Abstract
Purpose
To explore the effect of pulsatile flow in the decellularization process of small blood vessels.
Methods
A total of 30 human umbilical cords were used in the current study. The umbilical cords were flushed with 0.25% trypsin/0.01% EDTA for 30 minutes, followed by treatment with 1% sodium dodecyl sulfate sodium dodecyl sulfate for 3 hours. The effectiveness of decellularization on the umbilical artery wall was analyzed by mechanical analysis. The scaffolds’ biocompatibility was observed by co-culture with the human umbilical endothelial cells.
Results
The maximum stress of the arteries before and after denuding was 3.55 ± 0.42N and 3.50 ± 0.43N, respectively. Under the pressure of 300 mmHg, 28 pieces of umbilical cords remained intact before and after the flushing, while 2 pieces ruptured under 300 mmHg. There was no significant difference in mechanical properties between flushed and control arteries. Isolated human umbilical endothelial cells grow and spread well on the decellularized umbilical artery scaffolds.
Conclusions
Decellularization by pulsatile flow in human umbilical artery is a convenient, rapid and efficient approach to increase the availability of small caliber blood vessel scaffolds.
Keywords: Decellularization, Human umbilical artery, Scaffolds, Small diameter blood vessel, Vascular tissue engineering
INTRODUCTION
Research into the pathogenesis of and possible therapeutic options for vascular disease is the most active area of inquiry in the medical field due to its high rates of morbidity and mortality.1-7 Vascular grafts are either of synthetic or biological origin. However, high thrombogenic surface and poor mechanical properties of synthetic grafts limit their use as small-caliber grafts. Hence, the use of autologous vessels constitutes an attractive alternative for patients needing small-caliber arterial reconstruction. Nevertheless, the use of autografts is hampered by limited suitability and availability because of the prevalence of peripheral vascular diseases and/or their previous uses in bypass surgery. Limited availability of autologous vessels and inadequate performance of synthetic grafts in small diameter (≤ 5 mm) vascular replacement make current choices suboptimal. The fabrication of a tissue-engineered vascular graft appears to hold promise as an alternative.2-13
Over the last decade, various attempts have been made to develop a bio-artificial small-caliber vascular prosthesis by means of tissue engineering. The seeding of autologous vascular cells on an adequate scaffold material that resists arterial blood pressure and enables vascular cells to attach and spread on it has thereby become the basic principle. Biotic scaffolds present the following characteristics, but are not limited to: strong cellular affinity, providing extracellular matrix conditions similar to in-vivo conditions to support cell growth, proliferation and differentiation in addition to controlling tissue structure, and regulating cell phenotype and cell aggregation.14,15
The human umbilical artery (HUA) with an inner caliber of roughly 1.5 mm under collapsed conditions has attracted more and more attention recently in the field of tissue engineering. Studies have showed that denuding of the HUA might provide a new approach for generating small-diameter vascular grafts for vascular reconstruction surgery.16-18
Classically, enzymatic solution was flushed into the artery lumen to denude the vascular endothelial cells during decellularization.12-15 This method was effective in those vessels with a large caliber because the solution can be easily flushed into the lumen. However, this situation may not be applicable to smaller caliber vessels due to size constraints. Since vascular endothelial cells are the innermost layer of the vasculature, introduction of enzymatic solution into the vascular lumen presents the most efficient way to detach this superficial layer. However, the most convenient and efficient way to decellularize the small-caliber blood vessel for use in reconstruction surgery has not been studied. In this study, we explore the possibility of utilizing pulsatile flow in combination with the enzymatic digestion method to make the small-caliber blood vessel a possible clinical application in reconstruction surgery.
MATERIALS AND METHODS
Preparation and dissection of the human umbilical arteries
Human umbilical cords were collected from term placentas after written consent was obtained from the patients. Cords were stored between 0 and 4 °C and used within 24 hours after separation from the mother. Before dissection, the cords were cleaned and rinsed to remove residual blood within the respective vascular lumen. The cords were then cut from both ends for 10 mm to eliminate the end effect. Both the umbilical vein and the Wharton’s jelly within the cord were removed and the umbilical arteries were preserved for the subsequent experiments. The length of the artery available for experimental use was 200 mm. This study was conducted in accordance with the Declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Anzhen Hospital of Capital Medical University, and written informed consent was obtained from all participants.
