Abstract
Prion protein (PrP) is present at extremely low levels in the blood of animals and its detection is complicated by the poor sensitivity of current standard methodologies. Interesting results have been obtained with recent advanced technologies that are able to detect minute amounts of the pathological PrP (PrPSc), but their efficiency is reduced by various factors present in blood. In this study, we were able to extract cellular PrP (PrPC) from plasma-derived exosomes by a simple, fast method without the use of differential ultracentrifugation and to visualize it by Western blotting, reducing the presence of most plasma proteins. This result confirms that blood is capable of releasing PrP in association with exosomes and could be useful to better study its role in the pathogenesis of transmissible spongiform encephalopathies.
Transmissible spongiform encephalopathies (TSEs) are a family of rare progressive neurodegenerative disorders characterized by abnormal brain deposition of an insoluble and protease-resistant isoform of cellular prion protein (PrPC) named PrPSc. Invariably fatal, TSEs include a wide range of animal and human conditions of sporadic, genetic or infectious origin, such as bovine spongiform encephalopathy in cattle, scrapie in sheep and goats, chronic wasting disease in deer and elk, and Creutzfeldt–Jakob disease in humans.
In recent years, it has been demonstrated that PrP is associated with exosomes, small membrane vesicles of endocytic origin which play an important role in intercellular communication. PrP is associated with exosomes secreted from non-neuronal and neuronal cells (Fevrier et al., 2004; Leblanc et al., 2006; Wang et al., 2011), and exosomes containing PrPSc are capable of transferring infectivity to cells (Vella et al., 2007; Alais et al., 2008). Despite the unequivocal presence of blood infectivity of prion-affected individuals, PrP detection might be masked by proteins or soluble components of plasma (Abdel-Haq, 2015; Gregori et al., 2008; Orrú et al., 2011; Properzi et al., 2015; Saá et al., 2014).
In order to study the role of plasma-derived exosomes in the pathogenesis of TSEs and their potential as a tool for in vivo diagnosis, we devised a simple method to significantly enrich plasma-derived PrP after exosome extraction in blood. To do this, we used plasma samples (Table S1, available in the online Supplementary Material) from five sheep naturally infected with scrapie, confirmed PrPSc-positive by confirmatory tests (Mazza et al., 2010), and three healthy sheep, selected from livestock with no record of scrapie cases in the past 5 years and selected for scrapie resistance by genetic analysis (Hunter, 2007).
A crucial step in exosome preparations is to obtain highly purified vesicles as plasma is a complex and viscous fluid with a high protein concentration (60–80 mg ml − 1) (Momen-Heravi et al., 2012; Salazar Vázquez et al., 2009). The most commonly used method to isolate and purify exosomes is by differential centrifugation coupled with ultracentrifugation (Welton et al., 2010). However, some contaminants such as protein complexes and co-sedimenting vesicles might compact with exosomes when using this method (Tauro et al., 2012). In our modified precipitation method, 1 ml each plasma sample was incubated with 252 μl polymeric precipitation mixture according to the manufacturer's instructions (Fresenius Medical Care) at 4 °C overnight and then centrifuged at 1500 g for 30 min at 4 °C. The exosome pellets were lysed using 500 μl RIPA buffer (Sigma-Aldrich) and protease inhibitors were added (Pefabloc SC Plus; Roche). Protein quantification was performed using a BCA (bicinchoninic acid) Protein Assay kit (Thermo Scientific). This method of precipitation was compared with a classical differential ultracentrifugation protocol (10 000 g for 30 min followed by 100 000 g for 1 h) by nanoparticle tracking analysis. In particular, size and distribution of exosomes were evaluated by a NanoSight LM10 instrument (NanoSight) equipped with nta 2.0 analytic software (Dragovic et al., 2011). The NanoSight analysis showed that the profile, size and number of particles isolated by the two methods were comparable (Fig. S1a, b). These results demonstrate that the precipitation method did not affect the size and concentration of plasma particles. In addition, to confirm the vesicular nature of the plasma-derived exosomes, Western blotting analysis of flotillin-1 expression, a typical marker of exosomes (Vella et al., 2008a, b), was performed. As compared with tissue lysate [brain homogenate (BH)], extracted as described below, flotillin-1 was found to be enriched in exosome lysate (Fig. 1). Flottilin-1 is a constituent of lipid subdomains in recycling exosomes and is normally more enriched in exosome lysate than in cell/tissue lysate (Grey et al., 2015; Meister & Tikkanen, 2014; Vella et al., 2008a, b).
