Abstract
Background
Percutaneous coronary intervention (PCI) has become an alternative treatment for left main (LM) coronary artery disease. The aim of our study was to compare long-term clinical outcomes of patients undergoing unprotected LM PCI with bare-metal stent (BMS) or drug-eluting stent (DES) in a high-risk population.
Methods and Results
We enrolled 223 consecutive patients with unprotected LM coronary artery disease undergoing PCI (mean age: 71.1 ± 11.2 years, 187 male), including 94 patients receiving BMS and 129 patients receiving DES. The patients receiving DES had a significantly higher SYNTAX score (p = 0.05). During the mean follow-up period of 2.5 years, there were 31 cardiovascular deaths (BMS: 21 cases, DES: 10 cases, p = 0.04 by log-rank test), 56 major adverse cardiovascular events (MACE, including cardiovascular death, non-fatal myocardial infarction (MI) and clinical-driven target lesion revascularization; BMS: 33 cases, DES: 23 cases, p = 0.03 by log-rank test) and 6 cases with definite/probable stent thrombosis (BMS: 5 cases, DES: 1 cases, p = 0.09). In multivariate Cox analysis, the use of DES was identified as an independent protective factor against cardiovascular death [hazard ratio (HR) = 0.34, 95% confidence interval (Cl) = 0.15-0.79, p = 0.01] and MACE (HR = 0.50, 95% CI = 0.28-0.88, p = 0.02). The clinical outcome analyses in propensity-score matched the cohort (87 matched pair of patients receiving BMS and DES) and yielded similar results.
Conclusions
In the general practice among a high-risk population undergoing unprotected LM PCI, the use of DES appeared to be beneficial in reducing the risk of long-term cardiovascular death and MACE.
Keywords: Bare-mental stent, Drug-eluting stent, Left main coronary artery disease, Percutaneous coronary intervention
INTRODUCTION
Although coronary artery bypass grafting (CABG) remains the customary treatment in unprotected left main (LM) coronary artery disease,1-3 percutaneous coronary intervention (PCI) with stenting, especially using a drug-eluting stent (DES), has emerged as an alternative treatment with acceptable short-term and long-term clinical outcomes in recent studies.4-8 Although DES has been shown to apparently reduce the rate of restenosis and target lesion revascularization (TLR) as compared with bare-metal stents (BMS), it did not reduce the rate of cardiovascular death and myocardial infarction (MI).4,5,9,10 Furthermore, some conflicting evidence showed that, compared with BMS, DES might actually be associated with higher rate of late/very late stent thrombosis, which may be associated with catastrophic adverse events in the situation of LM stenting.11 Therefore, in this study we aimed to assess the long-term clinical outcomes of LM disease treated with PCI with BMS or DES stenting in a real-world high-risk population of a single center.
MATERIALS AND METHODS
This study included 223 consecutive patients with unprotected LM coronary artery stenosis (> 50% narrowing) undergoing PCI in Taipei Veterans General Hospital from January 2000 to December 2010. Patients who presented as acute ST segment elevation myocardial infarction and/or cardiogenic shock were excluded. Unprotected LM disease was defined as significant LM coronary artery stenosis without patent coronary artery bypass grafts to the left anterior descending or left circumflex arteries. PCI and ventriculography were performed using the standard procedure. Unfractionated heparin (10000 IU bolus) was administered before the procedure to achieve an activated clotting time > 300 seconds. Pre-dilation with balloon catheter was performed in all cases. For most LM lesions with distal bifurcation involved, stenting across the bifurcation toward the left anterior descending artery (cross-over technique) was attempted, followed by provisional stenting of the left circumflex artery (T-stenting or culottes stenting) if there was residual stenosis or dissection over the orifice of the left circumflex artery. Mini-crush stenting and V-stenting techniques were used in 4 and 3 cases, respectively, which was determined by preference of the interventional operator and the presence of suitable LM coronary artery anatomy. Post-dilation with kissing balloon technique was attempted except techniques difficulty or small non-dominant left circumflex artery. Stent deployment was performed by high pressure balloon dilatation to achieve optimal stent apposition. The choice of DES or BMS was made by the patient’s preference or stent availability. Debulking by means of rotablator was used only in highly calcified lesions, and the use of intravascular ultrasound and glycoprotein IIb-IIIa receptor antagonist were at the discretion of the interventional operators. Intra-aortic balloon pumps were utilized for patients with complex anatomy/ depressed left ventricular function/or unstable hemodynamic status. PCI was considered angiographically successful if residual stenosis < 30% with coronary Thrombolysis in Myocardial Infarction grade 3 flow was obtained at the end of the procedure. After the procedure, all patients received aspirin (100 mg/d) indefinitely and clopidogrel (300 mg loading dose, then 75 mg per day) or ticlopidine (500 mg loading dose, then 250 mg twice a day) for at least 1 month (BMS) or 6 months (DES). Longer treatment with clopidogrel was at the operator’s discretion. Medications for treatment of angina pectoris (calcium channel blockers, beta-blockers and nitrates) were continued.
