of time. The simple slopes were plotted to facilitate interpretation. We followed up these analyses with a model that included both ADHD symptoms and sensation seeking together to confirm whether each factor was a unique moderator of the within-person effects of time point while controlling for their shared variance.
Results
Descriptive Analyses
Figure 1 shows the observed mean BrAC readings prior to each administration of the go/no-go task and subjective questionnaires. At the 20 min mark, observed BrAC was 78.62 mg% (SD = 7.10). Figure 2 shows the observed means and standard errors for the go/no-go and subjective variables. The repeated-measures variables were all moderately to strongly autocorrelated (rs = .39-.92). ADHD and sensation seeking scale scores were moderately correlated (r=.39, p<.001).
Figure 2.
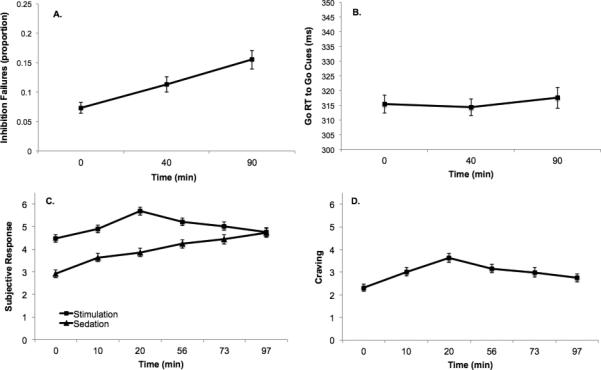
Main effects of time point on (a) inhibition failures (proportion) on the go/no-go task, (b) Go reaction time (RT) to go cues in ms (i.e., Response Activation), (c) subjective stimulant and sedative effects, and (d) craving. Markers and error bars represent observed means and standard errors. Subjective questionnaire scores were re-scaled to a 0-10 scale for analysis. G= Go/no-go time point; S=Subjective questionnaire time point. Times (mins) in parentheses on the x-axis represent approximate time elapsed since the start of the infusion.
Main Effects of Time Point
There was no significant within-person effect of time on response activation between G1 and G2 (B = −1.05, SE = 2.27, p =.645) or between G2 and G3 (B = 2.76, SE = 2.78, p =.324). In contrast, there was a significant within-person increase in inhibition failures between both G1 and G2 (B = .040, SE = .011, p = .001) and between G2 and G3 (B = .043, SE = .011, p < .001), indicating that impairments in inhibitory control continued to increase throughout the session (see Figure 2 for means). There was also evidence for differential effects of time on the subjective response variables in the MLM models (see Figure 2). While stimulation (B = 0.58, SE = .09, p < .001), sedation (B = 0.48, SE = 0.10, p < .001), and craving (B = 0.61, SE = 0.08, p < .001) all increased significantly during the ascending limb (S1 to S3), there were differential changes in these subjective effects during the plateau interval (S3 to S6). Specifically, both craving (B = −0.28, SE = 0.04, p < .001) and stimulation declined significantly (B = −0.29, SE = 0.06, p < .001), consistent with acute tolerance during the BAC plateau. Sedation continued to increase significantly, consistent with acute sensitization (B = 0.27, SE = 0.06, p < .001).
Within-Person Associations Among Subjective Variables
We next examined the MLM model of the within-person associations between stimulant and sedative effects and craving. The interaction between within-person changes in stimulation and BAC limb was significant (B = −0.27, SE = 0.10, p = .008), suggesting that within-person associations between stimulation and craving differed on the ascending limb vs. the BAC plateau. Simple slopes analyses revealed that within-person increases in stimulation were associated with increased craving on the ascending limb (B = 0.35, SE = 0.06, p < .001), but not during the BAC plateau interval (B = 0.08, SE = 0.09, p = .375). However, within-person changes in sedation did not interact significantly with time (B = −0.09, SE = 0.09, p = .30). After removing this interaction to facilitate interpretation of the main effect, it was found that within-person increases in sedation were not uniquely associated with increased craving (B = 0.08, SE = 0.05, p = .081). Finally, we also observed a significant association between individual differences in mean stimulation across all assessments and overall craving (B = 0.33, SE = 0.11, p = .002); no such association was observed for mean sedation (B = 0.14, SE = 0.10, p = .15).
Associations with Individual Differences
Response activation and inhibition
Table 2 shows the results of the MLM models including individual differences in ADHD and sensation seeking as predictors of go/no-go task variables. ADHD symptoms interacted significantly with the effect of time point during the plateau interval (i.e., G2 vs. G3), but not during the ascending limb (G1 vs. G2) in predicting inhibition failures (see Table 2). Sensation seeking showed a similar moderating effect on the within-person changes in inhibition failures; changes in inhibition failures between G2 and G3 (but not between G1 and G2) were dependent on sensation seeking. Neither ADHD symptoms nor sensation seeking interacted with time point in predicting response activation.
Table 2.
