Abstract
Background
Endothelial progenitor cells (EPCs) play a fundamental role in vascular repair and angiogenesis- related diseases. It is well-known that the process of angiogenesis is faulty in patients with diabetes. Long-term exposure of peripheral blood EPCs to high glucose (HG-EPCs) has been shown to impair cell proliferation and other functional competencies. Far infrared (FIR) therapy can promote ischemia-induced angiogenesis in diabetic mice and restore high glucose-suppressed endothelial progenitor cell functions both in vitro and in vivo. However, the detail mechanisms and global transcriptome alternations are still unclear.
Methods
In this study, we investigated the influences of FIR upon HG-EPC gene expressions. EPCs were obtained from the peripheral blood and treated with high glucose. These cells were then subjected to FIR irradiation and functional assays.
Results
Those genes responsible for fibroblast growth factors, Mitogen-activated protein kinases (MAPK), Janus kinase/signal transducer and activator of transcription and prostaglandin signaling pathways were significantly induced in HG-EPCs after FIR treatment. On the other hand, mouse double minute 2 homolog, genes involved in glycogen metabolic process, and genes involved in cardiac fibrosis were down-regulated. We also observed complex genetic networks functioning in FIR-treated HG-EPCs, in which several genes, such as GATA binding protein 3, hairy and enhancer of split-1, Sprouty Homolog 2, MAPK and Sirtuin 1, acted as hubs to maintain the stability and connectivity of the whole genetic network.
Conclusions
Deciphering FIR-affected genes will not only provide us with new knowledge regarding angiogenesis, but also help to develop new biomarkers for evaluating the effects of FIR therapy. Our findings may also be adapted to develop new methods to increase EPC activities for treating diabetes-related ischemia and metabolic syndrome-associated cardiovascular disorders.
Keywords: Endothelial progenitor cell, Far infrared, Microarray, Systems biology
INTRODUCTION
Deregulated angiogenesis has been proven to be involved in major complications in diabetes, leading to a diminished ability for collateral vessels to be formed in response to ischemia in the heart and peripheral tissues.1 The best way to maintain vascular access thereby becomes a critical medical issue to address in diabetes patients. Mounting evidence indicates that the administration of angiogenic growth factors increases nutrient perfusion through neovascularization, and also reduces ischemia-related organ damage.2 Nevertheless, clinical evidence has also shown that certain patients appear to be refractory to administration of exogenous growth factors and fail to develop collateral circulation after tissue ischemia.3 It is therefore necessary to develop alternative therapeutic approaches to restore endogenous angiogenesis and vasculogenesis more efficiently in patients.
Far infrared (FIR) therapy, a noninvasive and convenient therapeutic modality, is known to improve both blood flow and endothelial function.4-6 FIR rays are defined as invisible electromagnetic waves with a wavelength between 5.6 to 1000 μm that can be perceived as heat by thermoreceptors in the surrounding skin.7,8 Currently, FIR irradiation is implicated in the treatment of ischemic lesions and necrosis in skin tissue as a result of trauma, diabetes, and peripheral arterial occlusive disease. FIR treatment reduces the frequency of cardiovascular diseases (CVDs) as well as improves arteriovenous fistula (AVF) access flow in hemodialysis patients through both its thermal and non-thermal (endothelial-improving, anti-inflammatory, antiproliferative, antioxidative) effects.4 FIR radiation improves ventricular arrhythmias and endothelial function in patients with heart disease, and enhances access flow and patency of AVF in hemodialysis patients.5,9,10 In diabetic mice, FIR therapy also promotes ischemia-induced angiogenesis.6
Enhancement of the regenerative capacity of the injured endothelium appears to be one way to reduce the incidence of CVDs or AVF malfunction lesions. According to the traditional view, endothelium integrity is maintained by neighboring mature endothelial cells which migrate and proliferate to restore the injured endothelial cells. However, a series of clinical and basic studies prompted by the discovery of bone marrow-derived endothelial progenitor cells (EPCs) have demonstrated that the injured endothelial monolayer may be regenerated partly by circulating EPCs.11 These circulating EPCs are mobilized endogenously and triggered by tissue ischemia, or exogenously by cytokine stimulation such as vascular endothelial growth factor, matrix metalloproteinase-9 (MMP-9), and stromal cell-derived factor-1 (SDF-1).11-13 Reduced levels of circulating EPCs independently predict atherosclerotic disease progression and development of cardiovascular events.14 Clinical studies have demonstrated that levels of circulating EPCs are associated with vascular endothelial function and cardiovascular risk factors, and help to identify patients at increased cardiovascular risk.15,16 There are several ways to increase levels of circulating EPCs and improve their function by pharmacological strategies and lifestyle modification.13,17 Many studies have evaluated the possibility of EPC transplantation for improving disease recovery; for example, one investigation described the long-term stroke outcome in a mouse model of transient middle cerebral artery occlusion (MCAO).18,19
Recent evidence has indicated that levels of circulating EPCs and their associated arterial stiffness were closely related to glycemic control in patients with type 2 diabetes mellitus (DM).6 Long-term exposure of EPCs to elevated levels of glucose enhances cellular senescence and decreases cell numbers and functional competencies via nitric oxide (NO)-related mechanisms.20 These findings provide a rationale for developing potential therapeutic approaches regarding hyperglycemia-related EPC dysfunction. Far infra-red therapy, on the other hand, can promote ischemia-induced angiogenesis in diabetic mice and restores high glucose-suppressed endothelial progenitor cell functions both in vitro and in vivo.6 Pilot mechanistic studies showed that FIR radiation upregulates high glucose-impaired endothelial nitric oxide synthase (eNOS) production, as well as the phosphorylation and activation of protein kinase B (AKT), extracellular regulated protein kinases (ERK) and p38 Mitogen-activated protein kinases (MAPK).6 However, the detail mechanisms and global transcriptome alternations of this process have not yet been addressed. Deciphering FIR-affected genes will not only inform us of new knowledge relating to angiogenesis, but also help to develop new biomarkers for evaluating FIR therapy effects in vitro and in vivo. So, in this study, the main objective was to investigate the influences of FIR upon EPC gene expression profiles, working with the hypothesis that the levels of specific genes must be influenced in activated EPCs after FIR stimulation.
