Abstract
Background
Atrial fibrillation is the most common complication of cardiac surgery and is associated with significant morbidity and mortality. Recognizing patients at high risk for developing postoperative atrial fibrillation (POAF) may help identify those who could benefit from strategies to prevent POAF. This study was conducted to delineate outcomes and to assess risk factors for POAF among Taiwanese patients undergoing coronary artery bypass grafting (CABG).
Methods
From January 2009 until February 2012, this prospective study included 266 consecutive patients admitted to our hospital with coronary artery disease. All patients underwent isolated CABG. Patients with preoperative permanent atrial fibrillation and concomitant surgery were excluded. Multiple risk factors associated with the incidence of POAF were collected and evaluated.
Results
POAF occurred in 126 of 226 patients (47.37%). Univariate analysis revealed that significant risk factors for the condition were age, gender, diabetes, dyslipidemia, smoking, impaired renal function, impaired cardiac function, and increased serum electrolytes. Multivariate analysis showed dyslipidemia [hazard ratio (HR): 0.418; 95% confidence interval (Cl): 0.190-0.915, p = 0.029], impaired renal function as indicated by an estimated glomerular filtration rate < 60 mL/min/1.73 m2 (HR: 3.174; 95% CI: 1.432-7.037, p = 0.004), and serum sodium (HR: 1.112; 95% Cl: 1.047-1.182, p = 0.001) prior to cardiopulmonary bypass as significant. Moreover, POAF was associated with lower 30-day, 1- and 3-year cumulative survival rates and higher early postoperative complications.
Conclusions
Patients with isolated CABG who were administered β-blockers, angiotensin converting enzyme inhibitor/angiotensin receptor blockers treatment, and lipid therapy before CABG were associated with reduced POAF, while those with impaired renal function and higher serum sodium before CABG predisposed POAF in a Taiwanese population.
Keywords: Atrial fibrillation (AF), Coronary artery bypass grafting (CABG), Coronary artery disease (CAD), Postoperative atrial fibrillation (POAF)
INTRODUCTION
Postoperative atrial fibrillation (POAF) is the most common arrhythmia encountered after isolated coronary artery bypass grafting (CABG) surgery, with an incidence of 20-40%.1,2 Despite advances in surgery, anesthesia, and postoperative care, the incidence of POAF after cardiac surgery continues to rise. New-onset POAF has been reported to increase intensive care readmission, resource use,3 and morbidity and mortality in patients after cardiac surgery.4,5
The pathogenesis of POAF is a multifactorial process, which involves patient-related factors, cardiac status, inflammation, electrolyte status, and operation method. Currently, a risk profile obtained from non-Asian subjects has been employed as a reference to predict and assess the risk of POAF in Asian patients with CABG. However, racial-ethnic differences in risk factors for POAF need to be considered, and studies of risk factors for POAF in an Asian population with isolated CABG have been limited. We conducted this study to establish the risk profile of adult patients undergoing isolated CABG in Taiwan.
MATERIALS AND METHODS
Study population
The study protocol was approved by the Institutional Review Board of the Tri-Service General Hospital, National Defense Medical Center. In this prospective study, 266 consecutive patients who underwent isolated CABG between June 2009 and March 2012 were enrolled and followed up either until April 2014. All patients underwent physical examination, a review of their medical history, and anthropometrical evaluation. Clinical characteristics included height, weight, body mass index (BMI), ejection fraction, preoperative insertion of an intra-aortic balloon pump, and number of diseased vessels. Preexisting medical conditions were any history of cerebrovascular disease, chronic lung disease, diabetes mellitus, hypertension, renal function, smoking, congestive heart failure, myocardial infarction, and cardiogenic shock. Hypertension was established when systolic blood pressure was > 140 mmHg and diastolic blood pressure was > 90 mmHg at rest, or the patient had been prescribed medication for hypertension. Diabetes was established when the fasting blood glucose level was > 126 mg/dl, postprandial 2-h glucose level was > 200 mg/dl, or the patient had been prescribed medication for diabetes. Lipid therapy was established when the patient had been prescribed statin therapy at least 3 months before cardiac surgery or when one or more of the following levels were elevated: total cholesterol level, > 240 mg/dl; low-density lipoprotein cholesterol level, > 130 mg/dl; or triglyceride level > 200 mg/dl. Renal function was evaluated by the estimated glomerular filtration rate (eGFR) (mL/min-1/1.73 m2), which was calculated using the equation proposed by investigators in the Chronic Kidney Disease Epidemiology (CKD-EPI).6 An eGFR < 60 mL/min-1/1.73 m2 value was the cut-off value for impaired renal function. Emergency CABG was defined as the need for surgery in patients with acute myocardial infarction in whom primary percutaneous coronary interventions had failed or could not be performed, persistent ischemia of a significant area of myocardium at rest and/or hemodynamic instability refractory to nonsurgical therapy remained, and life-threatening ventricular arrhythmias or other indications that precluded the elective scheduling of CABG were present. Patients with preoperative permanent atrial fibrillation (AF) and concomitant surgery were also excluded. All patients underwent continuous electrocardiogram (EKG) telemetry monitoring with automatic arrhythmia detection software for 5 days following surgery to record all atrial and ventricular arrhythmias. The primary outcome was to determine the incidence of new-onset AF after CABG surgery. POAF was defined as any new-onset AF of any duration detected by EKG telemetry monitoring or requiring anti-arrhythmic treatment during the 5 days after the CABG procedure.
