Abstract
Background
Intracoronary nitroprusside and thrombus aspiration have been demonstrated to improve myocardial perfusion during percutaneous coronary interventions (PCI) for ST-segment elevation acute myocardial infarction (STEMI) However, no long-term clinical studies have been performed comparing these approaches.
Methods
A single medical center retrospective study was conducted to evaluate the effects of intracoronary nitroprusside administration before slow/no-reflow phenomena versus thrombus aspiration during primary PCI. Forty-three consecutive patients with STEMI were enrolled in the intracoronary nitroprusside treatment group. One hundred twenty-four consecutive STEMI patients who received thrombus aspiration were enrolled; ninety-seven consecutive STEMI patients who did not receive either thrombus aspiration or intracoronary nitroprusside treatment were enrolled and served as control subjects. Patients with cardiogenic shock, who had received platelet glycoprotein IIb/IIIa inhibitor, or intra-aortic balloon pump insertion were excluded. Thrombolysis in Myocardial Infarction (TIMI) flow grade, corrected TIMI frame count and TIMI myocardial perfusion grade (TMPG) were assessed prior to and following PCI by two independent cardiologists blinded to the procedures. The rate of major adverse cardiac events (MACE) at 30 days, 1 year, and 3 years after study enrollment as a composite of recurrent myocardial infarction, target-vessel revascularization, and cardiac death were recorded.
Results
The control group had a significantly lower pre-PCI TIMI flow (≤ 1; 49.5% vs. 69.8% vs. 77.4%; p = < 0.001) compared with the nitroprusside and thrombus aspiration groups. The thrombus aspiration group had a significantly higher pre-PCI thrombus score (> 4; 98.4% vs. 88.4% vs. 74.3%; p = < 0.001) and post-PCI TMPG (3; 39.5% vs. 16.3% vs. 20.6%; p = 0.001) compared with the nitroprusside and control groups. No significant differences were noted in the post-PCI thrombus score, 30-day, 1-year and 3-year MACE rate, and Kaplan-Meier curve among 3 groups of patients.
Conclusions
Although thrombus aspiration provided improved TMPG compared with early administration of intracoronary nitroprusside and neither of both during primary PCI, it did not have a significant impact on 30-day, 1-year and 3-year MACE rate.
Keywords: Acute myocardial infarction, Intracoronary nitroprusside, Thrombus aspiration
INTRODUCTION
Primary percutaneous coronary intervention (PCI) is an effective treatment for acute ST-segment elevation myocardial infarction (STEMI). Primary PCI has been recommended as a Class I-Level of Evidence, an indication by the 2012 European Heart Society Guidelines on the management of myocardial infarction in patients with persistent STEMI.1 However, the slow/no-reflow phenomenon that presents as inadequate myocardial perfusion was observed in 10-44% of cases and occurs after the target vessel has been adequately dilated without evidence of angiographic mechanical obstruction.2-4 This phenomenon has been associated with higher left ventricular dysfunction and adverse clinical outcomes.4-7 The pathogenesis of slow/no-reflow is multi-factorial and primarily attributed to vasospasm of the resistance arterioles, microcirculation dysfunction, and distal embolization.8-11 Among the available vasodilator agents, nitroprusside with a rapid and marked vasodilating effect has been proposed to be effective at restoring coronary blood flow, with limited systemic hypotension.12-14 Likewise, it has also been demonstrated that thrombus aspiration is associated with improved microvascular perfusion.11,15 However, no data exists comparing the long-term clinical beneficial effects of these 2 therapies in patients with American Megatrends (AMI) undergoing primary PCI. Thus, the current study was conducted to compare the early administration of intracoronary nitroprusside versus thrombus aspiration and controls in myocardial perfusion for STEMI.
METHODS
From late September 2006 to August 2008, 43 consecutive patients who underwent direct PCI within 12 hours from the onset of STEMI were retrospectively enrolled for intracoronary nitroprusside treatment. All cases were performed by an interventional cardiologist who had performed more than 400 cases of primary PCI from 1995 to 2006. Intracoronary nitroprusside (100 μg) was administered just before the guidewire was advanced through the infarct-related vessel with Thrombolysis in Myocardial Infarction (TIMI) flow grade > 0, or just after the guidewire was advanced through the distal portion of the totally occluded infarct-related vessel. If the vessel developed improved TIMI flow, then intracoronary nitroprusside was administered after subsequent angiogram. Otherwise, intracoronary nitroprusside was administered just as an un-inflated balloon catheter was introduced through the totally occluded site of the infarct-related artery to the distal portion of the vessel. During the remainder of the procedures, administration of intracoronary nitroprusside (100 μg) continued and was administered in short intervals. For the thrombus aspiration group, we retrospectively enrolled a total of 124 consecutive patients from January 2005 to December 2008 who had undergone direct PCI with thrombus aspiration within 12 hours of STEMI who neither received an intracoronary calcium channel blocker, adenosine or nitroprusside. In the thrombus aspiration group after passage of the guidewire, a 6-French Export Aspiration Catheter (crossing profile, 0.068”) (Medtronic, Minneapolis, MN, USA) was advanced into the target coronary segment with continuous aspiration, whereafter either balloon dilatation with or without subsequent stent implantation or direct stenting was performed.