Decellularization of human umbilical arteries
Vessels were placed in a circuit which pumped liquids into their lumen at the speed of 8 ml/min, where the rotation speed of the pump was 10 revolutions/min. The circuit was formed by the HUA, feeding needles, tubes and the solutions of the bottle (Figure 1). The blunt tip of both feeding needles (inner diameter: 1 mm) was inserted into the HUA which was tied on the needles by the thread. The other end of each needle was connected with a tube (inner diameter: 3 mm, thickness: 1 mm). Each tube’s free end was soaked in the solutions of the bottle. The vessels’ lumen were pumped by a solution of 0.25% trypsin (CAS#90002-07-7, Amresco, Solon, Ohio, USA.) with 0.01% EDTA (Sigma, German) in phosphate buffered saline (PBS) without Ca2+ and Mg2+ for 30 minutes. This procedure was followed by 10 g/L sodium dodecyl sulfate (SDS) (QiHuaSheng, Beijing, China) for 3 hours. The scaffolds were rinsed by PBS to clean the detergents. All steps were conducted under room temperature with continuous pulsatile flow through the vessels’ lumen.
Figure 1.
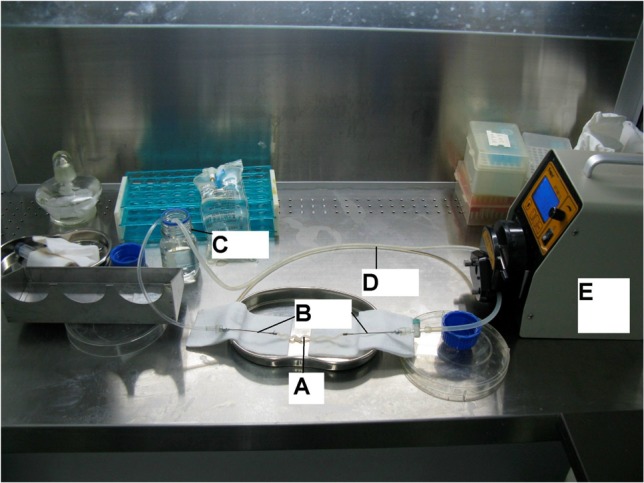
The apparatus of decellularization. (A) HUA. (B) Feeding needles. (C) Bottle and solution. (D) Tube. (E) Pump.
Burst pressure testing
Burst pressure was measured by progressive inflation of the vessel. The ends of each vessel section were attached to a stainless steel adapter and a circuit of heavily walled silicone tubing, respectively. A modified syringe pump was then attached to one end of the tubing with a pressure transducer attached to the distal end to monitor the change in pressure. The syringe pump injected PBS into the circuit at a rate of 8 ml/min until vessel rupture or the pressure remained at 300 mmHg. The number of the vessels that ruptured under the pressure of 300 mmHg was recorded.
Stress-strain testing
A uniaxial tensile testing rig (Electroforce 300, Bose, USA) was used for all stress-strain analyses to determine the stress-strain relationship. Artery samples were cut into 6 cm length and iced onto the clevis. Using the extension rate of 30 mm/min, samples were stressed until failure.
Scanning electron microscopy (SEM) analysis
Samples of the luminal and the abluminal surfaces of the HUA were gently washed with PBS three times for 5 minutes each, followed by fixation in 1% (v/v) glutaraldehyde for 4 hours. Samples were then washed in PBS three times for 5 minutes each. This was followed by a treatment of 1% osmium in PBS for 2 hours. Samples were washed and dehydrated in graded ethanol (30%, 50%, 70%, 90%, 95%, and 100%, v/v) for 10 minutes each. The critical point was dried and gold was sputtered. Samples were analyzed using scanning electron microscopy (S-520, Hitachi, Japan).