Multilevel models (MLM) examining ADHD symptoms and sensation seeking as predictors of go/no-go task performance during the alcohol infusion.
| Go/No Go Task | ||||||||
|---|---|---|---|---|---|---|---|---|
| Inhibition Failures (proportion) | Go RT to Go Cues (ms) | |||||||
| B | SE | t | p | B | SE | t | p | |
| ADHD Model | ||||||||
| Time (G1 vs. G2) | .040** | .012 | 3.44 | .001 | −1.08 | 2.29 | −0.47 | .639 |
| Time (G2 vs. G3) | .044** | .010 | 4.28 | .000 | 2.81 | 2.83 | 0.99 | .324 |
| ADHD | .001 | .009 | 0.01 | .993 | −3.94 | 2.92 | −1.35 | .180 |
| ADHD*Time (G1 vs. G2) | −.008 | .012 | −0.73 | .469 | −1.62 | 2.32 | −0.70 | .488 |
| ADHD* Time (G2 vs. G3) | .032** | .011 | 3.03 | .003 | 1.56 | 2.91 | 0.54 | .594 |
| SS Model | ||||||||
| Time (G1 vs. G2) | .040** | .012 | 3.43 | .001 | −1.04 | 2.29 | −0.45 | .653 |
| Time (G2 vs. G3) | .043** | .010 | 4.18 | .000 | 2.73 | 2.81 | 0.97 | .334 |
| SS | .008 | .009 | 0.91 | .364 | −0.36 | 2.96 | −0.12 | .903 |
| SS*Time (G1 vs. G2) | −.009 | .012 | −0.79 | .433 | 0.73 | 2.32 | 0.31 | .754 |
| SS*Time (G2 vs. G3) | .031** | .010 | 2.97 | .004 | −1.37 | 2.82 | −0.49 | .628 |
Note. G1=baseline; G2= approximately 40 min after the start of the alcohol infusion; G3 = approximately 90 min after the start of the infusion; ADHD=ADHD symptom count; SS = sensation seeking;
p<.05
p<.01.
Figure 3 shows the results of the simple slopes analyses. While inhibition failures increased significantly between G1 and G2 for both ADHD groups, inhibition failures continued to increase between G2 and G3 for participants high (1 SD above the mean) on ADHD symptoms (B = .077, SE = .015, p < .001), but not for participants low (1 SD below the mean) on ADHD symptoms (B = .012, SE = .014, p = .409). The same pattern was observed for sensation-seeking, with both high and low sensation-seekers showing initial increases in inhibition failures, but only high sensation seekers showing continued increases in inhibition failures (B = .074, SE = .015, p < .001) relative to low sensation seekers (B = .012, SE = .014, p = .395).
Figure 3.
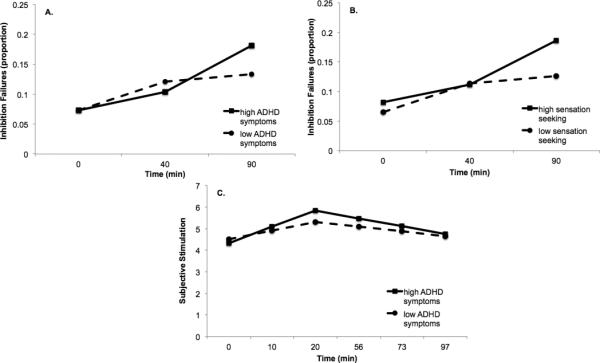
Simple slopes for changes in inhibition failures conditioned on high (1SD above mean) and low (1SD below mean) levels of (a) ADHD symptoms and (b) sensation seeking, and simple slopes for changes in subjective stimulation conditioned on high and low levels of ADHD symptoms (c). Subjective stimulation scores were re-scaled to a 0-10 scale for analysis.
Given that sensation seeking and ADHD symptoms were significantly correlated, we conducted follow-up analyses including both variables in the same model. When accounting for the shared variance, the unique moderating impact of each variable on within-person changes in inhibition failures between G2 and G3 was slightly weaker (ADHD symptom by time interaction: B = .022, SE = .012, p = .063; sensation seeking by time interaction: B = .021, SE = .012, p = .077). However, given that both interactions remained marginally significant, it appears that both ADHD and sensation seeking had some degree of independent impact on inhibition failures during the BAC plateau.
Subjective responses
Table 3 shows the results of the MLM models examining ADHD symptoms and sensation seeking as moderators of the effects of time on subjective responses. ADHD symptoms moderated within-person changes in subjective stimulation during the ascending limb (S1 to S3), but not during the BAC plateau period (S3 to S6). Sensation seeking did not moderate the effects of time point on subjective stimulation. Moreover, neither ADHD symptoms nor sensation seeking moderated the effects of time point on subjective sedation or craving. Simple slopes analyses for subjective stimulation showed that participants high on ADHD symptoms showed a steeper increase in subjective stimulation on the ascending limb (B = 0.76, SE = 0.13, p < .001) than participants low on ADHD symptoms (B = 0.40, SE = 0.13, p = .002). Finally, a follow up analysis showed that ADHD symptoms remained a significant, unique moderator of the effects of time on subjective stimulation after controlling for the moderating effect of sensation seeking (ADHD symptom by time interaction: B = 0.24, SE = 0.10, p = .014).
Table 3.