From January 2005 to December 2008, a total of 97 consecutive patients who had undergone primary PCI without either thrombus aspiration or intracoronary nitroprusside administration within 12 hours of STEMI and who had not received an intracoronary calcium channel blocker or adenosine, were retrospectively enrolled.
STEMI patients were included if they had experienced continuous chest pain for at least 30 minutes, if they had been sent for PCI within 12 hours of onset of chest pain and found to have ST-segment elevation ≥ 1 mm (0.1 mV) in two or more contiguous leads or new-onset of LBBB on the 12-lead electrocardiogram. Exclusion criteria included cardiogenic shock, treatment with an intra-aortic balloon pump, and platelet glycoprotein IIb/IIIa inhibitor use.
TIMI flow grade, corrected TIMI frame count (CTFC) and TIMI myocardial perfusion grade (TMPG) were assessed before and after PCI by two independent cardiologists blinded to the study protocol. The primary endpoints were: 1) the post-procedural frequencies of a TIMI flow grade of 3, 2) CTFC of < 14, 3) TMPG of 3, and 4) the major adverse cardiac events (MACE) rate. Here, MACE included recurrent myocardial infarction, target-vessel revascularization, and cardiac death by 30 days, 1-year and 3-year after the primary PCI procedure. Information on recurrent ischemic symptoms, death, target vessel myocardial infarction, and need for revascularization of the treated vessels was collected by chart review, outpatient visit, or telephone contact at 1-year and 3-year intervals after the primary PCI procedure. This retrospective study was approved by the Institutional Review Board at Kaohsiung Chang Gung Memorial Hospital.
Angiographic definitions
In general, thrombus score grading scales are used for qualification and quantification of the thrombus burden. Altogether, the TIMI classification relies on the angiographic assessment of the presence of thrombus and its relative size, utilizing a simple score ranging from grade 0 (no thrombus), to grade 5 (very large thrombus content that completely occludes vessel flow). Grade 0 was defined as no angiographic evidence of thrombus. Grade 1 was defined as angiographic features suggestive of thrombus with angiographic characteristics such as decreased contrast density, haziness of contrast, irregular lesion contour or a smooth convex meniscus at the site of a total occlusion suggestive, but not firmly diagnostic, of thrombus. Grade 2 was defined as definite thrombus present in multiple angiographic projections with marked irregular lesion contour with a significant filling defect, where the thrombus’ greatest dimension is < 1/2 of the vessel diameter. Grade 3 was defined as definite thrombus which appears in multiple angiographic views with the greatest dimension from > 1/2 to < 2 vessel diameters. Grade 4 was defined as definite large size thrombus present with a greatest dimension of > 2 vessel diameters. Grade 5 was defined as definite complete thrombotic occlusion of a vessel with a convex margin that stains with contrast, persisting for several cardiac cycles.
TIMI flow grade was classified into 4 groups: grade 0 (no flow); grade 1 (penetration without perfusion); grade 2 (partial perfusion); or grade 3 (complete perfusion). To objectively evaluate an index of coronary flow as a continuous quantitative variable, the number of cineframes required for contrast to first reach standardized distal coronary landmarks in the infarct-related artery (the TIMI frame count) was measured with a frame counter on the cineviewer (25 frames/sec). A CTFC < 14 was defined as a CTFC that was faster than the 95% confidence interval for normal flow (0-13 frames, hyperemia, TIMI 4 flow). A CTFC of 40 had been previously identified as the cutoff point between TIMI-3 and TIMI-2 flow.
The TMPG was used to assess the filling and clearance of contrast in the myocardium. TMPG-0 was defined as no apparent tissue-level perfusion (no ground-glass appearance of blush or opacification of the myocardium) in the distribution of the culprit artery. TMPG-1 indicated a presence of myocardial blush but no clearance from the microvasculature (blush or a stain present on the next injection). TMPG-2 indicated that the blush cleared slowly (blush strongly persistent and diminished minimally or not at all during 3 cardiac cycles of the washout phase). TMPG-3 indicated that blush began to clear during washout (blush minimally persistent after 3 cardiac cycles of washout).