The clinical follow-up data were collected by scheduled monthly clinic evaluations or direct telephone contact for the first-ever major adverse cardiac and event (MACE), which was defined as cardiovascular death, non-fatal MI and clinically-driven TLR. All patients were followed-up completely without any cases lost to follow-up. Myocardial infarction was defined as the presence of significant new Q waves in at least 2 electrocardiographic leads or of symptoms compatible with MI associated with an increase in creatine kinase-MB fraction ≥ 3 times the upper limit of the reference range. TLR was defined as any repeated percutaneous intervention of the target lesion performed for > 50% angiographic re-narrowing of the treated lesion from 5 mm proximal to 5 mm distal to the stent, or repeat bypass surgery. Stent thrombosis occurrence was classified as definite, probable, or possible according to Academic Research Consortium (ARC) criteria,12 and was considered as acute (within 24 hours), subacute (within 30 days), late (after 30 days and within 12 months), and very late (after 1 year). The additive EuroSCORE and SYNTAX score were used to stratify the risk of all-cause mortality and MACE at follow-up. The additive EuroSCORE was calculated based on the original methodology,13 and the SYNTAX score from the summation of the individual scorings for each separate lesion (defined as > 50% stenosis in vessels > 1.5 cm).14 The EuroSCORE and SYNTAX score were computed by 2 experienced cardiologists unaware of the clinical course of patients. Ultimately, patients were considered as high risk in the presence of additive EuroSCORE > 6 or SYNTAX score > 32. estimated glomerular filtration rate (eGFR) was calculated according to the simplified version of the Modification of Diet in Renal Disease Study prediction equation formula, which was further modified by Ma et al. for Chinese patients with chronic kidney disease [eGFR = 175 × plasma creatinine-1.234 × age-0.179× 0.79 (if female)].15 The study protocol was approved by the Institutional Review Board at Taipei-Veterans General Hospital and informed written consent was obtained from each participant.
Statistical analysis
All continuous variables were presented as mean ± standard deviation or with a 25-75% range, and categorical variables as numbers and percentages. In order to reduce the treatment-selection bias in this single-center observational study, propensity score matching was performed. The propensity score was computed using a logistic regression model including age, gender, diabetes, clinical presentation as acute coronary syndrome, eGFR, left ventricular ejection fraction, EuroSCORE, SYNTAX score, and involvement of LM bifurcation. The differences of continuous data between all patients receiving BMS and DES and propensity score matched cohort were compared by two-sample t-test or Mann-Whitney U test, when appropriate. Categorical data were compared by means of Chi-square or Fisher’s exact test. In the whole cohort and propensity-score matched cohort, long-term year actuarial event-free survival curves were estimated by using the Kaplan-Meier method and were compared using the log-rank test. Cox regression analysis was performed to determine the independent predictors of long-term cardiovascular death and MACE, with those variables with a p-value of < 0.10 in the univariate analysis being included in the multivariate model. The hazard ratio (HR) and 95% confidence intervals (CI) were calculated. A p-value of less than 0.05 was considered to be statistically significant. The SPSS 17.0 (SPSS Inc., Chicago, Illinois, USA) software package was used for statistical analysis.
RESULTS
Patient characteristics
We enrolled 223 LM patients from January 2000 to December 2010, with 94 patients receiving only BMS and 129 patients receiving at least 1 DES. The mean age of the whole population was 71.1 ± 11.3 years with male (187, 83.9%) predominance. Of note, nearly half of the patients presented as acute coronary syndrome [n = 111, 49.8% including 41 (18.4%) patients with unstable angina, and 70 (31.4%) patients with non-ST elevation MI]. Furthermore, the prevalence of diabetes, chronic kidney disease and old MI in the whole population were also high (diabetes: n = 105, 47.1%; chronic kidney disease: n = 91, 40.8%; old MI: n = 69; 30.9%). The baseline characteristics of the patients receiving BMS and DES implantation are summarized in Table 1. Comparing with patients receiving BMS, those receiving DES tended to be younger, and had a higher prevalence of old stroke. In addition, the patients receiving DES tended to have borderline higher SYNTAX score (p = 0.05) and lower EuroSCORE (p = 0.10), suggesting these patients had a more complex coronary artery anatomy (Table 1).