MATERIALS AND METHODS
Human EPC isolation and cultivation
All patients gave informed consent, and the study was approved by the local research ethics committee. The protocols of this study are consistent with the ethical guidelines of the 1975 Helsinki Declaration. Peripheral blood EPC isolation and characterization were done as described previously with minor modifications.20,21 Cell and motility assays were performed by Transwell assays as described.21 In brief, peripheral blood samples were obtained from healthy donors, and total mononuclear cells (MNCs) were isolated by density gradient centrifugation with Histopaque-1077 (1.077 g/ml, Sigma, St. Louis, MO, USA). MNCs (5 × 106) were plated in 2 ml endothelial growth medium (EGM-2 MV Cambrex Corp., East Rutherford, NJ, USA) with supplements on fibronectin-coated 6-well plates. After 4 days of culturing, the medium was changed, and EPC (or ECFCs, endothelial colonies forming cells) emerged 2-4 weeks after the start of the MNCs culture. These EPCs exhibited a cobblestone morphology and monolayer growth pattern typical of mature endothelial cells at confluence.11,21 For high glucose culture, EPCs/ECFCs were treated with 25 mM of glucose for 4 days before being subjected to functional assays or FIR irradiation as described.11
ECFC characterization
Tube formation assays were performed on EPCs/ECFCs to assess their capacity for EPC vasculogenesis, which is believed to be important in new vessel formation. In brief, the in vitro tube formation assay was performed by thawing Matrigel at 4 °C overnight, and then by placing it into a 96-well plate at 37 °C for 1 hour to allow the matrix solution to solidify. EPCs were harvested with trypsin/ethylenediaminetetraacetic acid, and 1 × 104 EPCs/ECFCs or human umbilical vein endothelial cells (HUVECs) were placed on Matrigel with EGM-2 medium or serum-free Dulbecco’s modified eagle medium and incubated at 37 °C for 3 hours. Tubule formation was inspected under an inverted light microscope (100x), and four representative fields were taken. For 3D angiogenesis assay, collagen type I acidic solution was mixed with 1/2 volume of basic conditioned medium with 0.2 ug/ml SDF-1α (R&D Systems, Minneapolis, MN USA) and solidify for 30 minutes in a 96-well plate at 37 °C in a 5% CO2 incubator. 105 cells per well were seeded and assayed.
FIR treatment on EPC
Treatment of EPCs with FIR was performed as described.5,6 In brief, human peripheral blood EPCs were exposed to FIR radiation using a WS TY101 FIR emitter (WS Far Infrared Medical Technology Co, Ltd.). This device generates electromagnetic waves with wavelengths between 3 and 25 μm (peak 5 to 6 μm). The radiator was set at a height of 25 cm above the bottom of the tissue culture plates and cells were exposed to FIR radiation for 30 minutes according to the parameters determined previously.6
RNA isolation and quantitative reverse transcription polymerase chain reaction (RT-PCR)
Total mRNA were extracted by the RNeasy mini kit (Qiagen, Germany), and 100 ng to 1 μg of total RNA were subjected to reverse transcription using a First cDNA Synthesis kit (Fermentas, USA). For quantitative real-time PCR analysis, human pre-messenger RNA sequence was obtained from the NCBI (National Center for Biotechnology Information) AceView program (www.ncbi.nlm.nih.gov/AceView/). All primers were designed to cross introns using the Primer3 website (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi/) or Primer Express software (Applied Biosystems, CA, USA). Thermodynamics and primer specificity analysis were performed by the Vector NTI suite (Invitrogen, CA, USA) and the NCBI reverse e-PCR program (http://www.ncbi.nlm.nih.gov/sutils/e-pcr/reverse.cgi/). Real-time PCR reactions were performed using MaximaTM SYBR Green qPCR Master Mix (Fermentas, USA), and the specific products of the PCRs were detected and analyzed using a StepOneTM sequence detector (Applied Biosystems, USA). The expression level of each gene was normalized against the expression level of glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Gene expression microarrays and data analysis
Array data for FIR-treated human peripheral blood EPCs were deposited in the Gene Expression Omnibus database with an accession number of GSE37044. Total RNA collection, cRNA probe preparation, array hybridization and data analysis were done as previously described.22 Return merchandise authorization (RMA) log expression units were calculated from AffymetrixTM HG-U133 Plus 2.0 whole genome array data using the ‘affy’ package included in the Bioconductor (http://www.bioconductor.org/) suite of software for the R statistical programming language (http://www.r-project. org/). The default RMA settings were used to background correct, normalize and summarize all expression values. Significant differences between the sample groups were identified using the ‘limma’ (Linear Models for Microarray Analysis) package of the Bioconductor suite, and an empirical Bayesian moderated t-statistic hypothesis test between the two specified phenotypic groups was performed. To control for multiple testing errors, we then applied a false discovery rate algorithm to these p values in order to calculate a set of q values, thresholds of the expected proportion of false positives, or false rejections of the null hypothesis.
Gene enrichment analysis was performed using the Gene Ontology database with the WebGestalt (http://bioinfo.vanderbilt.edu/webgestalt/).23 Differential gene expression profiles were imported into the Ingenuity Pathways Analysis (IPA) software (Ingenuity Systems, Redwood City, CA; http://www.ingenuity.com/) to obtain canonical pathways, biological functions, and genetic networks. The knowledge base behind IPA was built upon properly accumulated scientific evidence, manually curated from thousands of journal articles, textbooks, and other data sources. After a list of signature genes was uploaded, interaction among focus genes and interaction among interacting genes and molecules from the knowledge base were used to combine genes into networks according to their probability of having more focus genes than expected by chance. Networks are scored on the basis of the number of uploaded signature genes they contain. The network score is based on the hypergeometric distribution and is calculated with the right-tailed Fisher’s exact test. The score is the negative log of this p value. The higher the score, the lower the probability of finding the observed number of uploaded signature genes in a given network by random chance.
RESULTS
FIR radiation stimulates angiogenesis-related activities in human EPCs
EPCs (late EPCs, ECFCs) were obtained from the peripheral blood of healthy subjects using an established protocol.20,21 These cells have a cobblestone-like morphology, which is similar to mature endothelial cells (Figure 1A). Isolated EPCs/ECFCs, just like HUVECs, formed capillary-like structures in a 3-dimensional (3D) angiogenesis assay (Figure 1B).
Figure 1.
Effects of FIR therapy on eNOS, EPC migration, and tube formation in vitro. (A) Cultivation of EPCs/ECFCs. Blood mononuclear cells were isolated and plated on fibronectin-coated culture dishes. Twenty-one days after plating, late EPCs (EPCs, ECFCs) with a cobblestone-like morphology were selected, reseeded, and grown to confluence (left). (B) Both EPCs and matured HUVECs formed vessel structures in 3D-angiogenesis assays. Arrows: tubular structures; arrowheads: tip cell-like structures. (C) Induction of eNOS mRNA production in HG-EPCs. EPCs cultured in 25 mM glucose for 4 days were subjected into FIR irradiation for 30 min before RNA extraction. The enhanced expression of eNOS in HG-EPCs after FIR irradiation was verified by RT-qPCR. *: p < 0.05 by 2-way ANOVA. Data are means ± SEM; n = 3 in each experiment. (D) Modified Boyden chamber assay was used to assess the effects of FIR radiation on the motility of HG-EPCs. VEGF was used as a chemoattracting factor. (E) A MatrixGel tube formation assay to monitor the in vitro angiogenesis ability of FIR-treated and control HG-EPCs. (F) Validation of microarray data. RT-qPCR assays were performed either on the same batch of RNAs used in array hybridization (left) or an independent batch of HG-EPCs RNAs were used (right). *: p < 0.05 by 2-way ANOVA. Data are means ± SEM. ECFCs, endothelial colonies forming cells; eNOS, endothelial nitric oxide synthase; EPCs, endothelial progenitor cells; FIR, far infrared; HUVECs, human umbilical vein endothelial cells; SEM, standard error of mean.