Multilevel models (MLM) examining ADHD symptoms and sensation seeking as predictors of subjective response during the alcohol infusion.
| Self-Reported Subjective Response | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subjective Stimulation | Subjective Sedation | Craving | ||||||||||
| B | SE | t | p | B | SE | t | p | B | SE | t | p | |
| ADHD Model | ||||||||||||
| Time (S1 to S3) | 0.58** | 0.09 | 6.44 | .000 | 0.48** | 0.10 | 4.57 | .000 | 0.61** | 0.09 | 7.21 | .000 |
| Time (S3 to S6) | −0.29** | 0.06 | −5.35 | .000 | 0.27** | 0.06 | 4.43 | .000 | −0.27** | 0.04 | −6.17 | .000 |
| ADHD | −0.09 | 0.17 | −0.57 | .572 | −0.14 | 0.16 | −0.85 | .398 | 0.28 | 0.15 | 1.83 | .071 |
| ADHD*Time (S1 to S3) | 0.18* | 0.09 | 2.00 | .049 | 0.09 | 0.11 | 0.86 | .391 | −0.04 | 0.09 | −0.46 | .643 |
| ADHD*Time (S3 to S6) | −0.08 | 0.06 | −1.38 | .171 | −0.01 | 0.06 | −0.08 | .938 | −0.05 | 0.04 | −1.28 | .263 |
| SS Model | ||||||||||||
| Time (S1 to S3) | 0.58** | 0.09 | 6.31 | .000 | 0.48** | 0.10 | 4.56 | .000 | 0.61** | 0.08 | 7.23 | .000 |
| Time (S3 to S6) | −0.29** | 0.06 | −5.29 | .000 | 0.27** | 0.06 | 4.46 | .000 | −0.28** | 0.05 | −6.26 | .000 |
| SS | 0.32 | 0.16 | 1.98 | .051 | −0.21 | 0.16 | −1.30 | .196 | 0.36* | 0.15 | 2.34 | .021 |
| SS*Time (S1 to S3) | −0.06 | 0.09 | −0.69 | .490 | 0.06 | 0.11 | 0.56 | .578 | −0.08 | 0.09 | −0.94 | .349 |
| SS*Time (S3 to S6) | −0.01 | 0.06 | −0.05 | .962 | −0.07 | 0.06 | −1.08 | .283 | 0.01 | 0.05 | 0.01 | .991 |
Note. S1=baseline; S3= approximately 20 min after the start of the alcohol infusion; S6 = approximately 97 min after the start of the infusion; ADHD=ADHD symptom count; SS = sensation seeking;
p<.05
p<.01.
Sex
We examined whether changes in go/no-go task performance and subjective responses differed for men and women. Sex did not significantly interact with any time effects in any of the models (all p > .05). Moreover, controlling for the interactions between sex and time point did not impact the significance of any of the findings for ADHD or sensation seeking (all p > .05), suggesting that sex differences could not account for the results.
Discussion
This study investigated the time course of subjective responses and behavioral control during an extended alcohol administration session, also examining externalizing traits as moderators of these effects. The use of an alcohol clamp paradigm allowed examination of these effects in the absence of between-subjects differences in BAC level, limb, or rate of change. Results from the cued go/no-go task suggested impairments in response inhibition following alcohol administration, without evidence of acute recovery, consistent with prior studies using oral administration and a similar target BAC (Fillmore, et al., 2005; Ostling & Fillmore, 2010). While prior studies reported that impairments in response inhibition persisted (but did not worsen) during the descending limb of intoxication (e.g., Fillmore, et al., 2005; Fillmore & Weafer, 2012; Ostling & Fillmore, 2010), the current results suggested increases in impairment across the BAC plateau, consistent with acute sensitization (Morzorati, et al., 2002). Given the use of bolus oral administration in most studies, the possibility that inhibitory control shows acute sensitization when BAC remains elevated has not been investigated thoroughly. As with prior evidence suggesting a lack of acute tolerance on measures of response inhibition, the current evidence for acute sensitization during sustained BAC is potentially relevant for understanding intoxicated risk behavior. For instance, greater alcohol impairment of inhibitory control has been shown to predict higher alcohol self-administration and greater willingness to drink and drive on the descending limb (Weafer & Fillmore, 2008, 2012).
The present findings also inform knowledge about individual difference factors that moderate behavioral impairment during intoxication. Higher ADHD symptoms and sensation seeking scores predicted greater impairments in response inhibition, with this effect being specific to the BAC plateau. In research using the same go/no-go task, ADHD status predicted greater sensitivity to alcohol-related impairment of response inhibition relative to controls (Weafer, et al., 2009). Those with ADHD also showed greater acute impairment of motor control and slower acute recovery of motor control relative to controls, despite similar acute tolerance to perceived intoxication (Roberts, et al., 2013). Thus, the present findings are consistent with prior evidence implicating ADHD symptoms in acute responses to alcohol (Shirley & Sirocco, 2014). In similar research examining the role of sensation seeking, those with higher scores did not show differential impairment or recovery in response inhibition at either of two alcohol doses relative to placebo, instead showing greater impairment across beverage condition and ascending/descending limbs (Fillmore, et al., 2009). Notably, the moderating effect of sensation seeking in this study appeared independent of the moderating effect of ADHD. Overall, these results are consistent with the notion that externalizing traits might moderate acute alcohol effects, including impairments in response inhibition.