Statistics
Data were expressed as mean ± standard deviation unless otherwise noted. Categorical data were analyzed by Chi-square test or Fischer’s exact test. Continuous variables among the three groups were compared using one-way ANOVA followed by Bonferroni multiple comparison procedure. MACE-free survival during follow-up was estimated with the Kaplan-Meier curve. A p-value of less than 0.05 was considered to be significant. Statistical analyses were conducted using SPSS statistical software for Windows version 13 (SPSS Inc., Chicago, IL, USA).
RESULTS
Our study found that clinical characteristics were similar between the nitroprusside, thrombus aspiration, and the control groups, except there was significantly increased left anterior descending artery involvement in the nitroprusside and control groups compared with the thrombus aspiration group (p = 0.049; Table 1).The thrombus aspiration group had a significantly higher pre-PCI thrombus score ≥ 4 compared with the nitroprusside and control groups (p = < 0.001; Table 2). The control group had a significantly lower pre-PCI TIMI flow ≤ 1 compared with the nitroprusside and thrombus aspiration groups (p = < 0.001; Table 2). The degree of pre-PCI % stenosis was significantly higher in the thrombus aspiration and nitroprusside groups compared with the control group (p = < 0.001; Table 2). The thrombus aspiration group had a significantly higher pre-PCI total occlusion compared with the control group (p = 0.001; Table 2).
Table 1. Baseline characteristics of patients .
| Group 1 (43) | Group 2 (124) | Group 3 (97) | p-value | |
| General demographics | ||||
| Age (yr) | 60.74 ± 13.35 | 58.91 ± 12.72 | 61.57 ± 12.28 | 0.289 |
| Male sex (%) | 88.4 | 85.5 | 81.4 | 0.529 |
| BMI | 25.36 ± 2.87 | 25.14 ± 3.24 | 24.98 ± 4.32 | 0.844 |
| Risk factors for MI | ||||
| Diabetes mellitus (%) | 48.8 | 36.3 | 36.1 | 0.296 |
| Current smoker (%) | 62.8 | 48.4 | 40.2 | 0.171 |
| Hypertension (%) | 53.5 | 59.7 | 53.6 | 0.732 |
| Old MI (%) | 2.3 | 3.2 | 3.1 | 0.956 |
| HF (advanced) (%) | 4.6 | 5.6 | 8.2 | 0.647 |
| ESRD (%) | 2.3 | 1.6 | 3.1 | 0.764 |
| Dyslipidemia (%) | 37.2 | 33.1 | 37.9 | 0.736 |
| The severity of MI | ||||
| SBP | 156.30 ± 30.87a | 138.00 ± 30.07b | 135.70 ± 27.00b | < 0.001* |
| Killip class (%) | 0.325 | |||
| I | 76.7 | 75.8 | 68.0 | |
| II | 11.6 | 17.7 | 24.7 | |
| III | 11.6 | 6.5 | 7.2 | |
| Infarct-related vessel | a | b | a | 0.049* |
| LAD (%) | 51.2 | 43.5 | 64.9 | |
| LCX (%) | 7.0 | 8.1 | 8.2 | |
| RCA (%) | 41.9 | 48.4 | 26.8 | |
| Procedure | 0.586 | |||
| POBA (%) | 4.7 | 0.8 | 2.1 | |
| BMS (%) | 86.0 | 90.3 | 90.7 | |
| DES (%) | 9.3 | 8.9 | 7.2 | |
| Laboratory examination | ||||
| Blood fasting sugar (mg/dL) | 124.05 ± 51.99 | 130.5 ± 48.93 | 141.9 ± 59.88 | 0.206 |
| HbA1C (%) | 7.68 ± 2.19 | 7.19 ± 2.08 | 7.06 ± 2.08 | 0.382 |
| Serum creatinine (mg/dL) | 1.35 ± 1.11 | 1.26 ± 1.06 | 1.52 ± 1.92 | 0.410 |
| Total cholesterol (mg/dL) | 186.12 ± 43.03 | 190.01 ± 42.35 | 192.73 ± 44.47 | 0.703 |
| LDL (mg/dL) | 119.33 ± 37.92 | 119.63 ± 37.19 | 122.71 ± 38.73 | 0.812 |
| HDL (mg/dL) | 38.53 ± 7.95 | 41.16 ± 10.27 | 40.37 ± 10.61 | 0.340 |
| Cardiac biomarker | ||||
| Troponin-I (ng/mL) | ||||
| initial | 4.01 ± 2.58a,b | 2.20 ± 5.30a | 5.07 ± 7.95b | 0.008* |
| peak | 23.94 ± 26.59 | 19.20 ± 6.90 | 20.25 ± 11.88 | 0.415 |
| CK-MB (ng/mL) | ||||
| initial | 14.14 ± 19.89 | 15.79 ± 33.82 | 24.49 ± 42.68 | 0.152 |
| peak | 74.36 ± 85.86 | 80.25 ± 133.29 | 60.93 ± 60.58 | 0.410 |
| Peak CPK | 2081 ± 2258 | 2598 ± 2033 | 2314 ± 2505 | 0.416 |
| Timing of primary PCI | ||||
| Chest pain to reperfusion (minutes) | 298.7 ± 163.3 | 289.6 ± 164.5 | 296.4 ± 224.4 | 0.701 |
* Group 1: nitroprusside; Group 2: thrombectomy; Group 3: control.