Table 1. Baseline and procedural characteristics .
| BMS (n = 94) | DES (n = 129) | p value | |
| Age (years) | 72.5 ± 10.6 | 70.1 ± 11.6 | 0.11 |
| Gender (male) | 81 (86.2%) | 106 (82.2%) | 0.47 |
| Diabetes | 41 (43.6%) | 63 (48.8%) | 0.50 |
| Hypertension | 79 (84.0%) | 98 (76.0%) | 0.18 |
| Hypercholesterolemia | 49 (52.1%) | 68 (52.7%) | 1.00 |
| Smoking | 49 (52.1%) | 57 (44.2%) | 0.28 |
| Body mass index (Kg/m2) | 25.1 ± 4.2 | 25.0 ± 3.6 | 0.79 |
| PAOD | 13 (13.8%) | 15 (11.6%) | 0.68 |
| Previous MI | 29 (30.9%) | 40 (31.0%) | 1.00 |
| Old stroke | 17 (18.1%) | 11 (8.5%) | 0.04 |
| Chronic kidney disease | 44 (46.8%) | 48 (37.2%) | 0.17 |
| eGFR | 62.6 ± 30.9 | 69.7 ± 32.0 | 0.10 |
| LVEF (%) | 49.9 ± 12.6 | 48.0 ± 12.9 | 0.29 |
| Clinical presentation | |||
| Stable angina | 48 (51.1%) | 64 (49.6%) | 0.81 |
| Unstable angina | 17 (18.1%) | 24 (18.6%) | 1.00 |
| Non-ST elevation MI | 29 (30.9%) | 41 (31.8%) | 1.00 |
| SYNTAX score (25%-75% range) | 30 (21-39) | 34 (22-45) | 0.05 |
| SYNTAX score > 32 | 38 (40.4%) | 67 (51.9%) | 0.10 |
| EuroSCORE (25%-75% range) | 8 (4-10) | 7 (3-10) | 0.10 |
| EuroSCORE > 6 | 48 (51.1%) | 54 (41.9%) | 0.18 |
| Lesion location, n (%) | |||
| Ostial/shaft | 39 (42.5%) | 44 (34.1%) | 0.27 |
| Bifurcation | 63 (67.0%) | 87 (67.4%) | 1.00 |
| Extent of diseased vessel n (%) | |||
| LM only | 10 (10.6%) | 7 (5.4%) | 0.20 |
| LM plus 1-vessel disease | 18 (19.1%) | 18 (14.0%) | 0.36 |
| LM plus 2-vessel disease | 29 (30.9%) | 39 (30.2%) | 1.00 |
| LM plus 3-vessel disease | 37 (39.4%) | 65 (50.4%) | 0.13 |
| RCA disease n(%) | 45 (47.9%) | 66 (51.2%) | 0.69 |
| Stent length in LM (mm) | 17.3 ± 6.6 | 23.0 ± 6.9 | < 0.01 |
| Stent diameter (mm) | 3.8 ± 0.7 | 3.6 ± 1.2 | 0.24 |
| Bifuncation LM stenting (n = 150) | |||
| Cross-over tech with 1 stent | 51 (81.0%) | 64 (73.6%) | 0.33 |
| Technique with 2 stent | |||
| T-stenting, n (%) | 8 (12.7%) | 12 (13.8%) | 1.00 |
| Crush, n (%) | 0 | 4 (4.6%) | 0.14 |
| Culottes, n (%) | 3 (4.8%) | 6 (6.9%) | 0.74 |
| V stenting, n (%) | 1 (1.6%) | 2 (2.3%) | 1.00 |
| Kissing post-dilation | 38 (40.4%) | 66 (51.2%) | 0.14 |
| Use of glycoprotein IIb-IIIa inhibitors | 14 (14.9%) | 39 (30.2%) | 0.01 |
| Use of IABP | 11 (11.7%) | 15 (11.6%) | 1.00 |
| IVUS guidance | 5 (5.3%) | 36 (27.9%) | < 0.01 |
| Rotablation | 1 (1.1%) | 13 (10.1%) | < 0.01 |
eGFR, estimated glomerular filtration rate; IABP, intraaortic balloon pump; IVUS, intravascular ultrasound; LM, left main coronary artery; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PAOD, peripheral arterial obstructive disease; RCA, right coronary artery.