Anesthetic, surgical, and CPB techniques
The method of anesthesia was equally applied to all cases. Induction of anesthesia was provided through titration of 5 mg of midazolam, 1.5-3 μg/kg of fentanyl, 2-4 mg/kg of thiamylal or 0.5-1.5 mg/kg of propofol, and 0.1 mg/kg of cisatracurium for induction, and isoflurane or sevoflurane for maintenance after tracheal intubation. Procedures were performed using cardiopulmonary bypass (CPB) in the conventional CABG and on-pump beating-heart CABG groups. CPB was established by aortic inflow cannulation and right atrium outflow cannulation with a single two-stage cannula. Cardiopulmonary bypass was undertaken using a standardized extracorporeal circulation utilizing non-pulsatile flow. In the conventional CABG group, all anastomoses were performed under cardiac arrest using tepid blood antegrade or retrograde cardioplegia or both. Systemic perfusion was performed using a hypothermic bypass strategy of systemic cooling between 28 °C and 32 °C. In the on-pump beating-heart CABG group, all coronary anastomoses were created using a commercially available stabilizer and heart positioner. The CPB flow was maintained at 1.5-2.5 l/min/m2 during coronary anastomosis, depending on the hemodynamic status. In the off-pump CABG group, all coronary anastomoses were performed using a commercially available stabilizer and heart positioner without the assistance of the heart-lung machine. All aortic-side anastomoses for the free graft in these 3 groups were performed with partial aortic clamping.7
Statistical analysis
Data are expressed as means ± standard deviation. The results are shown as percentages for categorical variables. Demographic variables compared between groups included age, gender, weight, body mass index, left ventricular function, concomitant diseases, baseline serum chemistry, and number of diseased vessels. Chi-square contingency tables or the Fisher’s exact test was used to analyze categorical variables. Student’s t-test was used to analyze continuous variables between two groups. Cumulative survival rates were calculated using Kaplan-Meier methods and a log-rank test. Cox proportional hazard model was used to calculate the multivariable-adjusted hazard ratios (HRs) and their 95% confidence intervals (CIs) for influence of pre-operative and operative factors on POAF mortality. Differences were considered statistically significant when the p-value was less than 0.05. All analyses were performed using SPSS 21 statistical package (SPSS Inc., Chicago, IL, USA).
RESULTS
This prospective, single-institution study included 266 total patients. Of all eligible patients, 126 (47.4%) developed POAF. The demographic and clinical characteristics between the non-AF and the POAF groups were compared. Using univariate analysis, variables significantly associated with POAF included age (69.9 ± 11.6 vs. 61.8 ± 10.8, p < 0.001), impaired renal function (41.3% vs. 24.5%, p = 0.004), and smoking history (39.7% vs. 62.7%, p < 0.001), whereas gender, non-diabetes, preoperative β-blocker or angiotensin-converting enzyme inhibitors (ACEI)/angiotensin receptor blockers (ARBs) treatment and lipid therapy decreased the risk of POAF (Table 1). There was no difference between the two groups in terms of BMI, chronic obstructive pulmonary disease (COPD), and previous cerebral vascular accident (CVA).