* Data expressed as mean ± SD or % (n) of patients.
* Different letters (a, b, c) associated with different groups indicate significant difference (at 0.05 level) by Bonferroni multiple comparison procedure.
BMI, body mass index; BMS, bare metal stent; CK-MB, creatine kinase-MB; CPK, creatine phosphokinase; DES, drug-eluting stent; ESRD, end-stage renal disease; GRP, group; HDL, high-density lipoprotein; HF, heart failure; LAD, left anterior descending artery; LCX, left circumflex artery; LDL, low-density lipoprotein; RCA, right coronary artery; MI, myocardial infarction; PCI, percutaneous coronary interventions; POBA, pure balloon angioplasty; SBP, systolic blood pressure; SD, standard deviation.
Table 2. Angiographic data before and after PCI .
| Group 1 (43) | Group 2 (124) | Group 3 (97) | p-value | |
| Pre-PCI | ||||
| Pre-PCI stenosis (%) | 94.31 ± 8.53a | 95.78 ± 8.22a | 90.16 ± 10.64b | < 0.001* |
| Pre-PCI MLD (mm) | 0.16 ± 0.25a | 0.13 ± 0.27a | 0.29 ± 0.35b | 0.001* |
| Pre-PCI RLD (mm) | 3.07 ± 0.52 | 3.12 ± 0.51 | 3.00 ± 0.63 | 0.253 |
| Thrombus score (%) | < 0.001* | |||
| score ≥ 4 | 88.4a | 98.4b | 74.3a | |
| score ≤ 3 | 11.6 | 1.6 | 25.7 | |
| TIMI flow (%) | < 0.001* | |||
| grade ≤ 1 | 69.8a | 77.4a | 49.5b | |
| grade ≥ 2 | 30.1 | 22.6 | 50.5 | |
| Total occlusion (%) | 65.1a,b | 72.6a | 47.4b | 0.001* |
| Post-PCI | ||||
| Post-PCI stenosis (%) | 11.46 ± 7.69 | 12.33 ± 10.86 | 11.06 ± 7.78 | 0.594 |
| Post-PCI MLD (mm) | 2.82 ± 0.52 | 2.89 ± 0.57 | 2.96 ± 0.88 | 0.501 |
| Post-PCI RLD (mm) | 3.19 ± 0.52 | 3.28 ± 0.49 | 3.25 ± 0.58 | 0.634 |
| Thrombus score (%) | 0.478 | |||
| score ≥ 2 | 0 | 2.4 | 6.2 | |
| score ≤ 1 | 100 | 97.6 | 93.8 | |
| TIMI flow (%) | 0.068 | |||
| grade ≤ 2 | 0 | 4.1 | 8.9 | |
| grade 3 | 100 | 95.9 | 91.1 | |
| TMPG (%) | 0.001* | |||
| TMPG ≤ 2 | 83.7a | 60.5b | 79.4a | |
| TMPG 3 | 16.3 | 39.5 | 20.6 | |
| Mean CTFC | 15.40 ± 5.73a,b | 13.45 ± 5.78a | 15.84 ± 6.12b | 0.008* |
| CTFC range (%) | 0.180 | |||
| < 14 | 46.5 | 60.5 | 45.4 | |
| 14~40 | 52.5 | 38.7 | 53.6 | |
| > 40 | 0 | 0.8 | 1 | |
| Stent length | 25.71 ± 6.91 | 25.24 ± 6.71 | 25.80 ± 7.31 | 0.814 |
| Stent diameter | 3.12 ± 0.40 | 3.42 ± 0.22 | 3.13 ± 0.37 | 0.320 |
* Group 1: nitroprusside; Group 2: thrombectomy; Group 3: control; CTFC, corrected TIMI frame count; MLD, minimal luminal diameter; PCI, percutaneous coronary intervention; RLD, reference luminal diameter; TIMI, Thrombolysis in Myocardial Infarction flow grade; TMPG, TIMI myocardial perfusion grade.