Here, peripheral blood EPCs were treated with high glucose (25 mM) for 4 days (HG-EPCs) before 30 minute of FIR treatment was administered. Figure 1C shows that FIR radiation administrated on HG-EPCs upregulated significantly the level of eNOS transcripts. We also used the modified Boyden chamber assay (Figure 1D) and the MatriGel tube formation assay (Figure 1E) to verify the increased migration and microvasculature formation functions of FIR-irradiated HG-EPCs. Compared with the control group, the migratory and microtubule formation abilities in HG-EPCs receiving FIR irradiation were significantly increased.
Global gene expression profile alternations after FIR radiation on EPCs
To reveal the underlying mechanisms of FIR-mediated phenotypes, we identified signature genes for FIR-treated HG-EPCs as well as control un-irradiated cells using whole genome gene expression microarrays. In total, 627 probe sets were specifically enriched in HG-EPCs after FIR treatment, while another 623 ones were repressed by FIR. Up- and down-regulated transcripts with more than 2-fold changes are shown in Table 1 and Table 2, respectively. Results of RT-qPCR validation performed on 2 independent batches of HG-EPCs were shown in Figure 1F. Among the up-regulated genes, RDX (radixin) and ARHGEF7 (Rho guanine nucleotide exchange factor (GEF) 7) are involved in the enhanced metastatic potential of cancer cells.24,25 VIP (vasoactive intestinal peptide) enhances angiogenesis in rats with focal cerebral ischemia.26 and increases VEGF expression.27 It has been reported that FIR treatment on high glucose-repressed EPCs induced p38 MAPK phosphorylation,6 and MAP3K8 (mitogen-activated protein kinase kinase kinase 8) is also in our gene list (Table 1, genes discussed here are indicated by asterisks). FGF18 (fibroblast growth factor 18) is wildly expressed in the cardiovascular tissues and can induce endothelial cells migration, maybe via a short transient activation of the MAPK pathway.28 Another member of the FGF signaling pathway, FRS2 (fibroblast growth factor receptor substrate 2), was also induced by FIR irradiation (Table 1). Another angiogenesis pathway that may be involved in FIR functions is the famous prostaglandin signaling pathway: PTGS2 (prostaglandin-endoperoxide synthase 2, also known as cyclooxygenase-2 (COX2)), PTGER3 and PTGER4 (prostaglandin E receptor 3 and 4, respectively). All were increased after FIR irradiation. There has been no published report to date that shows the prostaglandin pathway is involved in FIR functions.
Table 1. Known genes stimulated by FIR irradiation in PB-EPC (> 2 folds) .
| Probe set ID | Uni gene ID | Gene title | Gene symbol | Location |
| 238825_at | Hs.135167 | Acidic repeat contauning | ACRC | chrXq13.1 |
| 235412_at | Hs.508738 | Rho guanine nucleotide exchange factor (GEF) 7 | *ARHGEF7 | chr13q34 |
| 204194_at | Hs.154276 | BTB and CNC homology1, basic leucine zipper transcription factor 1 | BACH1 | chr21q22.11 |
| 225551_at | Hs.368353 | Consortin, connexin sorting protein | CNST | chr1q44 |
| 239203_at | Hs.396189 | Chromosome 7 open reading frame 53 | C7 or f53 | chr7q31.1 |
| 206727_at | Hs.654443 | Complement component 9 | C9 | chr5p14-p12 |
| 1555411_a_at | Hs.4859 | Cyclin L1 | CCNL1 | chr3q25.32 |
| 204995_at | Hs.500015 | Cyclin-dependent kinase 5, regulatory subunit 1(p35) | CDK5R1 | chr17q11.2 |
| 215318_at | Hs.687692 | N4BP2L2 intronic transcript 1 (non-protein coding) | N4BP2L2-IT1 | chr13q12-q13 |
| 214683_s_at | Hs.433732 | CDC-like kinase 1 | CLK1 | chr2q33 |
| 210346_s_at | Hs.406557 | CDC-like kinase 4 | CLK4 | chr5q35 |
| 224831_at | Hs.127126 | Cytoplasmic polyadenylation element binding protein 4 | CPEB4 | chr5q21 |
| 206424_at | Hs.150595 | Cytochrome P450, family26, subfamily A, polypeptide 1 | CYP26A1 | chr10q23-q24 |
| 227335_at | Hs.517172 | Death inducer-obliterator 1 | DIDO1 | chr20q13.33 |
| 208891_at | Hs.298654 | Dual specificity phosphatase 6 | DUSP6 | chr12q22-q23 |
| 224973_at | Hs.10784 | Family with sequence similarity 46, member A | FAM46A | chr6q14 |
| 206987_x_at | Hs.87191 | Fibroblast growth factor 18 | *FGF18 | chr5q34 |
| 228250_at | Hs.483329 | Rap guanine nucleotide exchange factor (GEF) 6///folliculin interacting protein 1 | FNIP1///RAPGEF6 | chr5q23.3///chr5q31.1 |
| 226045_at | Hs.593446 | Fibroblast growth factor receptor substrate 2 | *FRS2 | chr12q15 |
| 204472_at | Hs.654463 | GTP binding protein overexpressed in skeletal muscle | GEM | chr8q13-q21 |
| 203159_at | Hs.116448 | Glutaminase | GLS | chr2q32-q34 |
| 211998_at | Hs.533624 | H3 histone, family 3B (H3.3B) | H3F3B | chr1q41 |
| 209315_at | Hs.378532 | HBS1-like (S. cerevisiae) | HBS1L | chr6q23-q24 |
| 210387_at | Hs.591809 | Histone cluster 1, H2bg | HIST1H2BG | chr6p21.3 |
| 214438_at | Hs.74870 | H2.0-like homeobox | HLX | chr1q41-q42.1 |
| 201566_x_at | Hs.180919 | Inhibitor of DNA binding 2, dominant negative helix-loop-helix protein | ID2 | chr2p25 |
| 221763_at | Hs.413416 | Jumonji domain containing 1C | JMJD1C | chr10q21.2-q21.3 |
| 206765_at | Hs.1547 | Potassium inwardly-rectifying channel, subfamily J, member 2 | KCNJ2 | chr17q23.1-q24.2 |