Procedural characteristics
The PCI procedure was angiographically successful in all patients and the procedural characteristics are shown in Table 1. There were no significant differences in LM lesion location, involvement of right coronary artery and extent of disease vessels between patients treated with BMS or DES, and the majority of patients with distal bifurcation involvement of both groups were treated with a single stent with cross-over technique (n = 115,78.2% among patients with distal bifurcation involvement). Furthermore, the stenting strategies by using 2 stents were similar between the BMS group and DES group (Table 1). There was no significant difference in post-LM stenting kissing balloon dilation between both groups (p = 0.14). In contrast, the length of LM stent was significantly longer in patients receiving DES (p < 0.01). In addition, intravascular ultrasound, rotablation, and glycoprotein IIb-IIIa receptors inhibitors were more frequently used in patients receiving DES.
Long-term clinical outcomes
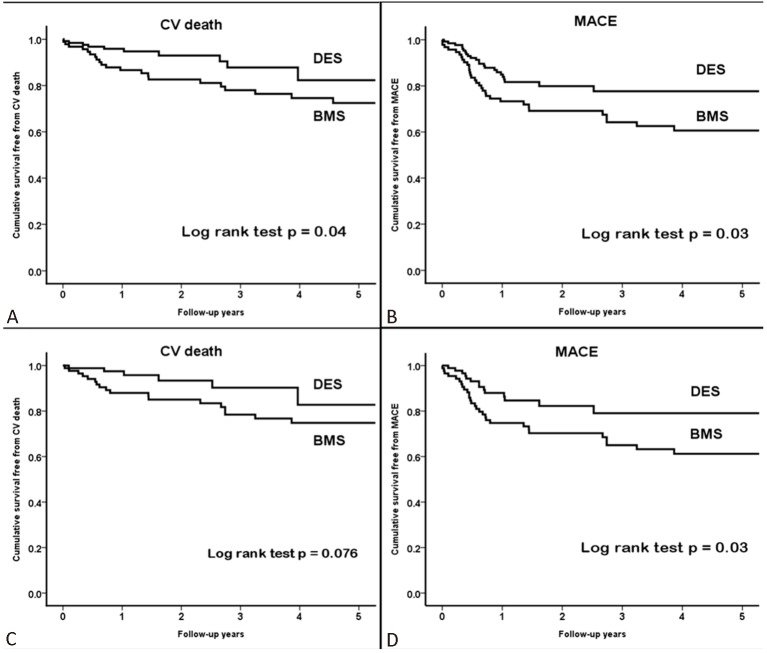
All patients were followed up completely for at least 1 year without loss of follow-up. The mean follow-up period was 2.5 years (25-75% range: 1.6 to 4.0 years). Table 2 summarizes the long-term clinical outcomes after unprotected LM PCI, and a total of 31 (13.9%) cardiovascular death and 56 (25.1%) MACE have been observed after LM PCI. Subacute definite/probably thrombosis occurred in 3 patients receiving BMS and 1 patient receiving DES. Furthermore, 2 probable late stent thrombosis cases occurred at 180 and 266 days after the index procedure, respectively, (cumulative definite/probable stent thrombosis cases: 6, 2.7%). Both patients underwent LM PCI using BMS and presented as sudden death. The cumulative incidence of definite or probable stent thrombosis was 0.8% (n = 1) after DES implantation and 5.3% (n = 5) after BMS implantation (p = 0.09). Figure 1A/B showed the cumulative survival curves free from cardiovascular death and MACE determined by using the Kaplan-Meier method in patients treated with BMS or DES respectively, with the outcomes being significantly worse in BMS group (cardiovascular death: p = 0.04; MACE: p = 0.03; by log-rank test, respectively). However, the endpoint of TLR was comparable between both groups (p = 0.84, data not shown). In multivariate Cox regression analysis adjusted for age, gender, SYNTAX score, EuroSCORE, diabetes, LM bifurcation involvement and clinical presentation as acute coronary syndrome, the use of DES was identified as a significant independent protective factor against cardiovascular death (p = 0.01) and MACE (p = 0.02), and the use of DES might reduce the risk of cardiovascular death and MACE by 66% and 50%, respectively (Table 3).
Table 2. Long-term clinical outcomes .
| BMS (n = 94) | DES (n = 129) | p value | |
| Cardiovascular death | 21 (22.3%) | 10 (7.8%) | < 0.01 |
| TLR | 14 (14.9%) | 18 (14.0%) | 0.85 |
| MACE | 33 (35.1%) | 23 (17.8%) | < 0.01 |
| Definite/probable stent thrombosis | 5 (5.3%) | 1 (0.8%) | 0.09 |
MACE, major adverse cardiovascular event, included cardiovascular death, non-fatal MI and TLR; TLR, target lesion revascularization.
Figure 1.