* Data expressed as mean ± SD or % (n) of patients.
* Different letters (a, b, c) associated with different groups indicate significant difference (at 0.05 level) by Bonferroni multiple comparison procedure.
The thrombus aspiration group had a significantly higher post-PCI TMPG 3 compared with the nitroprusside and control groups (p = 0.001; Table 2). The mean CTFC was significantly lower in the thrombus aspiration group compared with the control group (p = 0.008; Table 2). There were no significant differences in post-PCI TIMI flow, thrombus score, CTFC range, % stenosis, minimal luminal diameter, reference vessel luminal diameter, stent length and stent diameter (Table 2). Additionally, no significant differences were noted in the mean follow-up days, lost follow-up rate, 30-day, 1-year and 3-year MACE rate among the groups (Table 3). The Kaplan-Meier curve showed no significant difference among nitroprusside, thrombus aspiration and control groups (Figure 1).
Table 3. Clinical events 30 days or 1 year or 3 years later .
| Group 1 (43) | Group 2 (124) | Group 3 (97) | p-value | |
| Mean follow-up days | 1626 ± 931 | 1568 ± 712 | 1706 ± 818 | 0.439 |
| Lost follow-up rate after 3-years (%) | 9.3 | 8.9 | 15.5 | 0.277 |
| 30-day | ||||
| Major adverse cardiac event (%) | 0 | 1.6 | 3.3 | 0.402 |
| Recurrent myocardial infarction (%) | 0 | 0.8 | 1.3 | 0.404 |
| Target-vessel revascularization (%) | 0 | 0.8 | 0 | 0.631 |
| Cardiovascular mortality (%) | 0 | 0.8 | 2.1 | 0.508 |
| 1-year | ||||
| Major adverse cardiac event (%) | 7.0 | 13.7 | 10.9 | 0.476 |
| Recurrent myocardial infarction (%) | 2.9 | 3.5 | 2.6 | 0.923 |
| Target-vessel revascularization (%) | 6.7 | 14.8 | 9.6 | 0.436 |
| Cardiovascular mortality (%) | 0 | 0.8 | 2.4 | 0.512 |
| 3-year | ||||
| Major adverse cardiac event (%) | 11.6 | 21.0 | 17.4 | 0.381 |
| Recurrent myocardial infarction (%) | 6.3 | 6.4 | 9.2 | 0.776 |
| Target-vessel revascularization (%) | 7.0 | 19.4 | 15.1 | 0.548 |
| Cardiovascular mortality (%) | 0 | 1.6 | 3.1 | 0.442 |
* Plus-minus values are means ± SD; Group 1: nitroprusside; Group 2: thrombectomy; Group 3: control.
* Data expressed as % (n) of patients.
* Different letters (a, b, c) associated with different groups indicate significant difference (at 0.05 level) by Bonferroni multiple comparison procedure.
Figure 1.
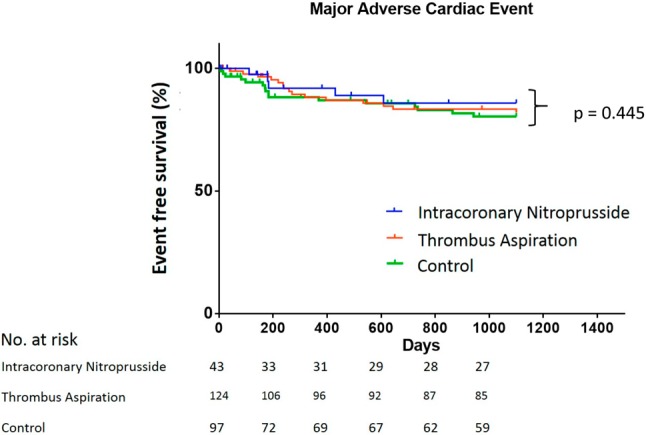
The Kaplan-Meier curve showed no significant difference among nitroprusside, thrombus aspiration and control groups (p = 0.445).