| 225582_at | Hs.523252 | Inositol 1, 4, 5-trisphosphate receptor interacting protein | ITPRIP | chr10q25.1 |
| 226370_at | Hs.495854 | Kelch-like 15 (Drosophila) | KLHL15 | chrXp22.1-p21 |
| 207247_s_at | Hs.522845 | Zinc finger protein, X-linked///zinc finger protein | ZFX///ZFY | chrXp21.3///chrYp11.3 |
| 226682_at | Hs.560343 | RAR-related orphan receptor A | RORA | chr15q21.3 |
| 205027_s_at | Hs.432453 | Mitogen-activated protein kinase kinase kinase 8 | *MAP3K8 | chr10p11.23 |
| 1556382_a_at | Hs.555985 | NMDA receptor regulated 1 | NARG1 | chr4q31.1 |
| 223218_s_at | Hs.319171 | Nuclear factor of kappaa light polypeptide gene enhancer in B-cells inhibitor, zeta | NFKBIZ | chr3p12-q12 |
| 224958_at | Hs.462598 | Nuclear fragile X mental retardation protein interacting protein 2 | NUFIP2 | chr17q11.2 |
| 231838_at | Hs.641481 | Poly(A) binding protein, cytoplasmic 1-like | PABPC1L | chr20 |
| 1569431_at | Hs.696131 | Platelet-activating factor acetylhydrolase, isoform lb, beta subunit 30 kDa | PAFAH1B2 | chr11q23 |
| 214415_at | Hs.652174 | Plasminogen-like B2///plasminogen-like B1 | PLGLB1///PLGLB2 | chr2p11-q11///chr2p11.2 |
| 242804_at | Hs.368454 | Polymerase (DNA directed) nu | POLN | chr4p16.3 |
| 201702_s_at | Hs.106019 | Protein phosphatase 1, regulatory (inhibitor) subunit 10 | PPP1R10 | chr6p21.3 |
| 202014_at | Hs.631593 | Protein phosphatase 1, regulatory (inhibitor) subunit 15A | PPP1R15A | chr19q13.2 |
| 235987_at | Hs.12250 | Protein kinase, X-linked, pseudogene 1 | PRKXP1 | chr15q26.3 |
| 1554997_a_at | Hs.196384 | Prostaglandin-endoperoxide synthase 2 | *PTGS2 | chr1q25.2-q25.3 |
| 205178_s_at | Hs.188553 | Retinoblastoma binding protein 6 | RBBP6 | chr16p12.2 |
| 244739_at | Hs.263671 | Radxin | *RDX | chr11q23 |
| 223707_at | Hs.523463 | Ribosomal protein L27a | RPL27A | chr11p15 |
| 218041_x_at | Hs.221847 | Solute carrier family 38, member 2 | SLC38A2 | chr12q |
| 213164_at | Hs.302742 | Solute carrier family 5 (inositol transporters), member 3 | SLC5A3 | chr21q22.12 |
| 219820_at | Hs.130949 | Solute carrier family 6, member 16 | SLC6A16 | chr19q13.1-q13.4 |
| 208127_s_at | Hs.468426 | Suppressor of cytokine signaling 5 | SOCS5 | chr2p21 |
| 243570_at | Hs.282700 | Signal peptidase complex subunit 2 homolog (S. cerevisiae) | SPCS2 | chr11q13.4 |
| 236123_at | Hs.201921 | Suppression of tumorigenicity 7 like | ST7L | chr1p13.2 |
| 1554250_s_at | Hs.661254 | Tripartite motif-containing 73 | TRIM73 | chr7q11.23 |
| 1558356_at | Hs.108049 | Uveal autoantigen with coiled-coil domains and ankyrin repeats | UACA | chr15q22-q24 |
| 206577_at | Hs.53973 | Vasoactive intestinal peptide | *VIP | chr6q25 |
| 205092_x_at | Hs.655536 | Zinc finger and BTB domain containing 1 | ZBTB1 | chr14q23.3 |
*, Discussed in the text.
Table 2. Known genes down-regulated in PB-EPC after FIR treatment (> 2 folds) .
| Probe set ID | Uni gene ID | Gene title | Gene symbol | Location |
| 220518_at | Hs.477015 | ABI gene family, member 3 (NESH) binding protein | ABI3BP | chr3q12 |
| 215483_at | Hs.651221 | A kinase (PRKA) anchor protein (yotiao) 9 | AKAP9 | chr7q21-q22 |
| 224797_at | Hs.24684 | Arrestin domain containing 3 | ARRDC3 | chr5q14.3 |
| 229694_at | Hs.144447 | Bromodomain and WD repeat domain containing 2 | BRWD2 | chr10q26 |
| 233106_at | Hs.645410 | FRMD6 antisense RAN 1 (non-protein coding) | FRMD6-AS1 | chr14q22.1 |
| 205525_at | Hs.490203 | Caldesmon 1 | CALD1 | chr7q33 |
| 1570571_at | Hs.653125 | Coiled-coil domain containing 91 | CCDC91 | chr12p11.22 |
| 239442_at | Hs.709257 | Centrosomal protein 68 kDa | CEP68 | chr2p14 |
| 215629_s_at | Hs.659291 | Deleted in lymphocytic leukemia, 2 | DLEU2 | chr13q14.3 |
| 230229_at | Hs.292549 | Discs, large homolog 1 (Drosophila) | DLG1 | chr3q29 |
| 236649_at | Hs.127432 | DTW domain containing 1 | DTWD1 | chr15q21.2 |
| 235405_at | Hs.485557 | Glutathione S-transferase A4 | GSTA4 | chr6p12.1 |
| 223412_at | Hs.63841 | Kelch repeat and BTB (POZ) domain containing 7 | KBTBD7 | chr13q14.11 |
| 215268_at | Hs.658760 | Hypothetical LOC643314 | KIAA0754 | chr1p34.2 |
| 215599_at | Hs.529793 | Glucuronidase, beta pseudogene///similar to Beta-glucuronidase-like protein SMA3 | LOC653188///SMA4 | chr5q13///chr5q13.2 |
| 1554155_at | Hs.709634 | Microcephalin 1 | MCPH1 | chr8p23.1 |
| 217373_x_at | Hs.567303 | Mdm2 p53 binding protein homolog (mouse) | *MDM2 | chr12q14.3-q15 |
| 213684_s_at | Hs.480311 | PDZ and LIM domain 5 | PDLIM5 | chr4q22 |
| 242248_at | Hs.78060 | Phosphorylase kinase, beta | PHKB | chr16q12-q13 |
| 214981_at | Hs.664318 | Periostin, osteoblast specific factor | POSTN | chr13q13.3 |
| 204284_at | Hs.303090 | Protein phosphatase 1, regulatory (inhibitor) subunit 3C | PPP1R3C | chr10q23-q24 |
| 201427_s_at | Hs.709405 | Selenoprotein P, plasm, 1 | SEPP1 | chr5q31 |
| 220983_s_at | Hs.323308 | Sprouty homolog 4 (Drosophila) | SPRY4 | chr5q31.3 |
| 236561_at | Hs.494622 | Transforming growth factor, beta receptor I | TGFBR1 | chr9q22 |
| 224565_at | Hs.523789 | Nuclear paraspeckle assembly transcript 1 (non-protein coding) | NEAT1 | chr11q13.1 |
| 244132_x_at | Hs.657337 | Zinc finger protein 518A | ZNF518A | chr10q23.33 |
*, Discussed in the text.