Fig. 1.
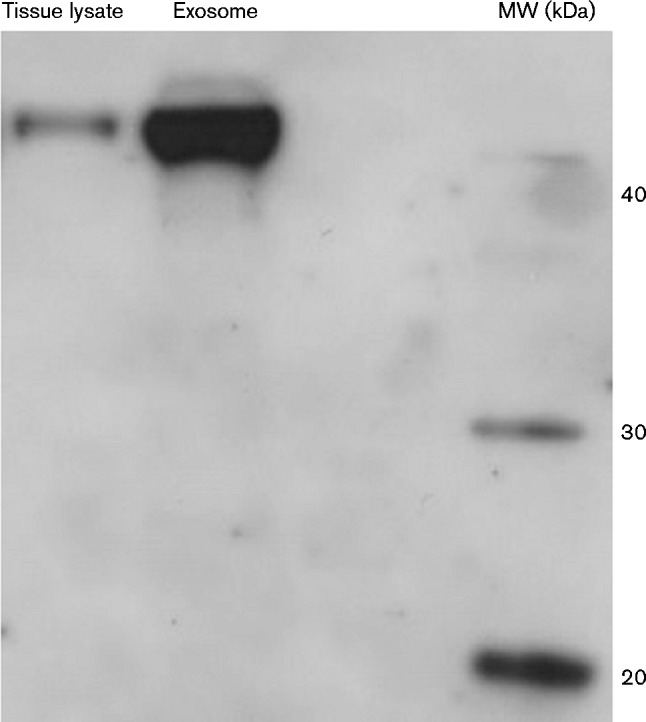
Expression of the plasma exosome marker flotillin-1 by Western blotting. Equivalent amounts (125 μg total protein) of scrapie-infected sheep BH (Tissue lysate) and exosome lysate (Exosome) were analysed by Western blotting using an anti-flotillin-1 (45 kDa) antibody (BD Biosciences). MW, molecular mass markers.
We next assessed the expression of PrP from exosome lysates by Western blotting using the anti-PrP mAb P4 (R-Biopharm), routinely used due to its high sensitivity and specificity in confirmatory Western blotting assays for active scrapie outbreak surveillance, as described previously (Arsac et al., 2007; Baron et al., 2007; Harmeyer et al., 1998; Mazza et al., 2010). However, the presence of plasma protein bands did not allow the identification of the typical PrP three-band Western blotting pattern (Raymond & Chabry, 2004) (Fig. S2), as reported previously (Gregori et al., 2008; Properzi et al., 2015; Saá et al., 2014).
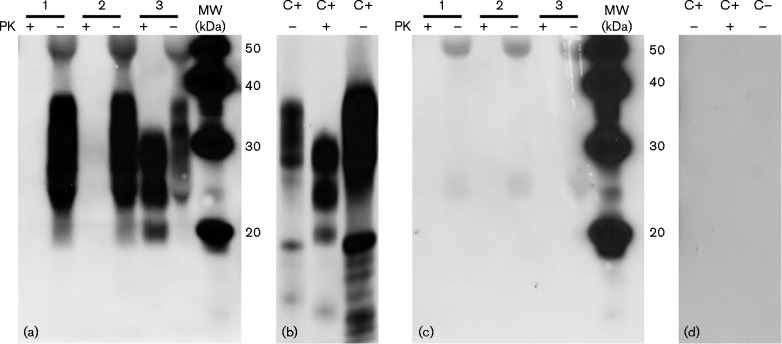
To improve PrP visualization, we performed a spike-in experiment where a known protein amount of sheep BH was added to the plasma from healthy animals. This experiment was done to test a method for PrP detection in dilute fluids such as plasma, but using samples effectively containing PrPC/Sc. The brains were homogenized in 10 % sarcosyl and clarified by ultracentrifugation as described previously (Bozzetta et al., 2004). After protein quantification, dilutions of total BH protein from scrapie-positive (SBH) and/or scrapie-negative (NBH) sheep were spiked into 500 μl healthy sheep plasma to give the following dilutions: (1) 500 pg NBH μl − 1 (100% negative), (2) 250 pg NBH μl − 1 (50% negative)+250 pg SBH μl − 1 (50% positive) and (3) 500 pg SBH μl − (100% positive).