Endothelial cells seeding
We used the enzymatic digestion technique to dislodge only inner surface cells of the HUV. These cells were then collected into a tube and centrifuged into a pellet. The pellet was then re-suspended and plated into culture flasks pre-coated with collagen I matrix. Endothelial cells isolated from human umbilical veins were cultured in endothelial cell growth medium (EGM) at 37 °C and 5% CO2 until they almost reached confluence. Decellularized umbilical artery segments were cut into 5 × 10 mm open patches and put into culture plates with the luminal surface facing up. The endothelial cells were trypsinized. Subsequently, the endothelial cells were resuspended and seeded to the aforementioned patches at a concentration of 2 × 106 cells/mL. Cells were then incubated at 37 °C and 5% CO2 for 5 days.
Statistical analysis
Continuous variables are expressed as mean ± SD. The paired-samples t-test was used for analysis. Differences between outcomes were evaluated by means of the non-parametric McNemar test. Statistical significance was considered at a p - level of less than 0.05. Statistical evaluation was performed using SPSS 13.0 for Windows.
RESULTS
Decellularization
Treatment of artery segments with 0.25% trypsin and 0.01% EDTA for 30 minutes followed by treatment of 1% SDS for 180 minutes led to complete acellularity of the arteries. The matrix, particularly cross-linking and elastic fibers, was preserved (Figure 2A-C and Figure 3A-D).
Figure 2.
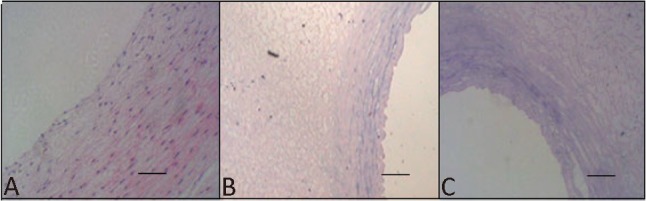
H&E staining of the human umbilical arteries before (A) and after (B) the treatment with SDS buffers for 2 h and after (C) the treatment with SDS buffers for 3 h. Original magnification, 200 × scale bar = 50 μm.
Figure 3.
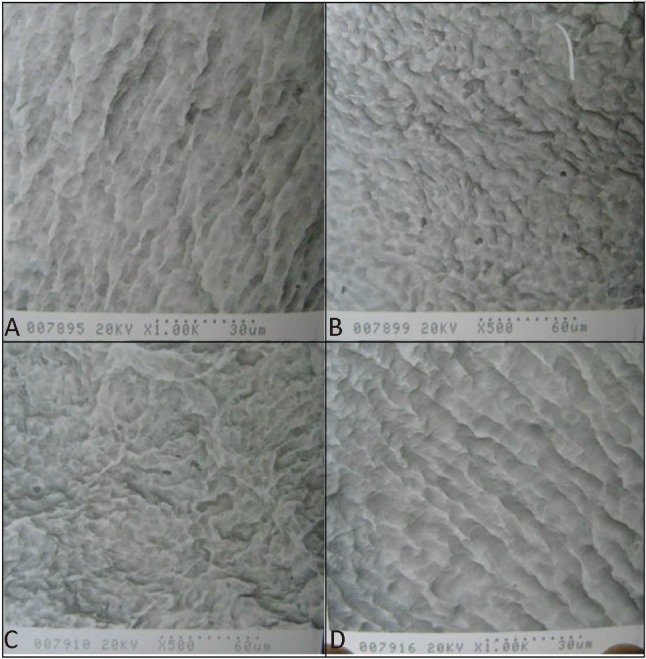
Scanning electron microscopy image of the luminal surface of the human umbilical arteries before the intervention (A), after the treatment with trypsin/EDTA buffers for 0.5 h (B), after the treatment with SDS buffers for 1.5 h (C) and after the treatment with SDS buffers for 3 h (D).
Burst pressure testing
Two vessels ruptured below the pressure of 300 mmHg and 28 vessels remained intact above the pressure of 300 mmHg in each group. There was no difference in burst pressure testing between the groups (p > 0.05).
Stress-strain testing
No significant differences in the maximum stress were found between the non-decellularized and decellularized umbilical arteries (Table 1).