Among the genes down-regulated by FIR in HG-EPCs, the p53-binding E3 ubiquitin ligase protein mouse double minute 2 homolog (MDM2) is present. The reduced levels of MDM2 and another gene PTK2 in 2 different batches of HG-EPCs by FIR were verified by RT-qPCR (Figure 1F).
Functional module analysis as a framework for the interpretation of FIR effects
The gene lists outlined above gave us preliminary insights into the functional consequences of detected differential gene expression. FGF, MAPK and prostaglandin signaling pathways were found to be associated with the effects of FIR. To understand more about how the gene expression profiles might be correlated with FIR functions as well as to provide quantitative evidence, the signature mRNAs were subjected to a Gene Ontology (GO) database search in order to find statistically overrepresented functional groups within the gene lists. The WebGestalt web tool23 was applied to provide statistical analysis and visual presentation of the results. The GO categories of biological processes that were statistically overrepresented (p < 0.05) among up or down genes are shown in Figure 2A and B, respectively. Consistent with the altered expression profile after FIR treatment, genes involved in cellular metabolism were significantly induced by FIR irradiation (118 genes, p = 1.4e-03; Figure 2A). Another significant biological process associated with this group is related to the protein phosphorylation processes (45 genes, p = 2.76e-6; Figure 2A), correlating with the status that FIR radiation could promote ERK and p38 MAPK activation in EPCs in response to high glucose stimulation. 6 Other predominant processes in the GO group include genes response to external stimulus (22 genes, p = 0.0014; gene names are shown in Figure 2A). Genes involved in cytokine (incl. IL6ST and IL20RB) and prostaglandin pathways (incl. PTGS2, PTGER3 and PTGER4) were induced and enriched by FIR, corresponding to the more angiogenic and activated status of treated EPCs.
Figure 2.
Significantly affected biological processes in HG-EPCs after FIR irradiation. Gene set enrichment analysis was performed according to the Gene Ontology (GO) classification. Up- (A) and down-regulated (B) probe sets were subjected to the GO database search via the WebGestalt interface. The number of genes, gene symbols, their percentages and the p values for each category that show significance (p < 0.05) are listed.
In contrast, the principal functions of the down-regulated genes include those related to the regulation of cellular macromolecule biosynthesis (75 genes, p = 7.3e-3; Figure 2B). The glycogen metabolic process, in particular, was repressed by FIR (7 genes, p = 8.5e-5; genes listed in Figure 2B), which may reflect the energy consumption requirement during the active migration and microtubular formation in FIR-treated EPCs.
Coordinated canonical pathway changes after FIR irradiation in human EPCs
It was shown that administration of FIR radiation promotes AKT, ERK and p38 MAPK activation and phosphorylation in EPCs in response to high glucose stimulation.6 However, additional signaling pathways may also be influenced by FIR. When the FIR-stimulated genes were subjected to a canonical pathway database using the IPA web tool, a total of 14 pathways were significantly enriched (Figure 3A). The most significant canonical pathway mapped is the “caveolar-mediated endocytosis signaling” pathway, and other top-ranked canonical pathways including G-protein coupled receptor signaling and JAK/Stat signaling pathways (Figure 3A). Four genes, including son of sevenless homolog 1 (SOS1), suppressors of cytokine signaling 3 (SOCS3), suppressors of cytokine signaling 5 (SOCS5), and protein inhibitors of activated stats 2 (PIAS2), are in the JAK/Stat signaling pathway, whose details are illustrated in Figure 3B.
Figure 3.
Induced canonical pathways and functional modules in HG-EPCs after FIR irradiation. FIR-induced probe sets were subjected to canonical pathway (A) and functional module (C) search via the IPA web tool. (B) Details of the JAK/Stat signal pathway. Genes highlighted: FIR-induced genes in this pathway.
To obtain more insights into these FIR-stimulated genes, a similar module analysis was performed according to the IPA toxicity database. As shown in Figure 3C, the top ranked functional groups included “Cardiac Hypertrophy”, “Liver Proliferation/Necrosis/Cell Death”, and “Cardiac Necrosis/Cell Death“. Genes regulating cell proliferation and apoptosis were therefore enriched among our gene list. Five genes involved in the “Gene regulation by peroxisome proliferators via PPARα” category were enriched (incl. inhibitor of kappa light polypeptide gene enhancer in B-cells, kinase beta, SOS1, fatty acid binding protein 1, prostaglandin-endoperoxide synthase 2, and nuclear receptor interacting protein 1; Figure 3C), indicated that the PPARα pathway is crucial for high glucose-incubated EPCs to respond to FIR.
Among genes down-regulated by FIR irradiation, the protein kinase A signaling canonical pathway was the most significantly affected one (Figure 4A). Other predominant pathways were Cardiac β-adrenergic signaling, Role of BRCA1 in DNA Damage Response, CDK5 signaling and others (Figure 4A). Genes involved in IGF1 signaling and Insulin receptor signaling, consistent with the phenotypes that Far infra-red therapy promotes ischemia-induced angiogenesis in diabetic mice and restores high glucose-suppressed endothelial progenitor cell functions.6 On the other hand, genes involved in hepatic (incl.collagen, type I, alpha 1, fibronectin 1, collagen, type IV, alpha 3, insulin-like growth factor binding protein 3, insulin-like growth factor binding protein 5, transmembrane protein 67, and chemokine (C-X-C motif) ligand 2) or cardiac (incl. protein tyrosine kinase 2, adenosine A3 receptor, fibronectin 1, adrenergic, beta-1-, receptor, periostin, osteoblast specific factor, immunoglobulin heavy constant mu, and dicer 1, ribonuclease type III) fibrosis were significantly repressed by FIR (Figure 4B).
Figure 4.