This study also sought to examine patterns of subjective responses (stimulation and sedation), and their association with craving, across ascending limb and BAC plateau intervals. Results implied acute tolerance to stimulant effects and acute sensitization to sedative effects during the BAC plateau. This pattern is consistent with two prior placebo-controlled studies that used alcohol clamp procedures with target BAC levels of 60mg% (Morzorati, et al., 2002), 40mg% and 100mg% (Kerfoot et al., 2013). Collectively, these and the current study indicate short-term adaptation to stimulant effects, but ongoing sensitization of sedative effects, during pseudo-constant BAC at various exposure levels. Notably, Morzorati et al. (2002) reported increases in sedative effects as long as three hours after establishing the BAC target. Also, these three studies each found significant increases in sedation during a short, controlled linear ascent to the target, underscoring that sedative effects emerge early in the course of acute alcohol exposure, rather than being specific to the descending limb.
Prior studies involving clamp paradigms have typically not modeled craving, a motivational index with central importance for the clinical phenomenology of addiction (e.g., Monti, Rohsenow, & Hutchison, 2000; Tiffany & Wray, 2012). Examining links between subjective responses and craving can clarify in-the-moment processes by which subjective responses correspond with consumption (Miranda, et al., 2014; Rose, et al., 2010). Although other alcohol challenge studies have reported associations of alcohol-induced stimulation with craving (e.g., Bujarski & Ray, 2014; Ray, et al., 2010; Rose, et al., 2010), the present study addressed this question by modeling two discrete intervals of the BAC trajectory. In addition, multi-level modeling evaluated within-person associations between subjective responses and craving over time. Increases in stimulation corresponded with increases in craving, but only as blood alcohol levels were rising. In contrast, changes in sedation did not relate to changes in craving. The differential association of stimulation and sedation with craving has potential clinical significance, warranting further study. Findings also showed that ADHD symptoms predicted steeper increases in stimulation, but only on the ascending limb. In contrast to prior studies of sensation seeking and related personality traits (e.g., Erblich & Earleywine, 2003; Fillmore, et al., 2009; Leeman, et al., 2014; Scott & Corbin, 2014), sensation seeking did not moderate changes in subjective stimulation. Because the association of subjective stimulant or sedative effects with motivational indicators (e.g., craving, wanting) may have etiological relevance (King, et al., 2011), further laboratory studies of these associations, including the examination of individual or pharmacological (e.g., BAC limb) moderators, is likely important.
A specific aim of this study was to examine these associations in participants within an age range that is typically excluded from alcohol administration research. Although the mean age of this sample is not drastically below the common cutoff of 21 years, we targeted a circumscribed window in late adolescence, which is uncommon in this literature. Given a dearth of laboratory research at this age, studying alcohol responses in this context could help to shed light on developmental processes relevant for individual differences in alcohol sensitivity. Notably, animal models not only support differences in responses to aversive and rewarding alcohol effects in adolescents versus adults, but also suggest developmental differences within adolescence, suggesting that ontogenetic differences may influence alcohol sensitivity continuously during adolescence (Spear & Varlinskaya, 2005). Consistent with animal findings, human adolescence is marked by particularly high rates of alcohol use, particularly in late adolescence, when peak incidence of AUD occurs (Brown, et al., 2008; Li, et al., 2004). The exclusion of this age group from most alcohol administration research necessarily limits a full developmental understanding of these processes (Miranda, et al., 2014; Morean & Corbin, 2010).
In the first study to examine adolescent responses to acute alcohol in the natural environment, Miranda and colleagues (2014) reported different patterns of subjective responses in adolescents (mean age: 18.3 years) relative to a comparison adult sample. Compared to adults, adolescents reported greater mean stimulation, particularly at the beginning of drinking episodes, but showed significant declines in stimulation as estimated BAC (eBAC) increased—a pattern absent among adults. Moreover, craving (but not stimulation or sedation) predicted event-level consumption in adolescents, and a significant correlation of stimulation with craving was limited to adolescents (Miranda, et al., 2014). However, changes in eBAC did not predict craving among adolescents. Using a somewhat older sample, the current study found the expected increases in stimulation and craving as BAC increased. Potential explanations for these differences include the disparate assessment contexts and the examination of a higher BAC range in the present study. Notably, the use of in vivo assessments in the former study revealed high levels of stimulation before BAC levels were appreciable, possibly reflecting stimulation from the social context, and perhaps explaining the decline in stimulation as BAC increased (Miranda, et al., 2014). In contrast, the use of intravenous alcohol in this study served to remove non-pharmacological cues. Future studies of in-the-moment associations of subjective effects, craving and self-administration, as well as age and contextual moderators, could further inform how acute subjective responses relate to risk for heavy drinking at different developmental stages.
The advantages of this study include the use of a behavioral task with extensive validation in alcohol administration studies and an experimental paradigm allowing exceptional pharmacokinetic control. Notably, because pharmacokinetic profiles resulting from single-dose bolus oral alcohol administration could differ substantially from those observed in real-world heavy drinking scenarios, laboratory studies involving extended alcohol administration can play a role in clarifying psychopharmacological effects as they might unfold in naturalistic episodes. Limitations of this study should also be considered—the major limitation being the lack of a placebo control condition. This design issue reflects the fact that the alcohol clamp sessions occurred in the context of a larger study, serving partly to ensure that participants could tolerate intravenous alcohol administration before proceeding further (Strang et al., 2015). Given the lack of a placebo control, the influence of expectancies or fatigue from participating in an extended alcohol infusion session, particularly in individuals with high ADHD scores, cannot be ruled out.