To concentrate the PrP protein, 40 μl of all spiked plasma samples were precipitated with thyroglobulin (TG; 5 mg ml − 1), a large unrelated soluble protein used as a carrier in protein precipitation methodologies (Kocisko et al., 1995), and 4 vols methanol at − 20 °C for 2 h, and then centrifuged. The spike-in samples, positive BH (C+) and negative BH (C − ) were analysed to detect PrP using the mAb P4 (Harmeyer et al., 1998) (Fig. 2a, b). To demonstrate the specificity of P4 and the possible interaction of secondary antibody, the samples were examined using only non-immune IgG as control (Fig. 2c, d). In no-proteinase K (PK) ( − )- and PK (+)-digested samples it was possible to compare the different PrP glycoform patterns (Raymond & Chabry, 2004): PrPC showed the three bands (di-, mono- and unglycosylated) between 20 and 37 kDa, and PrPSc between 17 and 29 kDa (Fig. 2a, b).
Fig. 2.
Detection of ovine brain-derived PrP spiked into healthy ovine plasma after TG/methanol precipitation. PK (+)- and no-PK ( − )-digested samples were analysed by Western blotting using the anti-PrP mAb P4. (a) Lane 1, 500 pg total NBH protein μl− 1 (100% negative); lane 2, 250 pg total NBH protein μl− 1 (50% negative)+250 pg total SBH protein μl− 1 (50% positive); lane 3, 500 pg total SBH protein μl− 1 (100% positive). (b) C+, scrapie-infected sheep BH (positive control); C − , healthy sheep BH (negative control). (c, d) Non-immune IgG Western blotting of the same samples. Images were acquired with two different exposure times: (a, c) 10 min and (b, d) 3 min. With regard to the PrP patterns: no-PK lane 1 ( − ) is similar to no-PK ( − ) C − , PK (+) lane 1 is negative; no-PK ( − ) lane 2 is similar to no-PK ( − ) C − , PK (+) lane 2 is negative; no-PK ( − ) lane 3 is similar to no-PK ( − ) C+, PK (+) lane 3 is similar to PK (+) C+. MW, molecular mass markers.
To verify the reduction of total plasma/exosome protein during the PrP isolation, silver staining after PAGE was performed. It was confirmed that the greater concentration of total protein was present in undiluted plasma, whereas exosome lysate, after TG/methanol precipitation, showed an evident reduction of total protein (Fig. S3).
As reported in other similar studies with blood samples (Bannach et al., 2012; Bellon et al., 2003; Caplazi et al., 2004; Gregori et al., 2008; Lau et al., 2007; Saá et al., 2014), different approaches can be used to concentrate PrP and spike-in experiments utilized to verify method sensitivity. In our study, our precipitation method proved useful to reduce the highly unspecific signal background and the spike-in experiment allowed us to determine the limit of detection (500 pg total BH protein μl − 1) in order to visualize PrPC/Sc by Western blotting.
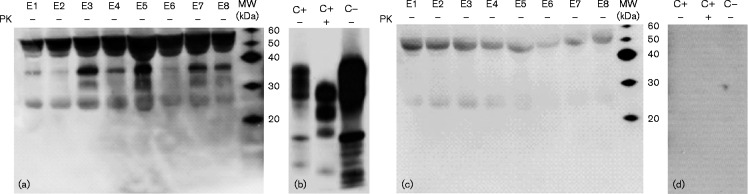
To detect PrP in the exosomes, the same protocol described above was used on exosomes derived from eight plasma samples of scrapie-infected or healthy sheep: 40 μl exosome lysate was precipitated with TG and 4 vols methanol at − 20 °C for 2 h and then centrifuged. The pellets were dissolved in Laemmli buffer and an equal volume (20 μl) was loaded for Western blotting. Although the heavy chain at 55 kDa and the light chain at 24 kDa of IgG were visible, it was possible to observe the diglycosylated band at ∼37 kDa in all eight no-PK-digested samples and the monoglycosylated band at ∼30 kDa in four samples (E3, E5, E7 and E8) (Fig. 3a). To confirm these results, the signal specificity was detected as described earlier (Fig. 3c). The different visualization of the PrPC bands could have been due to different protein concentrations of each sample; in fact, band intensity was more evident in the samples with a higher amount of protein (Table S1). In order to detect PrPSc, the same samples were PK-digested and loaded in a separate gel. Although the unspecific bands of IgG disappeared, it was not possible to visualize the PrPSc pattern bands (Fig. S4).