DISCUSSION
The slow/no-reflow phenomenon is a serious complication following direct PCI for AMI.16 Previous studies have demonstrated that both short- and long-term outcomes are poor in those who have developed the no-reflow phenomenon following direct PCI.17,18 Coronary thrombus material is associated with triggering thrombotic, inflammatory, vasoconstrictor, and other pathways. Evacuating a portion of the thrombus and plaque material only addresses a portion of the pathophysiological problem. Meta-analyses of randomized trials of thrombus aspiration have shown inconsistent results with respect to mortality.19-23 Systemic embolization can occur during thrombus aspiration.24 In a meta-analysis study conducted by De Luca et al., thrombus aspiration was shown to be associated with a trend toward an increased rate of stroke.21 In the Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS) trial, thrombus aspiration had been shown to improve myocardial reperfusion as compared with conventional PCI.1 At the 1-year follow-up, the secondary end point of mortality was lower in the thrombus aspiration group than in the conventional PCI group.25 However, in the Thrombus Aspiration in Myocardial Infarction Study (TASTE) trial, routine thrombus aspiration before PCI as compared with PCI alone did not reduce 30-day mortality among patients with STEMI.26 In this registry-based, randomized, controlled trial, no significant benefit of aspiration thrombectomy with respect to mortality or several other clinical outcomes at 30 days was observed.26 Currently, no large randomized controlled trial for long-term outcome after thrombus aspiration is available. A retrospective small outcome study in 89 patients originally randomized to thrombectomy vs conventional PCI showed a reduced long-term risk for death or cardiac re-hospitalization in STEMI patients treated with thrombectomy after a minimum follow-up of up to 1115 days, despite no echocardiographic improvement of left ventricular ejection fraction.27 However, the current study has demonstrated that the thrombus aspiration group had significantly higher post-PCI TMPG 3, but there were no significant difference in the 30-day, 1-year and 3-year MACE rate among patients treated with nitroprusside, thrombus aspiration and controls. The mechanism of thrombus aspiration by use of a 6-Fr Export Aspiration Catheter in improving the myocardial perfusion during primary PCI in STEMI patients is not clear. The ineffectiveness of percusurge which utilizes the same catheter to perform thrombus aspiration with the addition of a distal protection balloon in achieving improvement in myocardial perfusion as compared with aspiration alone is unreasonable. The possible mechanism might be due to the existence of controlled distal embolization while the dottering technique was employed during the aspiration process using a relatively large profile catheter (crossing profile, 0.068”).
The pathogenesis of the slow/no-reflow phenomenon is complex, and a single therapeutic approach is unlikely to prevent or treat this phenomenon effectively. Vasodilators such as verapamil, adenosine, nicorandil and nitroprusside have been shown to improve coronary flow and myocardial perfusion.9,12-14,28-31 However, no long-term follow-up study has compared intracoronary nitroprusside injection with thrombus aspiration during primary PCI in STEMI. In the current study, intracoronary nitroprusside failed to improve post-PCI TIMI flow, TMPG 3, CTFC range (%) and clinical outcomes compared with control. Furthermore, it achieved a significantly lower post-PCI TMPG 3 rate compared with thrombus aspiration. In a randomized study conducted by Amit et al., 60 μg of nitroprusside was injected once before balloon dilatation and distal to the occlusion site.31 Although, the study failed to improve coronary flow and myocardial tissue reperfusion, clinical outcomes were improved.31 Likewise, Sinozaki et al. reported nitroprusside (120 μg) was selectively administered through the drug delivery catheter into the distal coronary artery before balloon dilatation.13 Similar to the current study, nitroprusside was repeatedly administered throughout the procedure.13 In this study, nitroprusside administration was shown to prevent slow/no-reflows and improve reperfusion of infarcted myocardium.13 Thus, the administration of nitroprusside proximally through the guiding catheter instead of distal delivery as currently reported might explain the ineffectiveness of our approach.
Study limitation
Our study did have certain limitations. First, this study was a single center, non-randomized, retrospectively study with a small number of patients. Secondly, selection bias was probable. The presence of higher thrombus load may have predisposed the operators to select subjects for the thrombus aspiration group over the conventional PCI approach. This may have led to significantly higher pre-PCI thrombus scores, total occlusion rates, and TIMI ≤ 1 flow rates in the thrombus aspiration group compared with the control group.
CONCLUSIONS
Although thrombus aspiration provided improved TMPG compared with early administration of intracoronary nitroprusside during primary PCI, it did not have a significant impact on 30-day, 1-year and 3-year MACE rate.