While the current results are subject to the important caveat that pharmacological effects were not separable from expectancy or time effects, previous placebo-controlled studies can provide additional context for interpreting the current results. As described above, the pattern of stimulant and sedative effects observed here is consistent with results from initial placebo-controlled studies involving clamp paradigms. Notably, these prior studies did not suggest appreciable increases in subjective effects over time in placebo conditions. Also, several prior studies using the cued go/no-go task reported no significant decrements in performance over time in the placebo condition (Fillmore, et al., 2009; Fillmore & Weafer, 2012; Miller & Fillmore, 2014; Ostling & Fillmore, 2010), nor did differences in sensation seeking predict changes in task performance over time (Fillmore, et al., 2009). Moreover, research with this task suggests compensatory behavioral responses under the expectation of alcohol (Marczinski & Fillmore, 2005), suggesting that the current design might represent a conservative test for detecting response inhibition impairments. Finally, current evidence for selective impairment on response inhibition (and not response activation) is consistent with reported alcohol effects (e.g., Fillmore, et al., 2009; Weafer, et al., 2009). Nonetheless, expectancy and other non-pharmacological effects cannot be ruled out in this study. Notably, expectancy effects have not been characterized in the laboratory at this age range, necessitating placebo-controlled studies.
Additionally, while the current paradigm involved an extended assessment, longer sessions would allow for more cognitive assessments, perhaps clarifying the extent to which impairments in response inhibition continue to worsen or improve over longer periods (Miller & Fillmore, 2014). An additional limitation was the lack of diagnostic confirmation of ADHD diagnoses or medication status. However, assessment of ADHD symptomatology as a continuous outcome was accomplished with a validated self-report scale. Finally, while the current study examined subjective and behavioral alcohol effects in a relatively young sample, it did not aim to compare results with older participants. Given evidence for age group differences in subjective responses (Miranda, et al., 2014) and cognitive performance (e.g., Acheson, Stein, & Swartzwelder, 1998) during intoxication, further research is needed to characterize developmental aspects of acute alcohol effects, including the comparison of cohorts in late adolescence versus adulthood.
Acknowledgements
This study was supported by a grant from ABMRF/The Foundation for Alcohol Research (CH). The funding agency had no role in the study other than financial support. The authors also acknowledge support from the Canadian Institutes of Health Research (grants 260418, 288905/307742), the Ontario Mental Health Foundation, and the Canada Foundation for Innovation (CH), the NIAAA Division of Intramural Clinical and Biological Research (VAR), and NIH R21AA020304 (EC, CH).
All authors contributed significantly to the manuscript and all authors have read and approved the final manuscript.
The authors thank Sean O'Connor and Victor Vitvitskiy at the Indiana Alcohol Research Center (NIH P60 AA007611) for software support. The authors also express appreciation to Dr. Ariel Graff, Vanessa Garofalo, and Matthew McPhee for their assistance with data collection.
Footnotes
Disclosures
The authors declare no conflicts of interest.
Because there were only 3 observations for each participant in the go/no-go models, we were limited in the number of parameters we could specify in the random effects covariance matrix. Thus, we specified a diagonal matrix, which included estimates of the random intercept and random slopes for each time contrast. We had more degrees of freedom in the subjective response models, allowing us to specify an unstructured covariance matrix for the random effects, which included estimates of the random intercept, random slopes for the effects of time, and the covariances among these random effects.