Fig. 3.
PrPC in ovine plasma-derived exosomes after TG/methanol precipitation. Using Western blotting, an equal volume (20 μl) of samples was analysed for PrPC expression. (a) Eight no-PK ( − )-digested exosome lysates: five sheep naturally infected with scrapie (E1, E2, E3, E6 and E7) and three healthy sheep (E4, E5 and E8). (b) C+, PK (+)- and no-PK ( − )-digested scrapie-infected sheep BH (positive control); C − , no-PK ( − )-digested healthy sheep BH (negative control). (c, d) Non-immune IgG Western blotting of the same samples. Images were acquired with two different exposure times: (a, c) 40 min and (b, d) 3 min. MW, molecular mass markers.
The novelty of our study resides in the demonstration that PrPC is indeed associated with exosomes isolated from sheep plasma and can be visualized by Western blotting.
Low amounts of PrPC/Sc are present in a variety of peripheral tissues and body fluids (Franscini et al., 2006; Franz et al., 2012; Hampton, 2006; Henderson et al., 2015; Mulcahy et al., 2004; Murayama et al., 2014; WHO, 2010), but it is difficult to detect with current biochemical and immunohistochemical assays. Recently, it was possible to detect PrPTSE using an immunochemistry technique in plasma exosomes of prion-infected rodents; however, a pool of 5 ml hamster blood and a sucrose gradient by ultracentrifugation were needed in order to visualize the PrPC/Sc (Properzi et al., 2015). Our results indicate, instead, that it is possible to extract PrP from 1 ml plasma by a simple, fast method without the use of differential ultracentrifugation. In detail, we were able to extract a sufficient amount of purified exosomes by polymeric precipitation, with good quality total exosome protein, and TG/methanol precipitation permitted the elimination of most plasma protein contaminants that can interfere with PrP isolation.
Our results confirm that PrPC associated with exosomes might contribute to the pathogenesis and transmission of prion diseases. Targeting of exosomes containing PrPC could confer susceptibility to cells that do not express PrP and facilitate prion propagation (Porto-Carreiro et al., 2005). Moreover, cellular levels of PrPC are known to regulate the amount of secreted vesicles with a major role in health and disease (Dias et al., 2015). Further studies are needed to elucidate the intracellular trafficking route of glycosylphosphatidylinositol-anchored proteins, such as PrP, from the caveolar pathway to extracellular vesicles. From this point of view, our simple method to detect PrPC-associated exosomes in vivo could be useful to better study the role of PrPC in TSEs pathogenesis.
Further experiments combining our protocol as a (semi)purification step with other approaches could be useful to improve the detection of PrPSc in the blood of naturally TSE-infected animals. For example, good results have been demonstrated by recent PrPSc amplification technologies, such as real-time quaking-induced conversion (RT-QuIC) or protein misfolding cyclic amplification (PMCA) (Henderson et al., 2015; Orrú et al., 2014; Properzi & Pocchiari, 2013). PMCA was able to demonstrate the presence of PrPTSE in extracellular vesicles from the plasma of mice experimentally infected with mouse-adapted variant Creutzfeldt–Jakob disease (Saá et al., 2014).
Currently, most prion studies use experimentally infected animals. However, with our simple and fast approach, in vivo studies on naturally infected animals could provide novel insights into TSEs pathogenesis.
Acknowledgements
This study was funded by grants from the Italian Ministry of Health to C. Casalone and C. Corona (RF-2009-1474758, RF-2009-1474624 and IZS PLV 06/12 RC).