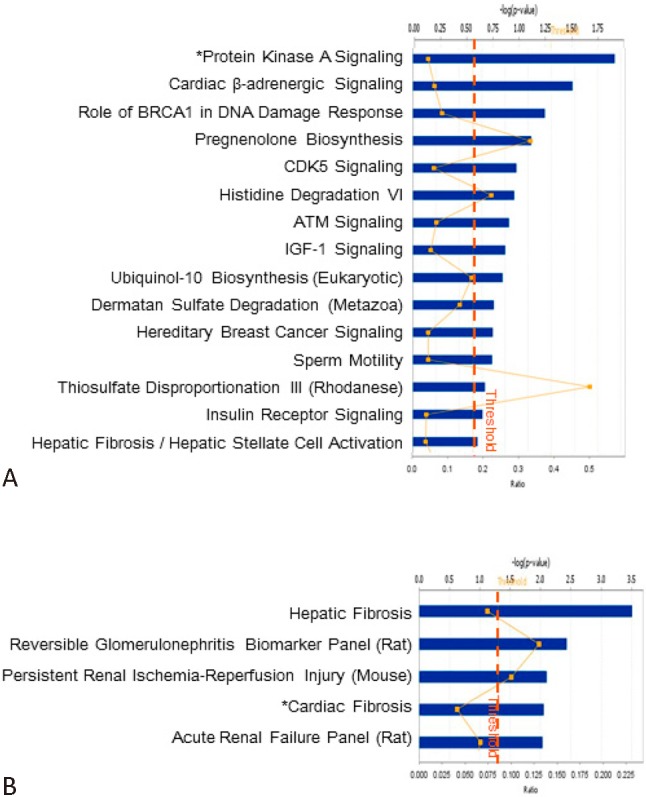
Repressed canonical pathways and functional modules in HG-EPCs after FIR irradiation. FIR-suppressed probe sets were subjected to canonical pathway (A) and functional module (B) search via the IPA web tool.
Genetic networks of FIR-treated EPCs
Increasing evidence has shown that genes do not act as individuals but collaborate in genetic networks. To better understand how FIR-affected genes form functional modules to regulate EPC biology, we performed systems biology genetic network analysis on signature genes. Identified up- or down-regulated genes were again inputted into the IPA tool to construct network modules. The knowledge base behind IPA summarizes known as molecular interactions are noted in published references (described in Materials and Methods). The term “network” in IPA is not the same as a biological or canonical pathway with a distinct function (i.e., angiogenesis) but a reflection of all interactions of a given protein as defined in the literature.
A major network consisting of 96 genes (96/627 = 15.31%) was identified (Figure 5A). This network was enriched with genes involved in hematological system development and function (20 genes incl. CASP8 and FADD-like apoptosis regulator, dual specificity phosphatase 4, eukaryotic translation initiation factor 4E, F-box protein 32, interleukin 6 signal transducer, integrin, beta 6, mitogen-activated protein kinase, Niemann-Pick disease, type C1, protein phosphatase, Mg2+/Mn2+dependent, 1D, PTGS2, prostaglandin E receptor 3, prostaglandin E receptor 4, Ras association (RalGDS/AF-6) domain family member 5, sirtuin (silent mating type information regulation 2 homolog) 1, SOCS3, thrombospondin 1, toll-like receptor 4, vasoactive intestinal peptide, X-box binding protein 1, and zinc finger CCCH-type containing 12A). MAPK was found to regulate most of these genes and functions as a “hub” gene, which had higher connectivity to others or resided in a position among submodules in the major network (Figure 5A). This observation was also consistent with the finding that FIR induces p38 MAPK phosphorylation and activation.6 XBP1, GATA binding protein 3, PTGS2, SIRT1, Histone H3, hairy and enhancer of split 1 and sprouty homolog 2 were also hubs connecting different submodules in the major network component (Figure 5A).
Figure 5A.
Interaction network analysis as a framework for the interpretation of FIR effects. FIR-induced probe sets were subjected to genetic network analysis via the Ingenuity Pathway Analysis (IPA) web tool. This network is displayed graphically as nodes (gene products) and edges (biological relationships between nodes) mapped by IPA. The intensity of the node color indicates the degree of differential expression. Hub genes in this genetic network are highlighted.
When a similar function network analysis was conducted on FIR-repressed genes, a major network consisting of 80 genes (80/623 = 12.84%) was also found (Figure 5B). Central to the network, there were significant hubs, including nuclear receptor subfamily 3 group C member 1, Mdm2 p53 binding protein homolog, GATA binding protein 4, transforming growth factor, beta receptor 1, FN1, COL1A1, brain-derived neurotrophic factor, and CXCL2 (Figure 5B, cellular compartments of these genes are also shown). MDM2 down-regulation has been verified by RT-qPCR in Figure 1. Top toxicity genes associated with this genetic network according to the IPA database are those involved in cardiac hypertrophy (incl. DICER1, GATA4, MDM2, POSTN, PTK2 and S100 calcium binding protein A10; indicated in Figure 5B), reflecting the healthy circumstance of these irradiated EPCs.
Figure 5B.
Interaction network analysis as a framework for the interpretation of FIR effects. A major functional genetic network composed of multiple genes down-regulated in HG-EPCs after FIR irradiation. Genes were arranged according to not only their relationships (indicated by lines) but also their cellular compartments. Hub genes are highlighted.
DISCUSSION
EPC research these years is quite active, partly due to the clinical application potential of these cells. These cells are potential sources for cell therapy that aim to enhance the neovascularization of tissue engineered constructs or ischemic tissues.29 It has been shown that both short-term and long-term FIR can increase access flow, in part due to the activation of both endothelial cells and EPCs.6 Infrared radiation is an invisible electromagnetic wave with a longer wavelength than that of visible light. According to the difference in wavelength, infrared radiation can be divided into 3 categories: near-infrared radiation (0.8-1.5 μ m); middle-infrared radiation (1.5-5.6 μ m); and FIR radiation (5.6-1,000 μ m). Many efforts have been undertaken to elucidate the mechanisms of FIR therapy: Akasaki et al. found that repeated FIR therapy upregulates eNOS expression and augments angiogenesis in an apolipoprotein E (ApoE)-deficient mouse hind limb ischemia model.4 Ikeda et al. reported that 4 weeks of sauna therapy significantly increased arterial eNOS expression and nitric oxide production in cardiomyopathic hamster.30 Huang et al. reported that FIR therapy enhanced blood flow recovery and new vessel formation in ischemic hindlimbs of diabetic mice, as well as the restoration of high glucose-suppressed functions of peripheral blood EPCs, presumably via activation of AKT, ERK and p38 MAPK pathways.6 Here we showed again a favorable effect of FIR treatment on EPCs derived from peripheral blood and provided for the first time the global transcriptomic alternations caused by FIR irradiation. Given that FIR therapy may counteract the detrimental effect of a diabetic environment on improvement of EPC functions, our results may provide some novel rationales for the potential clinical applications, such as the development of new biomarkers to monitor FIR effects in vivo or in vitro.