Table 1. Patient characteristics at baseline between non-post operative atrial fibrillation and post operative atrial fibrillation groups .
| Patient-related factors | Non-POAF N = 140 | POAF N = 126 | p-value |
| Age | 61.84 ± 10.76 | 69.90 ± 11.62 | < 0.001# |
| Gender (male %) | 115 (82.14%) | 90 (71.43%) | 0.042* |
| BMI | 26.01 ± 3.86 | 25.77 ± 4.14 | 0.625 |
| Smoking | 88 (62.86%) | 50 (39.68%) | < 0.001# |
| Diabetes mellitus | 68 (48.57%) | 77 (61.11%) | 0.049* |
| Lipid therapy | 80 (57.14%) | 56 (44.44%) | 0.049* |
| COPD | 12 (8.57%) | 10 (7.94%) | 0.999 |
| β-blocker | 53 (37.86%) | 33 (26.19%) | 0.042* |
| ACEI/ARBs | 15 (10.71%) | 3 (2.38%) | 0.007* |
| OLD CVA | 13 (9.29%) | 21 (16.67%) | 0.097 |
| eGFR < 60 mL/min/1.73 m2 | 34 (24.46%) | 52 (41.27%) | 0.004# |
ACEI, preoperative angiotensin-converting enzyme inhibitors administration; ARBs, preoperative angiotensin receptor blockers administration; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CVA, cerebral vascular accident; eGFR, estimated glomerular filtration rate; Non-POAF, non-post operative atrial fibrillation; POAF, post-operative atrial fibrillation;
β-blocker, preoperative β-blocker use.
* p-value < 0.05; # p-value < 0.01.
We next analyzed the preoperative cardiac status between the two groups. The results of univariate analysis showed that patients with POAF had a higher incidence of mechanical support by intra-aortic balloon pump (IABP) (14.3% vs. 5.0%, p = 0.011), preoperative left ventricular dysfunction (50.0% vs. 27.9%, p < 0.0001), moderate regurgitation of the mitral (27.0% vs. 12.9%, p = 0.005) or tricuspid valve (15.1% vs. 4.3%, p = 0.003), higher cardiac enzyme levels (troponin: 20.2 ± 30.5 vs. 9.7 ± 16.3, p = 0.001; creatine kinase-MB form (CK-MB): 109.0 ± 157.8 vs. 72.0 ± 99.6, p = 0.025), higher pulmonary arterial pressure (33.1 ± 11.9 vs. 28.4 ± 8.7, p < 0.0001), and lower left ventricular ejection fraction (48.9 ± 15.2 vs. 58.2 ± 13.0, p < 0.0001) (Table 2). There was no difference in terms of coronary stenosis location or atrial and ventricular volume. As shown in Table 3, the POAF group had higher cardiopulmonary serum CRP preoperatively (4.8 ± 6.7 vs. 1.8 ± 5.5, p < 0.0001) and higher serum sodium (142.2 ± 5.4 vs. 140.7 ± 3.5, p = 0.008), whereas serum potassium, magnesium, and calcium showed no difference. Our data revealed that there were statistically significant differences in emergency surgery and a longer extracorporeal circulation time between the two groups but no difference in the number of bypass grafts regardless of the aortic cross-clamp time or whether extracorporeal circulation was used (Table 4). The results of multivariate analysis revealed 3 variables to be the independent predictors of POAF: dyslipidemia (HR: 0.418; 95% CI: 0.190-0.915, p = 0.029), impaired renal function as eGFR < 60 mL/min/1.73 m2 (HR: 3.174; 95% CI: 1.432-7.037, p = 0.004) and serum sodium before CPB (HR: 1.112; 95% CI: 1.047-1.182, p = 0.001) (Table 5).
Table 2. Patients’ cardiac status before cardiopulmonary bypass between non-post operative atrial fibrillation and post operative atrial fibrillation groups .
| Cardiac status | Non-POAF N = 140 | POAF N = 126 | p-value |
| IABP | 7 (5.00%) | 18 (14.29%) | 0.011* |
| CK-MB | 72.00 ± 99.63 | 108.98 ± 157.82 | 0.025* |
| Troponin-I | 9.67 ± 16.27 | 20.15 ± 30.49 | 0.001# |
| LV EF (%) | 58.19 ± 13.02 | 48.94 ± 15.19 | < 0.001# |
| LV dysfunction | 39 (27.86%) | 63 (50.00%) | < 0.001# |
| PAP | 28.40 ± 8.75 | 33.09 ± 11.89 | < 0.001# |
| TR (≥ ++) | 6 (4.29%) | 19 (15.08%) | 0.003# |
| MR (≥ ++) | 18 (12.86%) | 34 (26.98%) | 0.005# |
CK-MB, creatine phosphokinase-MB; IABP, intra-aortic balloon pump; LV, left ventricular; LV EF, left ventricular ejection fraction; MR, mitral regurgitation; Non-POAF, non-post operative atrial fibrillation; PAP, pulmonary artery pressure; POAF, post-operative atrial fibrillation; TR, tricuspid regurgitation.
* p-value < 0.05; # p-value < 0.01.