Supplementary Data
Supplementary Data
References
- Abdel-Haq H. (2015). Factors intrinsic and extrinsic to blood hamper the development of a routine blood test for human prion diseases J Gen Virol 96 479–493 10.1099/vir.0.070979-0 . [DOI] [PubMed] [Google Scholar]
- Alais M., Simoes S., Baas D., Lehmann S., Raposo G., Darlix J. L., Leblanc P. (2008). Mouse neuroblastoma cells release prion infectivity associated with exosomal vesicles Biol Cell 100 603–615. [DOI] [PubMed] [Google Scholar]
- Arsac J. N., Andreoletti O., Bilheude J. M., Lacroux C., Benestad S. L., Baron T. (2007). Similar biochemical signatures and prion protein genotypes in atypical scrapie and Nor98 cases, France and Norway Emerg Infect Dis 13 58–65 10.3201/eid1301.060393 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannach O., Birkmann E., Reinartz E., Jaeger K. E., Langeveld J. P., Rohwer R. G., Gregori L., Terry L. A., Willbold D., Riesner D. (2012). Detection of prion protein particles in blood plasma of scrapie infected sheep PLoS One 7 e36620 10.1371/journal.pone.0036620 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron T., Biacabe A. G., Arsac J. N., Benestad S., Groschup M. H. (2007). Atypical transmissible spongiform encephalopathies (TSEs) in ruminants Vaccine 25 5625–5630 10.1016/j.vaccine.2006.10.058 . [DOI] [PubMed] [Google Scholar]
- Bellon A., Seyfert-Brandt W., Lang W., Baron H., Gröner A., Vey M. (2003). Improved conformation-dependent immunoassay: suitability for human prion detection with enhanced sensitivity J Gen Virol 84 1921–1925 10.1099/vir.0.18996-0 . [DOI] [PubMed] [Google Scholar]
- Bozzetta E., Acutis P. L., Martucci F., Nappi R., Casalone C., Mazza M., Caramelli M. (2004). Evaluation of rapid tests for the diagnosis of transmissible spongiform encephalopathies in sheep and goats Acta Neuropathol 107 559–562 10.1007/s00401-004-0849-8 . [DOI] [PubMed] [Google Scholar]
- Caplazi P. A., O'Rourke K. I., Baszler T. V. (2004). Resistance to scrapie in PrP ARR/ARQ heterozygous sheep is not caused by preferential allelic use J Clin Pathol 57 647–650 10.1136/jcp.2003.012203 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias M., Teixeira B., Rodrigues B., Arantes C., Roffe M., Hajj G., Martins V. (2015). Prion protein regulates autophagy and endocytic trafficking with essential roles for the biogenesis of extracellular vesicles J Extracell Vesicles 4 27783 10.3402/jev.v4.27783 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragovic R. A., Gardiner C., Brooks A. S., Tannetta D. S., Ferguson D. J., Hole P., Carr B., Redman C. W., Harris A. L., other authors (2011). Sizing and phenotyping of cellular vesicles using nanoparticle tracking analysis Nanomedicine (Lond) 7 780–788 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fevrier B., Vilette D., Archer F., Loew D., Faigle W., Vidal M., Laude H., Raposo G. (2004). Cells release prions in association with exosomes Proc Natl Acad Sci U S A 101 9683–9688 10.1073/pnas.0308413101 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franscini N., El Gedaily A., Matthey U., Franitza S., Sy M. S., Bürkle A., Groschup M., Braun U., Zahn R. (2006). Prion protein in milk PLoS One 1 e71 10.1371/journal.pone.0000071 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz M., Eiden M., Balkema-Buschmann A., Greenlee J., Schatzl H., Fast C., Richt J., Hildebrandt J. P., Groschup M. H. (2012). Detection of PrPSc in peripheral tissues of clinically affected cattle after oral challenge with bovine spongiform encephalopathy J Gen Virol 93 2740–2748 10.1099/vir.0.044578-0 . [DOI] [PubMed] [Google Scholar]
- Gregori L., Gray B. N., Rose E., Spinner D. S., Kascsak R. J., Rohwer R. G. (2008). A sensitive and quantitative assay for normal PrP in plasma J Virol Methods 149 251–259 10.1016/j.jviromet.2008.01.028 . [DOI] [PubMed] [Google Scholar]