In this study we have disclosed genes and functional modules affected by FIR, and hence maybe in angiogenesis. Besides reproducing what was already known for FIR functions and EPC biology, novel genes that may play crucial roles in EPC activities were also revealed. Among the many genes affected by FIR irradiation, we spotted some genes that have more interaction/connectivity to other genes. These “hub” genes are theoretically more crucial than other in terms of FIR functions. It is clear that dysregulation of hubs may eventually lead to the disruption of the genetic network and the malfunction of cells.31 Of these hub genes, 2 GATA family transcription factors, GATA3 and GATA4, were either induced or repressed by FIR (Figure 5A & Figure 5B). Both GATA factors play an important role in angiogenesis: GATA3 is known to mediate Tie2 expression and functions in large vessel endothelial cells,32 and GATA4 expression in endothelial-derived cells is crucial in the development of cardiac valves.33,34 The repression of GATA4 in glucose-treated EPCs may help to enhance EPC stemness and thereby motility. Other transcription factors including HES1, SPRY2 and SIRT1 are also hubs in the FIR-affected genetic network (Figure 5A). The Notch-HES1 signaling axis controls hemato-endothelial fate decisions of human embryonic and induced pluripotent cells,35 and SIRT1, together with nitric oxide, plays a critical role in vasodilation and atherosclerosis prevention.36 The FGF antagonist Sprouty2 (SPRY2) expression controls endothelial monolayer integrity and quiescence, which is fundamental to the formation of mature blood vessels.37 However, no report yet links these genes to EPC biology or FIR effects.
Infrared radiation transfers energy that is perceived as heat by thermoreceptors in the skin.38 According to the report by Hartel et al., the temperature can be increased up to 4 °C in 10 mm depth of tissue.39 In addition, infrared therapy may allow multiple energy transfers as deep as 2-3 cm into subcutaneous tissue without irritating or overheating the skin like unfiltered heat radiation.39 The skin temperature steadily increases to a plateau of around 38-39 °C during FIR treatment as long as the distance between the ceramic plate and the skin was more than 20 cm.40 On the other hand, cellular photosensors may also be involved in FIR functions. It has been shown that green light-emitting diode (LED) irradiation can activate directional motility in human orbital fat stem cells through activation of the ERK/MAP kinase/p38 signaling pathway.41 Two non-visual opsins, encephalopsin (OPN3) and short-wave-sensitive opsin 1 (OPN1SW), serve as photoreceptor responses to green LED irradiation.41 Elucidation of the levels of various opsins in healthy and high glucose-treated EPCs, as well as roles of opsins in FIR-induced phenotypes and gene expression alternations, will be a significant study to pursue in the future.
CONCLUSIONS
In summary, our research combined systems biology tools to decipher transcriptome expression alternations in high glucose-treated EPCs. With these findings, it will be possible to discover novel molecular mechanisms that are crucial to ECFC/late EPC functioning. In this context, our research will also lead to the development of new diagnosis/prognosis approaches for cardiovascular disorders as well as other EPC-related diseases such as diabetes. Novel diagnostic modalities that are able to trace FIR effects could also be developed accordingly.
Acknowledgments
This work was supported by a grant from the Tri-Service General Hospital (TSGH-C103-025).
CONFLICT-OF-INTEREST DISCLOSURE
The authors declare no competing financial interests.
REFERENCES
- 1.Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freedman SB, Isner JM. Therapeutic angiogenesis for ischemic cardiovascular disease. J Mol Cell Cardiol. 2001;33:379–393. doi: 10.1006/jmcc.2000.1329. [DOI] [PubMed] [Google Scholar]
- 3.Rosengart TK, Lee LY, Patel SR, et al. Angiogenesis gene therapy:phase I assessment of direct intramyocardial administration of an adenovirus vector expressing VEGF121 cDNA to individuals with clinically significant severe coronary artery disease. Circulation. 1999;100:468–474. doi: 10.1161/01.cir.100.5.468. [DOI] [PubMed] [Google Scholar]
- 4.Akasaki Y, Miyata M, Eto H, et al. Repeated thermal therapy up-regulates endothelial nitric oxide synthase and augments angiogenesis in a mouse model of hindlimb ischemia. Circ J. 2006;70:463–470. doi: 10.1253/circj.70.463. [DOI] [PubMed] [Google Scholar]
- 5.Lin CC, Liu XM, Peyton K, et al. Far infrared therapy inhibits vascular endothelial inflammation via the induction of heme oxygenase-1. Arterioscler Thromb Vasc Bio. 2008;28:739–745. doi: 10.1161/ATVBAHA.107.160085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang PH, Chen JW, Lin CP, et al. Far infra-red therapy promotes ischemia-induced angiogenesis in diabetic mice and restores high glucose-suppressed endothelial progenitor cell functions. Cardiovascular Diabetology. 2012;11:99. doi: 10.1186/1475-2840-11-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toyokawa H, Matsui Y, Uhara J, et al. Promotive effects of far-infrared ray on full-thickness skin wound healing in rats. Exp Biol Med (Maywood) 2003;228:724–729. doi: 10.1177/153537020322800612. [DOI] [PubMed] [Google Scholar]
- 8.Kawaura A, Tanida N, Kamitani M, et al. The effect of leg hyperthermia using far infrared rays in bedridden subjects with type 2 diabetes mellitus. Acta Medica Okayama . 2010;64:143–147. doi: 10.18926/AMO/32849. [DOI] [PubMed] [Google Scholar]
- 9.Kihara T, Biro S, Ikeda Y, et al. Effects of repeated sauna treatment on ventricular arrhythmias in patients with chronic heart failure. Circ J. 2004;68:1146–1151. doi: 10.1253/circj.68.1146. [DOI] [PubMed] [Google Scholar]
- 10.Imamura M, Biro S, Kihara T, et al. Repeated thermal therapy improves impaired vascular endothelial function in patients with coronary risk factors. J Am Coll Cardiol. 2001;38:1083–1088. doi: 10.1016/s0735-1097(01)01467-x. [DOI] [PubMed] [Google Scholar]
- 11.Huang PH, Chen YH, Wang CH, et al. Matrix metalloproteinase-9 is essential for ischemia-induced neovascularization by modulating bone marrow-derived endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2009;29:1179–1184. doi: 10.1161/ATVBAHA.109.189175. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi T, Kalka C, Masuda H, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi J, Kusano KF, Masuo O, et al. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 14.Briasoulis A, Tousoulis D, Antoniades C, et al. The role of endothelial progenitor cells in vascular repair after arterial injury and atherosclerotic plaque development. Cardiovasc Ther. 2011;29:125–139. doi: 10.1111/j.1755-5922.2009.00131.x. [DOI] [PubMed] [Google Scholar]