Table 3. Inflammation factor and electrolyte status before cardiopulmonary bypass between non-post operative atrial fibrillation and post operative atrial fibrillation groups .
| Electrolyte | Non-AF N = 140 | POAF N = 126 | p-value |
| CRP | 1.79 ± 5.45 | 4.77 ± 6.71 | < 0.001# |
| Na+ | 140.73 ± 3.47 | 142.22 ± 5.38 | 0.008# |
| K+ | 3.88 ± 0.55 | 3.86 ± 0.57 | 0.779 |
| Mg+ | 1.98 ± 0.34 | 1.92 ± 0.41 | 0.147 |
| Ca2+ | 0.98 ± 0.17 | 0.97 ± 0.22 | 0.753 |
Ca, calcium; CRP, c reactive protein; K, potassium; Mg, magnesium; Na, sodium; Non-POAF, non-post operative atrial fibrillation; POAF, post-operative atrial fibrillation.
* p-value < 0.05; # p-value < 0.01.
Table 4. Operative factors between non-post operative atrial fibrillation and post operative atrial fibrillation groups .
| Operation | Non-POAF N = 140 | POAF N = 126 | p-value |
| Emergency | 30 (21.43%) | 42 (33.33%) | 0.038* |
| PUMP/OFF PUMP/P+B | 99/25/16 (70.71%/17.86%/11.43%) | 86/16/24 (68.25%/12.70%/19.05%) | 0.152 |
| No. of grafts | 2.96 ± 0.88 | 2.94 ± 0.66 | 0.768 |
| CPB time (min) | 98.86 ± 55.01 | 113.24 ± 58.88 | 0.041* |
| Aortic cross-clamp time (min) | 46.29 ± 33.95 | 43.01 ± 32.87 | 0.426 |
CPB time, cardiopulmonary bypass time; Non-POAF, non-post operative atrial fibrillation; OFF PUMP, coronary bypass without cardiopulmonary bypass pump; P+B, on cardiopulmonary bypass pump with beating heart during coronary anastomosis; POAF, post-operative atrial fibrillation; PUMP, on cardiopulmonary bypass pump with cardiac arrest.
* p-value < 0.05; # p-value < 0.01.
Table 5. Cox proportional model for patient with post operative atrial fibrillation .
| Parameters | Hazard ratio | 95% CI | p value |
| Age (year) | 1.029 | 0.994-1.064 | 0.106 |
| Smoking (yes/no) | 0.928 | 0.421-2.048 | 0.854 |
| Gender (female/male) | 0.938 | 0.379-2.322 | 0.891 |
| DM (yes/no) | 1.378 | 0.614-3.092 | 0.437 |
| Lipid therapy (yes/no) | 0.418 | 0.190-0.915 | 0.029* |
| eGFR < 60 mL/min/1.73 m2 | 3.174 | 1.432-7.037 | 0.004# |
| IABP | 1.556 | 0.605-3.999 | 0.359 |
| Troponin-I | 1.014 | 0.998-1.031 | 0.083 |
| CRP | 1.011 | 0.964-1.059 | 0.656 |
| Emergency (yes/no) | 1.915 | 0.905-4.051 | 0.089 |
| CK-MB | 0.997 | 0.995-1.000 | 0.064 |
| LV dysfunction | 1.726 | 0.759-3.926 | 0.193 |
| Na+ | 1.112 | 1.047-1.182 | 0.001# |
CK-MB, creatine kinase-MB; CRP, c reactive protein; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; IABP, intra-aortic balloon pump; LV dysfunciton, left ventricular dysfunction; Na, sodium.
* p-value < 0.05; # p-value < 0.01.
The difference in the cumulative survival rate between non-AF and POAF groups was statistically significant (p < 0.001) (Figure 1). As shown in Table 6, the observed 30-day, 1-year, and 3-year cumulative survival rates were 98%, 96%, and 94% for the non-POAF group, respectively. The 30-day, 1-year, and 3-year cumulative survival rates were 83%, 79%, and 78%, respectively, in the POAF group. Patients with POAF had a higher frequency of perioperative infection (15.1% vs. 2.1%, p < 0.001), renal failure (12.8% vs. 2.9%, p = 0.002), and reoperation (6.4% vs. 1.4%, p = 0.05). The frequency of postoperative cerebral vascular accident (CVA) was similar for the 2 groups (1.6% in POAF vs. 0.7% in non-POAF, p = 0.605).
Figure 1.