- Grey M., Dunning C. J., Gaspar R., Grey C., Brundin P., Sparr E., Linse S. (2015). Acceleration of α-synuclein aggregation by exosomes J Biol Chem 290 2969–2982 10.1074/jbc.M114.585703 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton T. (2006). Prions found in deer body fluids JAMA 296 2543 . [DOI] [PubMed] [Google Scholar]
- Harmeyer S., Pfaff E., Groschup M. H. (1998). Synthetic peptide vaccines yield monoclonal antibodies to cellular and pathological prion proteins of ruminants J Gen Virol 79 937–945 10.1099/0022-1317-79-4-937 . [DOI] [PubMed] [Google Scholar]
- Henderson D. M., Davenport K. A., Haley N. J., Denkers N. D., Mathiason C. K., Hoover E. A. (2015). Quantitative assessment of prion infectivity in tissues and body fluids by real-time quaking-induced conversion J Gen Virol 96 210–219 10.1099/vir.0.069906-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter N. (2007). Scrapie: uncertainties, biology and molecular approaches Biochim Biophys Acta 1772 619–628 10.1016/j.bbadis.2007.04.007 . [DOI] [PubMed] [Google Scholar]
- Kocisko D. A., Priola S. A., Raymond G. J., Chesebro B., Lansbury P. T., Jr, Caughey B. (1995). Species specificity in the cell-free conversion of prion protein to protease-resistant forms: a model for the scrapie species barrier Proc Natl Acad Sci U S A 92 3923–3927 10.1073/pnas.92.9.3923 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau A. L., Yam A. Y., Michelitsch M. M., Wang X., Gao C., Goodson R. J., Shimizu R., Timoteo G., Hall J., other authors (2007). Characterization of prion protein (PrP)-derived peptides that discriminate full-length PrPSc from PrPC Proc Natl Acad Sci U S A 104 11551–11556 10.1073/pnas.0704260104 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc P., Alais S., Porto-Carreiro I., Lehmann S., Grassi J., Raposo G., Darlix J. L. (2006). Retrovirus infection strongly enhances scrapie infectivity release in cell culture EMBO J 25 2674–2685 10.1038/sj.emboj.7601162 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza M., Iulini B., Vaccari G., Acutis P. L., Martucci F., Esposito E., Peletto S., Barocci S., Chiappini B., other authors (2010). Co-existence of classical scrapie and Nor98 in a sheep from an Italian outbreak Res Vet Sci 88 478–485 10.1016/j.rvsc.2009.11.015 . [DOI] [PubMed] [Google Scholar]
- Meister M., Tikkanen R. (2014). Endocytic trafficking of membrane-bound cargo: a flotillin point of view Membranes (Basel) 4 356–371 10.3390/membranes4030356 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momen-Heravi F., Balaj L., Alian S., Trachtenberg A. J., Hochberg F. H., Skog J., Kuo W. P. (2012). Impact of biofluid viscosity on size and sedimentation efficiency of the isolated microvesicles Front Physiol 3 162 10.3389/fphys.2012.00162 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahy E. R., Bartz J. C., Kincaid A. E., Bessen R. A. (2004). Prion infection of skeletal muscle cells and papillae in the tongue J Virol 78 6792–6798 10.1128/JVI.78.13.6792-6798.2004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama Y., Masujin K., Imamura M., Ono F., Shibata H., Tobiume M., Yamamura T., Shimozaki N., Terao K., other authors (2014). Ultrasensitive detection of PrPSc in the cerebrospinal fluid and blood of macaques infected with bovine spongiform encephalopathy prion J Gen Virol 95 2576–2588 . [DOI] [PubMed] [Google Scholar]