- 15.Jialal I, Devaraj S, Singh U. Decreased number and impaired functionality of endothelial progenitor cells in subjects with metabolic syndrome:implications for increased cardiovascular risk. Atherosclerosis. 2010;211:297–302. doi: 10.1016/j.atherosclerosis.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang PH, Tsai HY, Wang CH, et al. Moderate intake of red wine improves ischemia-induced neovascularization in diabetic mice — roles of endothelial progenitor cells and nitric oxide. Atherosclerosis. 2010;212:426–435. doi: 10.1016/j.atherosclerosis.2010.06.034. [DOI] [PubMed] [Google Scholar]
- 17.Iwaguro H, Yamaguchi J, Kalka C, et al. Endothelial progenitor cell vascular endothelial growth factor gene transfer for vascular regeneration. Circulation. 2002;105:732–738. doi: 10.1161/hc0602.103673. [DOI] [PubMed] [Google Scholar]
- 18.Fan Y, Shen F, Frenzel T, et al. Endothelial progenitor cell transplantation improves long-term stroke outcome in mice. Ann Neurol. 2010;67:488–497. doi: 10.1002/ana.21919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moubarik C, Guillet B, Youssef B, et al. Transplanted late outgrowth endothelial progenitor cells as cell therapy product for stroke. Stem Cell Rev. 7:208–220. doi: 10.1007/s12015-010-9157-y. [DOI] [PubMed] [Google Scholar]
- 20.Chen YH, Lin SJ, Lin FY, et al. High glucose impairs early and late endothelial progenitor cells by modifying nitric oxide-related but not oxidative stress-mediated mechanisms. Diabetes. 2007;56:1559–1568. doi: 10.2337/db06-1103. [DOI] [PubMed] [Google Scholar]
- 21.Cheng CC, Lo HH, Huang TS, et al. Genetic module and miRNome trait analyses reflect the distinct biological features of endo-thelial progenitor cells from different anatomic locations. BMC Genomics. 2012;13:447. doi: 10.1186/1471-2164-13-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu YH, Hu TF, Chen YC, et al. The manipulation of miRNA-gene regulatory networks by KSHV induces endothelial cell motility. Blood. 2011;118:2896–2905. doi: 10.1182/blood-2011-01-330589. [DOI] [PubMed] [Google Scholar]
- 23.Zhang B, Kirov S, Snoddy J. WebGestalt:an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res. 2005;33:W741–W748. doi: 10.1093/nar/gki475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu YH, Lin WL, Hou YT, et al. Podocalyxin EBP50 ezrin molecular complex enhances the metastatic potential of renal cell carcinoma through recruiting Rac1 guanine nucleotide exchange factor ARHGEF7. Am J Pathol. 2010;176:3050–3061. doi: 10.2353/ajpath.2010.090539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valderrama F, Thevapala S, Ridley AJ. Radixin regulates cell migration and cell-cell adhesion through Rac1. J Cell Sci. 2012;125:3310–3319. doi: 10.1242/jcs.094383. [DOI] [PubMed] [Google Scholar]
- 26.Yang J, Zong CH, Zhao ZH, et al. Vasoactive intestinal peptide in rats with focal cerebral ischemia enhances angiogenesis. Neuroscience. 2009;161:413–421. doi: 10.1016/j.neuroscience.2009.03.052. [DOI] [PubMed] [Google Scholar]
- 27.Yang J, Shi QD, Song TB, et al. Vasoactive intestinal peptide increases VEGF expression to promote proliferation of brain vascular endothelial cells via the cAMP/PKA pathway after ischemic insult in vitro. Peptides. 2013;42:105–111. doi: 10.1016/j.peptides.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Antoine M, Wirz W, Tag CG, et al. Fibroblast growth factor 16 and 18 are expressed in human cardiovascular tissues and induce on endothelial cells migration but not proliferation. Biochem Biophys Res Commun. 2006;346:224–233. doi: 10.1016/j.bbrc.2006.05.105. [DOI] [PubMed] [Google Scholar]
- 29.Fuchs S, Dohle E, Kolbe M, Kirkpatrick CJ. Outgrowth endothelial cells:sources,characteristics and potential applications in tissue engineering and regenerative medicine. Adv Biochem Eng Biotechnol. 2010;123:201–217. doi: 10.1007/10_2009_65. [DOI] [PubMed] [Google Scholar]
- 30.Ikeda Y, Biro S, Kamogawa Y, et al. Repeated sauna therapy increases arterial endothelial nitric oxide synthase expression and nitric oxide production in cardiomyopathic hamsters. Circ J. 2005;69:722–729. doi: 10.1253/circj.69.722. [DOI] [PubMed] [Google Scholar]
- 31.Gursoy A, Keskin O, Nussinov R. Topological properties of protein interaction networks from a structural perspective. Biochem Soc Trans. 2008;36:1398–1403. doi: 10.1042/BST0361398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song H, Suehiro J, Kanki Y, et al. Critical role for GATA3 in mediating Tie2 expression and function in large vessel endothelial cells. J Biol Chem. 2009;284:29109–29124. doi: 10.1074/jbc.M109.041145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rivera-Feliciano J, Lee KH, Kong SW, et al. Development of heart valves requires Gata4 expression in endothelial-derived cells. Development. 2006;133:3607–3618. doi: 10.1242/dev.02519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moskowitz IP, Wang J, Peterson MA, et al. Transcription factor genes Smad4 and Gata4 cooperatively regulate cardiac valve development. [corrected] Proc Natl Acad Sci U S A. 2011;108:4006–4011. doi: 10.1073/pnas.1019025108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JB, Werbowetski-Ogilvie TE, Lee JH, et al. Notch-HES1 signaling axis controls hemato-endothelial fate decisions of human embyronic and induced pluripotent cells. Blood. 2013;122:1162–1173. doi: 10.1182/blood-2012-12-471649. [DOI] [PubMed] [Google Scholar]
- 36.Stein S, Matter CM. Protective roles of SIRT1 in atherosclerosis. Cell Cycle. 2011;10:640–647. doi: 10.4161/cc.10.4.14863. [DOI] [PubMed] [Google Scholar]
- 37.Peier M, Walpen T, Christofori G, et al. Sprouty2 expression controls endothelial monolayer integrity and quiescence. Angiogenesis. 2013;16:455–468. doi: 10.1007/s10456-012-9330-9. [DOI] [PubMed] [Google Scholar]
- 38.Capon A, Mordon S. Can thermal lasers promote skin wound healing? Am J Clin Dermatol. 2003;4:1–12. doi: 10.2165/00128071-200304010-00001. [DOI] [PubMed] [Google Scholar]
- 39.Hartel M, Hoffmann G, Wente MN, et al. Randomized clinical trial of the influence of local water-filtered infrared A irradiation on wound healing after abdominal surgery. Br J Surg. 2006;93:952–960. doi: 10.1002/bjs.5429. [DOI] [PubMed] [Google Scholar]
- 40.Yu SY, Chiu JH, Yang SD, et al. Biological effect of far-infrared therapy on increasing skin microcirculation in rats. Photodermatol Photoimmunol. 2006;22:78–86. doi: 10.1111/j.1600-0781.2006.00208.x. [DOI] [PubMed] [Google Scholar]
- 41.Ong WK, Chen HF, Tsai CT, et al. The activation of directional stem cell motility by green light-emitting diode irradiation. Biomaterials. 2013;34:1911–1920. doi: 10.1016/j.biomaterials.2012.11.065. [DOI] [PubMed] [Google Scholar]