References
- Acheson SK, Stein RM, Swartzwelder HS. Impairment of semantic and figural memory by acute ethanol: Age-dependent effects. Alcoholism: Clinical and Experimental Research. 1998;22(7):1437–1442. doi: 10.1111/j.1530-0277.1998.tb03932.x. [DOI] [PubMed] [Google Scholar]
- Amlung MT, Morris DH, McCarthy DM. Effects of acute alcohol tolerance on perceptions of danger and willingness to drive after drinking. Psychopharmacology. 2014;231(22):4271–4279. doi: 10.1007/s00213-014-3579-1. doi: 10.1007/s00213-014-3579-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babor TF, Biddle-Higgins JC, Saunders JB, Monteiro MG. AUDIT: The alcohol Use Disorders Identification Test: Guidelines for Use in Primary Care. World Health Organization; Geneva, Switzerland: 2001. [Google Scholar]
- Behar D, Berg CJ, Rapoport JL, Nelson W, Linnoila M, Cohen M, Marshall T. Behavioral and physiological effects of ethanol in high-risk and control children: a pilot study. Alcoholism, clinical and experimental research. 1983;7(4):404–410. doi: 10.1111/j.1530-0277.1983.tb05495.x. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcoholism: Clinical and Experimental Research. 1995;19(3):600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Brown SA, McGue M, Maggs J, Schulenberg J, Hingson R, Swartzwelder S, Murphy S. A developmental perspective on alcohol and youths 16 to 20 years of age. Pediatrics. 2008;121(Suppl 4):S290–310. doi: 10.1542/peds.2007-2243D. doi: 10.1542/peds.2007-2243D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski S, Ray LA. Subjective response to alcohol and associated craving in heavy drinkers vs. alcohol dependents: An examination of Koob's allostatic model in humans. Drug and Alcohol Dependence. 2014;140:161–167. doi: 10.1016/j.drugalcdep.2014.04.015. doi: 10.1016/j.drugalcdep.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL, editors. Rochester symposium on developmental psychopathology, Volume 7: Adolescence: Opportunities and challenges. University of Rochester Press; Rochester, NY: 1996. [Google Scholar]
- Cromer JR, Cromer JA, Maruff P, Snyder PJ. Perception of alcohol intoxication shows acute tolerance while executive functions remain impaired. Experimental and Clinical Psychopharmacology. 2010;18(4):329–339. doi: 10.1037/a0019591. doi: 10.1037/a0019591. [DOI] [PubMed] [Google Scholar]
- Erblich J, Earleywine M. Behavioral undercontrol and subjective stimulant and sedative effects of alcohol intoxication: Independent predictors of drinking habits? Alcoholism: Clinical and Experimental Research. 2003;27(1):44–50. doi: 10.1097/01.ALC.0000047300.46347.CE. doi: 10.1097/01.ALC.0000047300.46347.CE. [DOI] [PubMed] [Google Scholar]
- Field M, Wiers RW, Christiansen P, Fillmore MT, Verster JC. Acute alcohol effects on inhibitory control and implicit cognition: Implications for loss of control over drinking. Alcoholism: Clinical and Experimental Research. 2010;34:1346–1352. doi: 10.1111/j.1530-0277.2010.01218.x. doi: 10.1111/j.1530-0277.2010.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore M, Ostling E, Martin C, Kelly T. Acute effects of alcohol on inhibitory control and information processing in high and low sensation-seekers. Drug and Alcohol Dependence. 2009;100(1-2):91–99. doi: 10.1016/j.drugalcdep.2008.09.007. doi: 10.1016/j.drugalcdep.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, Marczinski CA, Bowman AM. Acute tolerance to alcohol effects on inhibitory and activational mechanisms of behavioral control. Journal of Studies on Alcohol. 2005;66(5):663–672. doi: 10.15288/jsa.2005.66.663. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M. Behavioral impairment under alcohol: Cognitive and pharmacokinetic factors. Alcoholism: Clinical and experimental research. 1998;22(7):1476–1482. [PubMed] [Google Scholar]
- Fillmore MT, Weafer J. Acute tolerance to alcohol in at-risk binge drinkers. Psychology of Addictive Behaviors. 2012;26(4):693–702. doi: 10.1037/a0026110. doi: 10.1037/a0026110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Behavioral disinhibition and the development of early-onset addiction: Common and specific influences. Annual Review of Clinical Psychology. 2008;4:325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- Kaplan HL, Sellers EM, Hamilton C, Naranjo CA, Dorian P. Is there acute tolerance to alcohol at steady state? Journal of Studies on Alcohol. 1985;46(3):253–256. doi: 10.15288/jsa.1985.46.253. [DOI] [PubMed] [Google Scholar]
- Kerfoot K, Pittman B, Ralevski E, Limoncelli D, Koretski J, Newcomb J, Petrakis IL. Effects of family history of alcohol dependence on the subjective response to alcohol using the intravenous alcohol clamp. Alcoholism: Clinical and Experimental Research. 2013;37(12):2011–2018. doi: 10.1111/acer.12199. doi: 10.1111/acer.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Adler L, Ames M, Demler O, Faraone S, Hiripi E, Walters EE. The World Health Organization Adult ADHD Self-Report Scale (ASRS): A short screening scale for use in the general population. Psychological Medicine. 2005;35(2):245–256. doi: 10.1017/s0033291704002892. [DOI] [PubMed] [Google Scholar]