Table 1. Max. stress of human umbilical arteries before and after decellularization.
| Umbilical arteries | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Non-decellularized vessels | 3.8 | 3.9 | 3 | 2.9 | 3.5 | 3.4 | 4 | 4.1 | 3.7 | 3.2 |
| Decellularized vessels | 3.7 | 3.7 | 3.1 | 2.8 | 3.4 | 3.2 | 4 | 4 | 3.9 | 3.1 |
The maximum stress of the arteries before and after denuding was 3.55 ± 0.42N and 3.50 ± 0.43N respectively (p = 0.17).
Human endothelial cells compatibility of the decellularized HUA
Light microscope examination showed that endothelial cells adhered to and spread well on the surface of the decellularized umbilical artery patches (Figure 4). These data support the potential use of decellularized HUA as bio-scaffolds for vascular tissue engineering.
Figure 4.
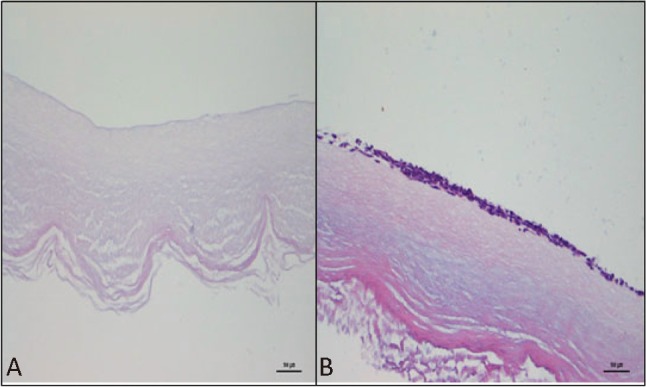
Light microscope examination of the decellularized umbilical artery patches before seeding (A) and seeded with human endothelial cells 5 days (B). Original magnification, 400 × scale bar = 500 μm.
DISCUSSION
From a clinical perspective, a biocompatible bypass grafting material with the appropriate mechanical properties that are resistant to thrombotic and hyperplastic responses that are used in small caliber vasculatures has yet to be found. The use of ex-vivo blood vessels as scaffolds for guided organ regeneration aims to provide an ideal chemical and physical environment that promotes biologic function and integration. However, the use of these materials does necessitate a degree of tissue processing to stabilize, sterilize, and prevent chronic foreign material responses. Two approaches have generally been taken in synthesizing a new material: 1) cross-linking, and 2) removal of host epitopes by decellularization. The technique of decellularization is used to decrease antigenicity, thereby reducing subsequent graft rejection and host allosensitization.19 There are several methods to remove cells from tissue including hypotonic cell lysis, enzymatic digestion, mechanical stripping and tissue fixation. Generally, solutions are used to decellularize the vessels, and these solutions are usually incubated and shaken with the vessels during the decellularization process. This method was effective on large diameter vessels because solutions can enter the vasulature easily. However, the same is not true with small diameter vessels as their constricted size may block the entrance of the solutions. It is well-known that the endothelial cells are located at the luminal surface of the vasculature. Introduction of the enzymatic solution into the vasculature enables the solutions to have direct contact with the cells and denude the luminal surface. Additionally, shear stress from pulsatile flow used in the current study would rinse the vessels’ lumen, which would strengthen the effectiveness of the decellularization. Only three and half hours were required to completely decellularize these vessels, as compared to several days to several weeks as previously indicated. The decellularization process was convenient and easily scalable, thus allowing the preparation of large amounts of decellularized umbilical arteries within several hours. Compared to other tissue engineering approaches, the short production time using decellularization approaches is also cost and labor efficient.