Cumulative incidence curves of cardiovascular death (A) and MACE (B) by Kaplan-Meier method in the whole population. Cumulative incidence curves of cardiovascular death (C) and MACE (D) by Kaplan-Meier method in the propensity score matched cohort. P values by log-rank test are shown. BMS, bare metal stent; CV, cardiovascular; DES, drug eluting stent; MACE, major adverse cardiovascular event.
Table 3. Multivariate Cox regression analysis for long-term clinical outcomes .
| All cohort | Propensity-score matched cohort | |||
| HR (95% CI) | p value* | HR (95% CI) | p value* | |
| Cardiovascular death | 0.34 (0.15-0.79) | 0.01 | 0.33 (0.13-0.87) | 0.03 |
| MACE | 0.50 (0.28-0.88) | 0.02 | 0.44 (0.23-0.83) | 0.01 |
| TLR | 0.83 (0.40-1.70) | 0.63 | 0.64 (0.28-1.46) | 0.29 |
Cl, confidence interval; HR, hazard ratio; MACE, major adverse cardiovascular event, included cardiovascular death, non-fatal MI and TLR; TLR, target lesion revascularization.
* p value adjusted for age, gender, SYNTAX score, EuroSCORE, diabetes, LM bifurcation lesion, and presentation as acute coronary syndrome.
As previous studies showed the use of new generation DES was associated with better clinical outcome compared with first generation DES (like Cypher, Taxes),16 we compared the clinical outcome in subgroups implanted with BMS, 1st generation DES and new generation DES, and found that there were no significant differences among their long-term cardiovascular (CV) death and MACE rate (CV death: p = 0.28; MACE: p = 0.11). In contrast, we investigated the impact of LM vessel/stent diameter to the long-term outcome, and found that in LM vessel diameter ≥ 3.5 mm (BMS = 69, DES = 79), the use of DES remained significantly associated with less long-term MACE rate (p = 0.05), but the long-term CV death rate of DES and BMS group was comparable (p = 0.10).
Propensity-score analysis
On the basis of similar propensity scores, there were 87 matched pairs of patients treated with BMS or DES. There were no significant differences in the clinical or procedure characteristics between the propensity-score matched cohort treated with BMS or DES, except that the stent length was significantly longer in patients receiving DES than those receiving BMS. In addition, intravascular ultrasound (lVUS) and rotablation were still used more frequently in the DES group (Table 4). Figure 1C/D showed that the use of DES resulted in a non-significant reduction in long-term cardiovascular death (p = 0.076) and significant reduction in MACE (p = 0.03), respectively, compared with the use of BMS. In contrast, the risk of TLR remained similar between both groups (p = 0.37). The multivariate Cox regression analysis in the propensity-score matched cohort revealed that the use of DES remained a significant independent protective factor against cardiovascular death (p = 0.03) and MACE (p = 0.01), and the use of DES might reduce the risk of cardiovascular death and MACE by 67% and 56%, respectively (Table 3).
Table 4. Baseline and procedural characteristics of propensity score matched cohort .
| BMS (n = 87) | DES (n = 87) | p value | |
| Age (years) | 72.5 ± 10.8 | 72.1 ± 11.3 | 0.99 |
| Gender (male) | 74 (85.1%) | 76 (87.4%) | 0.83 |
| Diabetes | 38 (43.7%) | 39 (44.8%) | 1.00 |
| Hypertension | 74 (85.1%) | 67 (77.0%) | 0.25 |
| Hypercholesterolemia | 45 (52.1%) | 68 (52.7%) | 1.00 |
| Smoking | 49 (51.7%) | 39 (44.8%) | 0.45 |
| Body mass index (Kg/m2) | 25.3 ± 4.3 | 25.0 ± 3.7 | 0.69 |
| PAOD | 11 (12.6%) | 10 (11.5%) | 1.00 |
| Previous MI | 26 (31.3%) | 26 (31.3%) | 1.00 |
| Old stroke | 15 (17.2%) | 7 (8.0%) | 0.11 |
| Chronic kidney disease | 38 (43.7%) | 34 (39.1%) | 0.64 |
| eGFR | 65.5 ± 29.6 | 66.8 ± 32.3 | 0.78 |
| LVEF (%) | 50.0 ± 12.4 | 51.0 ± 11.5 | 0.57 |
| Clinical presentation | |||
| Stable angina | 46 (52.9%) | 44 (50.6%) | 0.88 |
| Unstable angina | 17 (19.5%) | 15 (17.2%) | 0.85 |
| Non-ST elevation MI | 24 (27.6%) | 28 (32.2%) | 0.62 |
| SYNTAX score (25%-75% range) | 30 (21-39) | 30 (20-39) | 0.98 |
| SYNTAX score > 32 | 37 (42.5%) | 34 (39.1%) | 0.76 |
| EuroSCORE (25%-75% range) | 7 (4-9) | 7 (4-10) | 0.78 |
| EuroSCORE > 6 | 42 (48.3%) | 39 (44.8%) | 0.76 |
| Lesion location, n (%) | |||
| Ostial/shaft | 35 (40.2%) | 32 (36.8%) | 0.76 |
| Bifurcation | 58 (66.7%) | 62 (71.3%) | 0.62 |
| Extent of diseased vessel n (%) | |||
| LM only | 9 (10.3%) | 4 (4.6%) | 0.25 |
| LM plus 1-vessel disease | 17 (19.5%) | 12 (13.8%) | 0.42 |