- King AC, de Wit H, McNamara PJ, Cao D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Archives of General Psychiatry. 2011;68(4):389–399. doi: 10.1001/archgenpsychiatry.2011.26. doi: 10.1001/archgenpsychiatry.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, Ralevski E, Limoncelli D, Pittman B, O'Malley SS, Petrakis IL. Relationships between impulsivity and subjective response in an IV ethanol paradigm. Psychopharmacology. 2014;231(14):2867–2876. doi: 10.1007/s00213-014-3458-9. doi: 10.1007/s00213-014-3458-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner RM, Steinberg L, editors. Handbook of adolescent psychology. 2nd ed. John Wiley & Sons Inc.; Hoboken, NJ: 2004. [Google Scholar]
- Li TK, Hewitt BG, Grant BF. Alcohol use disorders and mood disorders: a National Institute on Alcohol Abuse and Alcoholism perspective. Biological Psychiatry. 2004;56(10):718–720. doi: 10.1016/j.biopsych.2004.03.006. doi: 10.1016/j.biopsych.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Combs SW, Fillmore MT. Increased sensitivity to the disinhibiting effects of alcohol in binge drinkers. Psychology of Addictive Behaviors. 2007;21(3):346–354. doi: 10.1037/0893-164X.21.3.346. doi: 10.1037/0893-164x.21.3.346. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT. Compensating for alcohol-induced impairment of control: Effects on inhibition and activation of behavior. Psychopharmacology. 2005;181(2):337–346. doi: 10.1007/s00213-005-2269-4. doi: 10.1007/s00213-005-2269-4. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the biphasic alcohol effects scale. Alcoholism: Clinical and Experimental Research. 1993;17(1):140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Martin CS, Moss HB. Measurement of acute tolerance to alcohol in human subjects. Alcoholism: Clinical and Experimental Research. 1993;17(2):211–216. doi: 10.1111/j.1530-0277.1993.tb00751.x. [DOI] [PubMed] [Google Scholar]
- Mellanby E. Alcohol: Its absorption into and disappearance from the blood under different conditions. In: Mellanby E, editor. Medical Research Commitiee, Special Report Series No. 31. London, England: 1919. [Google Scholar]
- Miller MA, Fillmore MT. Protracted impairment of impulse control under an acute dose of alcohol: a time-course analysis. Addictive Behaviors. 2014;39(11):1589–1596. doi: 10.1016/j.addbeh.2013.10.035. doi: 10.1016/j.addbeh.2013.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda R, Jr., Monti PM, Ray L, Treloar HR, Reynolds EK, Ramirez J, Magill M. Characterizing subjective responses to alcohol among adolescent problem drinkers. Journal of Abnormal Psychology. 2014;123(1):117–129. doi: 10.1037/a0035328. doi: 10.1037/a0035328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Hutchison KE. Toward bridging the gap between biological, psychobiological and psychosocial models of alcohol craving. Addiction. 2000;95(Suppl 2):S229–236. doi: 10.1080/09652140050111799. [DOI] [PubMed] [Google Scholar]
- Morean ME, Corbin WR. Subjective response to alcohol: A critical Review of the literature. Alcoholism: Clinical and Experimental Research. 2010;34(3):385–395. doi: 10.1111/j.1530-0277.2009.01103.x. doi: 10.1111/j.1530-0277.2009.01103.x. [DOI] [PubMed] [Google Scholar]
- Morzorati SL, Ramchandani VA, Flury L, Li TK, O'Connor S. Self-reported subjective perception of intoxication reflects family history of alcoholism when breath alcohol levels are constant. Alcoholism: Clinical and Experimental Research. 2002;26(8):1299–1306. doi: 10.1097/01.ALC.0000025886.41927.83. doi: 10.1097/01.alc.0000025886.41927.83. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Renton RM. High risk groups often have higher levels of alcohol response than low risk: the other side of the coin. Alcoholism: Clinical and Experimental Research. 2010;34(2):199–202. doi: 10.1111/j.1530-0277.2009.01081.x. doi: doi:10.1111/j.1530-0277.2009.01081.x. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB. Alcohol challenge with sons of alcoholics: A critical review and analysis. Psychological Bulletin. 1990;108(3):383–402. doi: 10.1037/0033-2909.108.3.383. [DOI] [PubMed] [Google Scholar]
- O'Connor S, Morzorati S, Christian J, Li TK. Clamping breath alcohol concentration reduces experimental variance: Application to the study of acute tolerance to alcohol and alcohol elimination rate. Alcoholism: Clinical and Experimental Research. 1998;22(1):202–210. [PubMed] [Google Scholar]
- Ostling EW, Fillmore MT. Tolerance to the impairing effects of alcohol on the inhibition and activation of behavior. Psychopharmacology. 2010;212(4):465–473. doi: 10.1007/s00213-010-1972-y. doi: 10.1007/s00213-010-1972-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokorny AD, Miller BA, Kaplan HB. The brief MAST: a shortened version of the Michigan Alcoholism Screening Test. The American Journal of Psychiatry. 1972;129(3):342–345. doi: 10.1176/ajp.129.3.342. [DOI] [PubMed] [Google Scholar]
- Quinn PD, Fromme K. Subjective response to alcohol challenge: A quantitative review. Alcoholism: Clinical and Experimental Research. 2011 doi: 10.1111/j.1530-0277.2011.01521.x. doi: 10.1111/j.1530-0277.2011.01521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radlow R. A quantitative theory of acute tolerance to alcohol. Psychopharmacology. 1994;114(1):1–8. doi: 10.1007/BF02245438. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Bolane J, Li TK, O'Connor S. A physiologically-based pharmacokinetic (PBPK) model for alcohol facilitates rapid BrAC clamping. Alcoholism: Clinical and Experimental Research. 1999;23(4):617–623. [PubMed] [Google Scholar]
- Ramchandani VA, O'Connor S, Blekher T, Kareken D, Morzorati S, Nurnberger J, Li TK. A preliminary study of acute responses to clamped alcohol concentration and family history of alcoholism. Alcoholism: Clinical and Experimental Research. 1999;23(8):1320–1330. [PubMed] [Google Scholar]