REFERENCES
- 1.Steg G, James SK, Atar D, et al. Management of myocardial infarction in patients with persistent ST-segment elevation. Eur Heart J. 2012;33:2569–2619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- 2.Piana RN, Paik GY, Moscucci M, et al. Incidence and treatment of ‘no-reflow’ after percutaneous transluminal coronary intervention. Circulation. 1994;89:2514–2518. doi: 10.1161/01.cir.89.6.2514. [DOI] [PubMed] [Google Scholar]
- 3.Ito H, Iwakura K. Assessing the relation between coronary reflow and myocardial reflow. Am J Cardiol. 1998;81:8G–12G. doi: 10.1016/s0002-9149(98)00046-0. [DOI] [PubMed] [Google Scholar]
- 4.Ito H, Maruyama A, Iwakura K, et al. Clinical implications of the ‘no-reflow’ phenomenon: a predictor of complications and left ventricular remodeling in reperfused anterior wall myocardial infarction. Circulation. 1996;93:223–228. doi: 10.1161/01.cir.93.2.223. [DOI] [PubMed] [Google Scholar]
- 5.Abbo KM, Dooris M, Glazier S. Features and outcome of no-reflow after percutaneous coronary intervention. Am J Cardiol. 1995;75:778–782. doi: 10.1016/s0002-9149(99)80410-x. [DOI] [PubMed] [Google Scholar]
- 6.Morishima I, Sone T, Mokuno S, et al. Clinical significance of no-reflow phenomenon observed on angiography after successful treatment of acute myocardial infarction with percutaneous transluminal coronary angioplasty. Am Heart J. 1995;130:239–243. doi: 10.1016/0002-8703(95)90434-4. [DOI] [PubMed] [Google Scholar]
- 7.Morishima I, Sone T, Okumura K, et al. Angiographic no-reflow phenomenon as a predictor of adverse long-term outcome in patients treated with percutaneous transluminal coronary angioplasty for first acute myocardial infarction. J Am Coll Cardiol. 2000;36:1202–1209. doi: 10.1016/s0735-1097(00)00865-2. [DOI] [PubMed] [Google Scholar]
- 8.Taniyama Y, Ito H, Iwakura K, et al. Beneficial effect of intracoronary verapamil on microvascular and myocardial salvage in patients with acute myocardial infarction. J Am Coll Cardiol. 1997;30:1193–1199. doi: 10.1016/s0735-1097(97)00277-5. [DOI] [PubMed] [Google Scholar]
- 9.Hang CL, Wang CP, Yip HK, et al. Early administration of intracoronary verapamil improves myocardial perfusion during percutaneous coronary intervention for acute myocardial infarction. Chest. 2005;128:2593–2598. doi: 10.1378/chest.128.4.2593. [DOI] [PubMed] [Google Scholar]
- 10.Burzotta F, Crea F. Thrombus-aspiration: a victory in the war against no re-flow. Lancet. 2008;371:1889–1990. doi: 10.1016/S0140-6736(08)60808-9. [DOI] [PubMed] [Google Scholar]
- 11.Svilaas T, Vlaar PJ, van der Horst IC, et al. Thrombus aspiration during primary percutaneous intervention. N Engl J Med. 2008;358:557–567. doi: 10.1056/NEJMoa0706416. [DOI] [PubMed] [Google Scholar]
- 12.Hillegass WB, Dean NA, Liao L, et al. Treatment of no-reflow and impaired flow with the nitric oxide donor nitroprusside following percutaneous coronary interventions: initial human clinical experience. J Am Coll Cardiol. 2001;37:1335–1343. doi: 10.1016/s0735-1097(01)01138-x. [DOI] [PubMed] [Google Scholar]
- 13.Shinozaki N, Ichinose H, Yahikozawa K, et al. Selective intracoronary administration of nitroprusside before balloon dilatation prevents slow reflow during percutaneous coronary intervention in patients with acute myocardial infarction. Int Heart J. 2007;48:423–433. doi: 10.1536/ihj.48.423. [DOI] [PubMed] [Google Scholar]
- 14.Pasceri V, Pristipino C, Pelliccia F, et al. Effects of the nitric oxide donor nitroprusside on no-reflow phenomenon during coronary interventions for acute myocardial infarction. Am J Cardiol. 2005;95:1358–1361. doi: 10.1016/j.amjcard.2005.01.082. [DOI] [PubMed] [Google Scholar]
- 15.Burzotta F, Testa L, Giannico F, et al. Adjunctive devices in primary or rescue PCI: a meta-analysis of randomized trials. Int J Cardiol. 2008;123:313–321. doi: 10.1016/j.ijcard.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 16.Abbo KM, Dooris M, Glazier S. Features and outcome of no-reflow after percutaneous coronary intervention. Am J Cardiol. 1995;75:778–782. doi: 10.1016/s0002-9149(99)80410-x. [DOI] [PubMed] [Google Scholar]