| LM plus 2-vessel disease | 26 (29.9%) | 31 (35.6%) | 0.52 |
| LM plus 3-vessel disease | 35 (40.2%) | 40 (46.0%) | 0.44 |
| RCA disease n (%) | 42 (48.3%) | 49 (56.3%) | 0.36 |
| Stent length in LM (mm) | 17.3 ± 6.7 | 22.6 ± 7.1 | < 0.01 |
| Stent diameter (mm) | 3.8 ± 0.7 | 3.7 ± 1.4 | 0.55 |
| Bifuncation LM stenting (n = 120) | |||
| Cross-over tech with 1 stent | 46 (79.3%) | 44 (77.2%) | 0.82 |
| Technique with 2 stent | |||
| T-stenting, n (%) | 8 (13.8%) | 7 (11.3%) | 0.79 |
| Crush, n (%) | 0 | 5 (8.1%) | 0.06 |
| Culottes, n (%) | 3 (5.2%) | 5 (8.7%) | 0.72 |
| V stenting , n (%) | 1 (1.7%) | 1 (1.6%) | 1.00 |
| Kissing post-dilation | 35 (40.2%) | 46 (52.9%) | 0.13 |
| Use of glycoprotein IIb-IIIa inhibitors | 13 (14.9%) | 26 (29.9%) | 0.03 |
| Use of IABP | 9 (10.3%) | 11 (12.6%) | 0.81 |
| IVUS guidance | 5 (5.7%) | 26 (29.9%) | < 0.01 |
| Rotablation | 1 (1.1%) | 10 (11.5%) | < 0.01 |
eGFR, estimated glomerular filtration rate; IABP, intraaortic balloon pump; IVUS, intravascular ultrasound; LM, left main coronary artery; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PAOD, peripheral arterial obstructive disease; RCA, right coronary artery.
DISCUSSION
The main findings of this study showed that, compared with the use of BMS, unprotected LM PCI using DES might be associated with lower long-term risks of cardiovascular death and MACE both in the entire population and propensity-matched cohort. In contrast, there was no difference in TLR rate between the BMS group and DES group. In addition, the incidence of very late stent thrombosis was similar in both groups.
Recent studies have suggested that PCI might be an alternative treatment to CABG for patients with LM disease with suitable anatomy,17-19 and this has been reflected in the current American College of Cardiology/ American Heart Association guidelines, which have upgraded the LM PCI from a Class III indication to a Class IIa indication.20 Although the use of DES in LM PCI has been consistently associated with lower revascularization rate, compared with the BMS, LM stenting with DES was not associated with reduced risk of short-term or long-term cardiovascular death/MI/stent thrombosis risk.4,5,9 In contrast, Tamburino et al.21 reported that the adjusted 3-year rates of TLR were similar in the DES group and BMS group (11.4% vs. 10.7%; HR, 0.79; 95% CI 0.33-1.90; p = 0.60). The adjusted hazard ratio for the risk of mortality after DES implantation was 0.37 (95% CI 0.15-0.96; p = 0.04). The reason for this discrepancy in the TLR or death rate remains unclear, and the advantage of DES from the improvement of both a general PCI stenting strategies/techniques as well as the ancillary medical therapy, such as prolonged dual antiplatelet therapy, might be one of the contributing factors.21 Besides, our results were similar to those of Tamburino. In our high-risk population with higher EuroSCORE compared with previous studies, the use of DES remained associated with reduced risks of cardiovascular death and MACE. However, even adjusted with propensity score, the use of glycoprotein IIb-IIIa receptor inhibitors, IVUS and rotablation remained significantly more frequent in the DES group. In contrast, there was a trend toward higher EuroSCORE in the BMS group. Finally, final kissing balloon dilatation was used less frequently in the BMS group. Taken together, all of these clinical/procedural characteristics might contribute to the discrepancy in clinical outcomes of both groups.22,23 Interestingly, the TLR rate was similar in both the DES and BMS groups, which was different from previous reports.5,6,9 As the follow-up angiography was done by clinical indications and the angiographic follow-up rate was relatively low, the restenosis rate might be underestimated, especially in the BMS group. In addition, compared with BMS group, the patients treated with DES appeared to have more complex coronary anatomy, with higher SYNTAX score and more triple vessel disease, which might increase the TLR rate of the DES group. Finally, considering the paramount anatomical importance of LM, restenosis of stent might result in catastrophic adverse events, including MI or cardiovascular death; therefore, a low angiographic follow-up rate might reasonably lead to a higher rate of cardiovascular death and MACE but with similar TLR rate in the BMS group. Nevertheless, the comparison of BMS and DES for LM PCI remains to be further investigated in a larger study.