The enzymatic solution we chose in this study was trypsin/EDTA, which can detach the endothelial cell from the vasculature. SDS is a tissue detergent that lyses cell membranes and it can lyze cells uniformly within the vasculature. Furthermore, its mechanism of action may make it less damaging than other enzymatic, mechanical, or tissue fixation methods. Decellularization with SDS resulted in uniform cell removal throughout the tissue. Removal of the smooth muscle cells required higher concentrations of SDS. Examination of the extracellular matrix and basement membrane demonstrated that SDS did not significantly alter the morphology of these two components. This finding is important in that collagen is responsible for vessel strength, and elastin imparts distensibility and recoil. Adequate initial strength is an absolute requirement for any arterial bypass graft. Results of the in-vitro strength tests suggest that SDS decellularization did not significantly alter artery strength. From a functional standpoint, this finding supports our findings that no significant change was found in collagen morphology.17,20,21
Mechanical redundancy is critical for long-term resilience to physiologic stresses. It is adequate for the blood vessel scaffolds to endure 300 mmHg pressure since the blood pressure is about 100 mmHg under normal physiological conditions and does not exceed 300 mmHg even where pathological conditions are involved. For this reason, we test the HUA by the standard of 300 mmHg. In our study, the majority of the artery segments (28/30) endured the pressure of 300 mmHg; there was no difference in burst pressure testing between both treated and control groups. Also, there were no significant differences in the maximum stress. These results showed that decellularization did not impact HUA’s mechanical characteristics.
Biocompatibility is another essential property of the scaffold which ensures that the scaffold can be reseeded. The decellularized HUA can be reseeded with smooth muscle cells and endothelial cells.17,18 We observed that endothelial cells become confluent on the umbilical artery scaffold after three days in culture. These results indicate that the decellularized HUA has good biocompatibility.
The umbilical artery has a uniform caliber throughout the whole length at about 1.5 mm under collapsed conditions, and 4.5-5.5 mm under physiological pressure. Native tissues, including human saphenous veins, have been decellularized and have shown potential use as small-caliber vascular grafts; however, the human umbilical artery might represent a more attractive source in that it is widely available and easily isolated.17 The decellularization process applied in our study was convenient and could be easily scaled up, thus allowing the preparation of large amounts of decellularized umbilical arteries within a few hours. Also, compared to other tissue engineering approaches, where preparation of a mechanically robust vascular graft takes several weeks, this short production time using decellularization approaches appears to be cost and labor efficient. Therefore, decellularization of human umbilical arteries might represent a more appropriate approach for preparing small-caliber vascular grafts. The potential ability to grow, repair and remodel might allow the graft to adapt to the physical properties, promote biological function and minimize degradation over time. In comparison to other decellularization methods, our approach is convenient and does not impact tissue mechanical characteristics.
CONCLUSIONS
In conclusion, decellularization of the widely available human umbilical artery by the pulsatile flow provides a convenient and efficient approach for developing a potential small-caliber vascular graft.
Acknowledgments
The study was supported by the foundation of Beijing Municipal Commission of Education (KM200710025022).
CONFLICT OF INTEREST
None declared.
REFERENCES
- 1.Naito Y, Shinoka T, Duncan D, et al. Vascular tissue engineering: towards the next generation vascular grafts. Adv Drug Deliv Rev. 2011;63:312–323. doi: 10.1016/j.addr.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Sheridan WS, Duffy GP, Murphy BP. Mechanical characterization of a customized decellularized scaffold for vascular tissue engineering. J Mech Behav Biomed Mater. 2012;8:58–70. doi: 10.1016/j.jmbbm.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Wang CC, Hsu LA, Chen WJ, et al. Global risk classification predicts the clinical outcomes of patients with significant left main coronary artery disease undergoing coronary bypass surgery. Acta Cardiol Sin. 2012;28:118–128. [Google Scholar]