- 17.Morishima I, Sone T, Mokuno S, et al. Clinical significance of no-reflow phenomenon observed on angiography after successful treatment of acute myocardial infarction with percutaneous transluminal coronary angioplasty. Am Heart J. 1995;130:239–243. doi: 10.1016/0002-8703(95)90434-4. [DOI] [PubMed] [Google Scholar]
- 18.Ito H, Maruyama A, Iwakura K, et al. Clinical implications of “no reflow” phenomenon: a predictor of complications and left ventricular remodeling in reperfused anterior wall myocardial infarction. Circulation. 1996;93:223–228. doi: 10.1161/01.cir.93.2.223. [DOI] [PubMed] [Google Scholar]
- 19.Burzotta F, De Vita M, Gu YL, et al. Clinical impact of thrombectomy in acute ST-elevation myocardial infarction: an individual patient-data pooled analysis of 11 trials. Eur Heart J. 2009;30:2193–2203. doi: 10.1093/eurheartj/ehp348. [DOI] [PubMed] [Google Scholar]
- 20.Bavry AA, Kumbhani DJ, Bhatt DL. Role of adjunctive thrombectomy and embolic protection devices in acute myocardial infarction: a comprehensive meta-analysis of randomized trials. Eur Heart J. 2008;29:2989–3001. doi: 10.1093/eurheartj/ehn421. [DOI] [PubMed] [Google Scholar]
- 21.De Luca G, Navarese EP, Suryapranata H. A meta-analytic overview of thrombectomy during primary angioplasty. Int J Cardiol. 2013;166:606–612. doi: 10.1016/j.ijcard.2011.11.102. [DOI] [PubMed] [Google Scholar]
- 22.Mongeon FP, Bélisle P, Joseph L, et al. Adjunctive thrombectomy for acute myocardial infarction: a Bayesian meta-analysis. Circ Cardiovasc Interv. 2010;3:6–16. doi: 10.1161/CIRCINTERVENTIONS.109.904037. [DOI] [PubMed] [Google Scholar]
- 23.Tamhane UU, Chetcuti S, Hameed I, et al. Safety and efficacy of thrombectomy in patients undergoing primary percutaneous coronary intervention for acute ST elevation MI: a meta-analysis of randomized controlled trials. BMC Cardiovasc Disord. 2010;10:10. doi: 10.1186/1471-2261-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin MS, Wu LS, Cheng NJ, et al. Thrombus aspiration complicated by systemic embolization in patients with acute myocardial infarction. Circ J. 2009;73:1356–1358. doi: 10.1253/circj.cj-08-0569. [DOI] [PubMed] [Google Scholar]
- 25.Vlaar PJ, Svilaas T, van der Horst IC, et al. Cardiac death and reinfarction after 1 year in the thrombus aspiration during percutaneous coronary intervention in acute myocardial infarction study (TAPAS): a 1-year follow-up study. Lancet. 2008;371:1915–1920. doi: 10.1016/S0140-6736(08)60833-8. [DOI] [PubMed] [Google Scholar]
- 26.Fröbert O, Lagerqvist B, Olivecrona GK. Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med. 2013;369:1587–1597. doi: 10.1056/NEJMoa1308789. [DOI] [PubMed] [Google Scholar]
- 27.Adlbrecht C, Distelmaier K, Bonderman D, et al. Long-term outcome after thrombectomy in acute myocardial infarction. Eur J Clin Invest. 2010;40:233–241. doi: 10.1111/j.1365-2362.2009.02253.x. [DOI] [PubMed] [Google Scholar]
- 28.Lee HC, An SG, Choi JH, et al. Effect of intra-coronary nicorandil administration prior to reperfusion in acute ST segment elevation myocardial infarction. Cir J. 2008;72:1425–1429. doi: 10.1253/circj.cj-08-0212. [DOI] [PubMed] [Google Scholar]
- 29.Iwakura K, Ito H, Okamura A, et al. Nicorandil treatment in patients with acute myocardial infarction. Circ J. 2009;73:925–931. doi: 10.1253/circj.cj-08-1059. [DOI] [PubMed] [Google Scholar]
- 30.Grygier M, Araszkiewicz A, Lesiak M, et al. New method of intracoronary adenosine injection to prevent microvascular reperfusion injury in patients with acute myocardial infarction undergoing percutaneous coronary intervention. Am J Cardiol. 2011;107:1131–1135. doi: 10.1016/j.amjcard.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 31.Amit G, Cafri C, Yaroslavtsev S, et al. Intracoronary nitroprusside for the prevention of the no-reflow phenomenon after primary percutaneous coronary intervention in acute myocardial infarction. A randomized, double-blinded, placebo-controlled clinical trial. Am Heart J. 2006;152:887.e9–887.e14. doi: 10.1016/j.ahj.2006.05.010. [DOI] [PubMed] [Google Scholar]