Stent thrombosis, especially the very late stent thrombosis, has become a major safety concern about stenting in the current DES era. A large registry has suggested that, compared with BMS, DES implantation may increase mortality as a result of very late stent thrombosis.24 A meta-analysis showed there were no differences in the early and late stent thrombosis rates between the DES and BMS groups. However, it demonstrated a higher incidence of very late stent thrombosis after DES implantation in comparison with BMS (0.7% vs. 0.1%, p = 0.006),11 and the potential of very late stent thrombosis has become the major concern about the use of DES for unprotected LM PCI. In our study, we showed a comparably low risk of late or very late stent thrombosis in DES group (0.8%) and the cumulative stent thrombosis rate appeared to be even lower in the DES group (p = 0.09). Larger LM coronary artery diameter, the reduced use of two-stent strategies and the elevated use of IVUS might be the contributing factors, but the exact mechanisms warranted further studies.
Limitations
There were several limitations in this study. First, this study was a retrospective and observational study with a relatively small cohort. Furthermore, our study findings derived mainly from a single-center observation based on the patients with higher surgical risks, these results could not be applicable to other LM PCI scenarios. Second, due to a very long 10-year enrollment period, a significant heterogeneity in treatment/stenting strategy could exist. Actually, tremendous changes happened during the ten years regarding UPLM PCI, including treatment strategy changes, technique and device improvements, medications, and even guideline recommendations. All of these factors may have significantly affected the results owing to unmeasured confounders, procedure bias, or detection bias. Specifically, patients receiving DES later could have benefited from improvements in PCI procedures and adjunctive medications for coronary artery disease, including long-term treatment of clopidogrel, compared with the patients who earlier received BMS. In fact, no statistical method of adjustment can completely abolish this limitation. Moreover, the technical features adopted, such as the use of IVUS – guided stenting or a selective rather than a systematic 2-stent strategy for bifurcation lesions may be associated with favorable outcomes. Third, as mentioned above, the angiographic follow-up rate of our population was relatively low, such that incomplete angiographic follow-up related potential bias might have a substantial impact on the long-term clinical outcomes in both groups.
CONCLUSIONS
In the real-world practice of unprotected LM PCI in a high risk population from a single center, we found that DES implantation appeared to be associated with reduced cardiovascular death and MACE risks in comparison with BMS.
REFERENCES
- 1.Yusuf S, Zucker D, Peduzzi P, et al. Effect of coronary artery bypass graft surgery on survival: overview of 10-year results from randomised trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet. 1994;344:563–570. doi: 10.1016/s0140-6736(94)91963-1. [DOI] [PubMed] [Google Scholar]
- 2.Seung KB, Park DW, Kim YH, et al. Stents versus coronary-artery bypass grafting for left main coronary artery disease. N Engl J Med. 2008;358:1781–1792. doi: 10.1056/NEJMoa0801441. [DOI] [PubMed] [Google Scholar]
- 3.Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–972. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 4.Chieffo A, Stankovic G, Bonizzoni E, et al. Early and mid-term results of drug-eluting stent implantation in unprotected left main. Circulation. 2005;111:791–795. doi: 10.1161/01.CIR.0000155256.88940.F8. [DOI] [PubMed] [Google Scholar]
- 5.Kim YH, Park DW, Lee SW, et al. Long-term safety and effectiveness of unprotected left main coronary stenting with drug-eluting stents compared with bare-metal stents. Circulation. 2009;120:400–407. doi: 10.1161/CIRCULATIONAHA.108.800805. [DOI] [PubMed] [Google Scholar]
- 6.Kubo S, Kadota K, Shimada T, et al. Seven-year clinical outcomes of unprotected left main coronary artery stenting with drug-eluting stent and bare-metal stent. Circ J. 2010;77:2497–2504. doi: 10.1253/circj.cj-13-0032. [DOI] [PubMed] [Google Scholar]