- Ray LA, Mackillop J, Monti PM. Subjective responses to alcohol consumption as endophenotypes: Advancing behavioral genetics in etiological and treatment models of alcoholism. Substance Use & Misuse. 2010;45(11):1742–1765. doi: 10.3109/10826084.2010.482427. doi: 10.3109/10826084.2010.482427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W, Milich R, Fillmore MT. Reduced acute recovery from alcohol impairment in adults with ADHD. Psychopharmacology. 2013;228(1):65–74. doi: 10.1007/s00213-013-3016-x. doi: 10.1007/s00213-013-3016-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AK, Hobbs M, Klipp L, Bell S, Edwards K, O'Hara P, Drummond C. Monitoring drinking behaviour and motivation to drink over successive doses of alcohol. Behavioural Pharmacology. 2010;21(8):710–718. doi: 10.1097/FBP.0b013e32833fa72b. doi: 10.1097/FBP.0b013e32833fa72b. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. The relationships of a family history of alcohol dependence, a low level of response to alcohol and six domains of life functioning to the development of alcohol use disorders. Journal of Studies on Alcohol. 2000;61(6):827–835. doi: 10.15288/jsa.2000.61.827. [DOI] [PubMed] [Google Scholar]
- Schweizer TA, Vogel-Sprott M. Alcohol-impaired speed and accuracy of cognitive functions: A review of acute tolerance and recovery of cognitive performance. Experimental and Clinical Psychopharmacology. 2008;16(3):240–250. doi: 10.1037/1064-1297.16.3.240. doi: 10.1037/1064-1297.16.3.240. [DOI] [PubMed] [Google Scholar]
- Scott C, Corbin WR. Influence of sensation seeking on response to alcohol versus placebo: Implications for the acquired preparedness model. Journal of Studies on Alcohol and Drugs. 2014;75(1):136–144. doi: 10.15288/jsad.2014.75.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley MC, Sirocco KY. Introduction to special section: ADHD, impulsivity, and alcohol abuse. Experimental and Clinical Psychopharmacology. 2014;22(2):97–99. doi: 10.1037/a0036124. doi: 10.1037/a0036124. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption: Psychosocial and biochemical methods. Humana Press; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- Spear LP. Adolescent neurobehavioral characteristics, alcohol sensitivities, and intake: Setting the stage for alcohol use disorders? Child Development Perspectives. 2011;5(4):231–238. doi: 10.1111/j.1750-8606.2011.00182.x. doi: 10.1111/j.1750-8606.2011.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Adolescence. Alcohol sensitivity, tolerance, and intake. Recent Developments in Alcoholism. 2005;17:143–159. [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Sensitivity to ethanol and other hedonic stimuli in an animal model of adolescence: Implications for prevention science? Developmental Psychobiology. 2010;52:236–243. doi: 10.1002/dev.20457. doi: 10.1002/dev.20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang NM, Claus ED, Ramchandani VA, Graff-Guerrero A, Boileau I, Hendershot CS. Dose-dependent effects of intravenous alcohol administration on cerebral blood flow in young adults. Psychopharmacology (Berl) 2015;232(4):733–744. doi: 10.1007/s00213-014-3706-z. doi: 10.1007/s00213-014-3706-z. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. 5th ed. Allyn & Bacon/Pearson Education; Boston, MA: 2007. [Google Scholar]
- Tiffany ST, Wray JM. The clinical significance of drug craving. Annals of the New York Academy of Sciences. 2012;1248:1–17. doi: 10.1111/j.1749-6632.2011.06298.x. doi: 10.1111/j.1749-6632.2011.06298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, Fillmore MT. Individual differences in acute alcohol impairment of inhibitory control predict ad libitum alcohol consumption. Psychopharmacology (Berl) 2008;201(3):315–324. doi: 10.1007/s00213-008-1284-7. doi: 10.1007/s00213-008-1284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, Fillmore MT. Acute tolerance to alcohol impairment of behavioral and cognitive mechanisms related to driving: drinking and driving on the descending limb. Psychopharmacology (Berl) 2012;220(4):697–706. doi: 10.1007/s00213-011-2519-6. doi: 10.1007/s00213-011-2519-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, Fillmore MT, Milich R. Increased sensitivity to the disinhibiting effects of alcohol in adults with ADHD. Experimental and Clinical Psychopharmacology. 2009;17(2):113–121. doi: 10.1037/a0015418. doi: 10.1037/a0015418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiers RW, Bartholow BD, van den Wildenberg E, Thush C, Engels R, Sher KJ, Stacy AW. Automatic and controlled processes and the development of addictive behaviors in adolescents: A review and a model. Pharmacology Biochemistry and Behavior. 2007;86(2):263–283. doi: 10.1016/j.pbb.2006.09.021. doi: 10.1016/j.pbb.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Zoethout RW, Delgado WL, Ippel AE, Dahan A, van Gerven JM. Functional biomarkers for the acute effects of alcohol on the central nervous system in healthy volunteers. British Journal of Clinical Pharmacology. 2011;71(3):331–350. doi: 10.1111/j.1365-2125.2010.03846.x. doi: 10.1111/j.1365-2125.2010.03846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M, Kuhlman D, Joireman J, Teta P, Kraft M. A comparison of three structural models for personality: The Big Three, the Big Five, and the Alternative Five. Journal of Personality and Social Psychology. 1993;65:757–768. [Google Scholar]