- 4.Cleary MA, Geiger E, Grady C, et al. Vascular tissue engineering:the next generation. Trends Mol Med. 2012;18:394–404. doi: 10.1016/j.molmed.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Soletti L, Hong Y, Guan J, et al. A bilayered elastomeric scaffold for tissue engineering of small diameter vascular grafts. Acta Biomater. 2010;6:110–122. doi: 10.1016/j.actbio.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pooyan P, Tannenbaum R, Garmestani H. Mechanical behavior of a cellulose-reinforced scaffold in vascular tissue engineering. J Mech Behav Biiomed Mater. 2012;7:50–59. doi: 10.1016/j.jmbbm.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Huang SS, Leu HB, Lu TM, et al. The impacts of in-hospital invasive strategy on long-term outcome in elderly patients with non-st-elevation myocardial infarction. Acta Cardiol Sin. 2013;29:115–123. [PMC free article] [PubMed] [Google Scholar]
- 8.Tschoeke B, Flanagan TC, Cornelissen A, et al. Development of a composite degradable/nondegradable tissue-engineered vascular graft. Artif Organs. 2008;32:800–809. doi: 10.1111/j.1525-1594.2008.00601.x. [DOI] [PubMed] [Google Scholar]
- 9.Grigioni M, Daniele C, Avenio G, Barbaro V. Biomechanics and hemodynamics of grafting In: Tura A, editor. Vascular grafts: experiment and modeling. Boston: WIT Press; 2003. pp. 41–82. [Google Scholar]
- 10.Davids L, Dower T, Zilla P. The lack of healing in conventional vascular grafts. In: Zilla P, Greisler HP, editors. Tissue engineering of vascular prosthetic grafts. Austin: R.G. Landes; 1999. pp. 3–45. [Google Scholar]
- 11.Williams SK, Rose DG, Jarrell BE. Microvascular endothelial cell sodding of ePTFE vascular grafts: improved patency and stability of the cellular lining. J Biomed Mater Res. 1994;28:203–212. doi: 10.1002/jbm.820280210. [DOI] [PubMed] [Google Scholar]
- 12.Weintraub WS, Jones EL, Craver JM, Guyton RA. Frequency of repeat coronary bypass or coronary angioplasty after coronary artery bypass surgery using saphenous venous grafts. Am J Cardiol. 1994;73:103–112. doi: 10.1016/0002-9149(94)90198-8. [DOI] [PubMed] [Google Scholar]
- 13.Konner K. Primary vascular access in diabetic patients: an audit. Nephrolo Dial Transplant. 2000;15:1317–1325. doi: 10.1093/ndt/15.9.1317. [DOI] [PubMed] [Google Scholar]
- 14.Nerem RM, Seliktar D. Vascular tissue engineering. Annu Rev Biomed Eng. 2001;3:225–243. doi: 10.1146/annurev.bioeng.3.1.225. [DOI] [PubMed] [Google Scholar]
- 15.Thomas AC, Campbell GR, Campbell JH. Advances in vascular tissue engineering. Cardiovasc Pathol. 2003;12:271–276. doi: 10.1016/s1054-8807(03)00086-3. [DOI] [PubMed] [Google Scholar]
- 16.Markus H, Volker RJ, Georgine H, et al. Advantages of human umbilical vein scaffolds derived from cesarean section vs. vaginal delivery for vascular tissue engineering. Biomaterials. 2008;29:1075–1084. doi: 10.1016/j.biomaterials.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Gui L, Muto A, Chan SA, et al. Development of decellularized human umbilical arteries as small-diameter vascular grafts. Tissue Engineering. 2009;15:2665–2676. doi: 10.1089/ten.tea.2008.0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kerdjoudj H, Berthelemy N, Rinckenbach S, et al. Small vessel replacement by human umbilical arteries with polyelectrolyte film-treated arteries. J Am Coll Cardiol. 2008;52:1589–1597. doi: 10.1016/j.jacc.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Daniel J, Abe K, McFetridge PS. Development of the human umbilical vein scaffold for cardiovascular tissue engineering applications. ASAIO J. 2005;51:252–261. doi: 10.1097/01.mat.0000160872.41871.7e. [DOI] [PubMed] [Google Scholar]
- 20.Schaner PJ, Martin ND, Tulenko TN, et al. Decellularized vein as a potential scaffold for vascular tissue engineering. J Vasc Surg. 2004;40:146–153. doi: 10.1016/j.jvs.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 21.Huynh T, Abraham G, Murray J, et al. Remodeling of an acellular collagen graft into a physiologically responsive neovessel. Nat Biotechnol. 1999;17:1083–1086. doi: 10.1038/15062. [DOI] [PubMed] [Google Scholar]