- Orrú C. D., Wilham J. M., Raymond L. D., Kuhn F., Schroeder B., Raeber A. J., Caughey B. (2011). Prion disease blood test using immunoprecipitation and improved quaking-induced conversion MBio 2 e00078–e00011 10.1128/mBio.00078-11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrú C. D., Bongianni M., Tonoli G., Ferrari S., Hughson A. G., Groveman B. R., Fiorini M., Pocchiari M., Monaco S., other authors (2014). A test for Creutzfeldt-Jakob disease using nasal brushings N Engl J Med 371 519–529 10.1056/NEJMoa1315200 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porto-Carreiro I., Février B., Paquet S., Vilette D., Raposo G. (2005). Prions and exosomes: from PrPc trafficking to PrPsc propagation Blood Cells Mol Dis 35 143–148. [DOI] [PubMed] [Google Scholar]
- Properzi F., Pocchiari M. (2013). Identification of misfolded proteins in body fluids for the diagnosis of prion diseases Int J Cell Biol 2013 839329 10.1155/2013/839329 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Properzi F., Logozzi M., Abdel-Haq H., Federici C., Lugini L., Azzarito T., Cristofaro I., di Sevo D., Ferroni E., other authors (2015). Detection of exosomal prions in blood by immunochemistry techniques J Gen Virol 96 1969–1974 10.1099/vir.0.000117 . [DOI] [PubMed] [Google Scholar]
- Raymond G. J., Chabry J. (2004). Purification of the pathological isoform of prion protein (PrPSc or PrPres) from transmissible spongiform encephalopathy-affected brain tissue. In Methods and Tools in Biosciences and Medicine Techniques in Prion Research, pp. 16–26. Edited by Lehmann S., Grassi J. Basel: Birkhäuser. [Google Scholar]
- Saá P., Yakovleva O., de Castro J., Vasilyeva I., De Paoli S. H., Simak J., Cervenakova L. (2014). First demonstration of transmissible spongiform encephalopathy-associated prion protein (PrPTSE) in extracellular vesicles from plasma of mice infected with mouse-adapted variant Creutzfeldt-Jakob disease by in vitro amplification J Biol Chem 289 29247–29260 10.1074/jbc.M114.589564 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar Vázquez B. Y., Martini J., Chávez Negrete A., Cabrales P., Tsai A. G., Intaglietta M. (2009). Microvascular benefits of increasing plasma viscosity and maintaining blood viscosity: counterintuitive experimental findings Biorheology 46 167–179 . [DOI] [PubMed] [Google Scholar]
- Tauro B. J., Greening D. W., Mathias R. A., Ji H., Mathivanan S., Scott A. M., Simpson R. J. (2012). Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes Methods 56 293–304 10.1016/j.ymeth.2012.01.002 . [DOI] [PubMed] [Google Scholar]
- Vella L. J., Sharples R. A., Lawson V. A., Masters C. L., Cappai R., Hill A. F. (2007). Packaging of prions into exosomes is associated with a novel pathway of PrP processing J Pathol 211 582–590. [DOI] [PubMed] [Google Scholar]
- Vella L. J., Greenwood D. L., Cappai R., Scheerlinck J. P., Hill A. F. (2008a). Enrichment of prion protein in exosomes derived from ovine cerebral spinal fluid Vet Immunol Immunopathol 124 385–393 10.1016/j.vetimm.2008.04.002 . [DOI] [PubMed] [Google Scholar]
- Vella L. J., Sharples R. A., Nisbet R. M., Cappai R., Hill A. F. (2008b). The role of exosomes in the processing of proteins associated with neurodegenerative diseases Eur Biophys J 37 323–332 10.1007/s00249-007-0246-z . [DOI] [PubMed] [Google Scholar]
- Wang G. H., Zhou X. M., Bai Y., Yin X. M., Yang L. F., Zhao D. (2011). Hsp70 binds to PrPC in the process of PrPC release via exosomes from THP-1 monocytes Cell Biol Int 35 553–558 10.1042/CBI20090391 . [DOI] [PubMed] [Google Scholar]
- Welton J. L., Khanna S., Giles P. J., Brennan P., Brewis I. A., Staffurth J., Mason M. D., Clayton A. (2010). Proteomics analysis of bladder cancer exosomes Mol Cell Proteomics 9 1324–1338 10.1074/mcp.M000063-MCP201 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2010). WHO tables on tissue infectivity distribution in transmissible spongiform encephalopathies. Update 2010. http://www.who.int/bloodproducts/tablestissueinfectivity.pdf?ua = 1. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data