- 7.Pandya SB, Kim YH, Meyers SN, et al. Drug-eluting versus bare-metal stents in unprotected left main coronary artery stenosis a meta-analysis. JACC Cardiovasc Interv. 2010;3:602–611. doi: 10.1016/j.jcin.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toyofuku M, Kimura T, Morimoto T, et al. Three-year outcomes after sirolimus-eluting stent implantation for unprotected left main coronary artery disease: insights from the j-Cypher registry. Circulation. 2009;120:1866–1874. doi: 10.1161/CIRCULATIONAHA.109.873349. [DOI] [PubMed] [Google Scholar]
- 9.Buszman PE, Buszman PP, Kiesz RS, et al. Early and long-term results of unprotected left main coronary artery stenting: the LE MANS (Left Main Coronary Artery Stenting) registry. J Am Coll Cardiol. 2009;54:1500–1511. doi: 10.1016/j.jacc.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Stone GW, Ellis SG, Cox DA, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004;350:221–231. doi: 10.1056/NEJMoa032441. [DOI] [PubMed] [Google Scholar]
- 11.Roukoz H, Bavry AA, Sarkees ML, et al. Comprehensive meta-analysis on drug-eluting stents versus bare-metal stents during extended follow-up. Am J Med. 2009;122:581 e581–e510. doi: 10.1016/j.amjmed.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 12.Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 13.Nashef SA, Roques F, Michel P, et al. European system for cardiac operative risk evaluation (EuroSCORE) Eur J Cardiothorac Surg. 1999;16:9–13. doi: 10.1016/s1010-7940(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 14.Sianos G, Morel MA, Kappetein AP, et al. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1:219–227. [PubMed] [Google Scholar]
- 15.Ma YC, Zuo L, Chen JH, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17:2937–2944. doi: 10.1681/ASN.2006040368. [DOI] [PubMed] [Google Scholar]
- 16.Kedhi E, Joesoef KS, McFadden E, et al. Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice (COMPARE): a randomised trial. Lancet. 2010;375:201–209. doi: 10.1016/S0140-6736(09)62127-9. [DOI] [PubMed] [Google Scholar]
- 17.Kappetein AP, Feldman TE, Mack MJ, et al. Comparison of coronary bypass surgery with drug-eluting stenting for the treatment of left main and/or three-vessel disease: a 3-year follow-up of the SYNTAX trial. Eur Heart J. 2011;32:2125–2134. doi: 10.1093/eurheartj/ehr213. [DOI] [PubMed] [Google Scholar]
- 18.Park SJ, Kim YH, Park DW, et al. Randomized trial of stents versus bypass surgery for left main coronary artery disease. N Engl J Med. 2011;364:1718–1727. doi: 10.1056/NEJMoa1100452. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe S, Komiya T, Sakaguchi G, Shimamoto T. Unprotected left main coronary artery disease in patients with low predictive risk of mortality. Ann Thorac Surg. 2012;94:1927–1933. doi: 10.1016/j.athoracsur.2012.06.060. [DOI] [PubMed] [Google Scholar]
- 20.Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58:e44–e122. doi: 10.1016/j.jacc.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Tamburino C, Di Salvo ME, Capodanno D, et al. Are drug-eluting stents superior to bare-metal stents in patients with unprotected non-bifurcational left main disease? Insights from a multicentre registry. Eur Heart J. 2009;30:1171–1179. doi: 10.1093/eurheartj/ehp052. [DOI] [PubMed] [Google Scholar]
- 22.Claessen BE, Mehran R, Mintz GS, et al. Impact of intravascular ultrasound imaging on early and late clinical outcomes following percutaneous coronary intervention with drug-eluting stents. JACC Cardiovasc Interv. 2011;4:974–981. doi: 10.1016/j.jcin.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Kang SJ, Ahn JM, Song H, et al. Comprehensive intravascular ultrasound assessment of stent area and its impact on restenosis and adverse cardiac events in 403 patients with unprotected left main disease. Circ Cardiovasc Interv. 2011;4:562–569. doi: 10.1161/CIRCINTERVENTIONS.111.964643. [DOI] [PubMed] [Google Scholar]
- 24.Lagerqvist B, James SK, Stenestrand U, et al. Long-term outcomes with drug-eluting stents versus bare-metal stents in Sweden. N Engl J Med. 2007;356:1009–1019. doi: 10.1056/NEJMoa067722. [DOI] [PubMed] [Google Scholar]