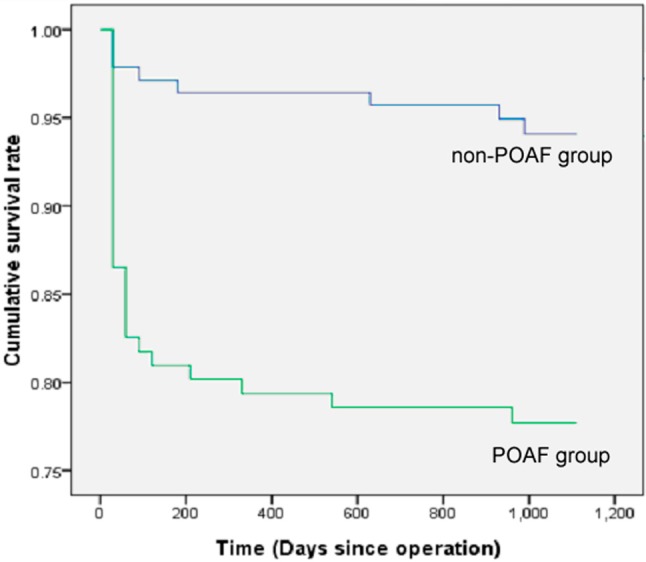
Cumulative survival rate after operation, a significant difference (p < 0.001) was observed between non-POAF and POAF groups.
Table 6. Cumulative survival rate at three different time points and early complications between Non-POAF and POAF groups .
| Non-POAF N = 140 | POAF N = 126 | p-value | ||
| Cumulative survival rate | ||||
| 30-day | 0.98 | 0.83 | < 0.001 | |
| 1-year | 0.96 | 0.79 | < 0.001 | |
| 3-year | 0.94 | 0.78 | < 0.001 | |
| Early complication | ||||
| Perioperative Infection | 3 (2.1%) | 19 (15.1%) | < 0.001 | |
| Postoperative renal failure | 4 (2.9%) | 16 (12.8%) | 0.002 | |
| Cerebral vascular accident | 1 (0.7%) | 2 (1.6%) | 0.605 | |
| Reoperation | 2 (1.4%) | 8 (6.4%) | 0.050 |
* p-value < 0.05; # p-value < 0.01.
DISCUSSION
Postoperative atrial fibrillation is a complex condition that involves a host of modifiable and nonmodifiable risk factors that are not yet well known. Recent studies suggest that several factors are considered to be linked with the development of POAF including predisposing factors, such as advanced age, obesity, diabetes, hypertension, and metabolic syndrome; intraoperative factors, such as surgical methods, venous cannula, pulmonary vein vent, and acute volume changes; and postoperative factors, such as hypotension, volume overload, and increased afterload.4,8-11 As risk profiles about the risk of POAF in Asian patients undergoing isolated CABG are limited, we conducted this study to establish the risk profile and determine the early and long-term mortality of patients in Taiwan. In the present study, the incidence of POAF was 47.4% among these isolated CABG patients. Our data indicated an increase in the incidence of POAF with advanced age, which is in line with other published studies.10,11 Smoking, gender, diabetes, dyslipidemia, impaired renal function, congestive heart failure, profound myocardial injury, and decreased ventricular ejection fraction, as well as the need for mechanical support, were shown to be predisposing factors to POAF. We also found that dyslipidemia, impaired renal function as eGFR < 60 and before cardiopulmonary bypass serum sodium were independent factors for POAF using a multivariate Cox proportional hazard model.
POAF and off-pump
Although the deleterious effects of CPB are known, it remains inconclusive whether CABG without CPB reduces the prevalence of POAF.12,13 In the present study, we compared the outcomes from different surgical techniques, namely conventional CABG with CPB and cardioplegic arrest, off-pump CABG, and on-pump beating-heart CABG, as well as the CPB time. While avoiding cardioplegic arrest or CPB did not aid the reduction of POAF at our institution, we found the CPB time to be significantly shorter in the non-POAF group compared with the POAF group.
POAF and preoperative medicines therapy
Preoperative β-blocker,14,15 ACEI/ARBs,16,17 or statins18,19 have been reported to affect POAF. Nevertheless, the clinical effect of this therapy is still controversial.20-22 This study evaluated 266 patients; 86 (32.33%) of these received a preoperative β-blocker, while 180 (67.67%) did not. Patients with non-POAF were more likely to take a preoperative β-blocker compared with patients with POAF (37.86% in the non-POAF vs. 26.19% in the POAF groups, p = 0.042). Only 18 (6.77%) of the 266 evaluated patients received an ACEI or ARB preoperatively; the remaining 248 (93.23%) did not. Of these 18 patients, 15 were in the non-POAF group, and 3 were in the POAF group. The portion of patients receiving ACEI/ARBs preoperatively was low in this study, but it also showed the effects in the prevention of POAF with p = 0.007. In the present study, 136 patients undergoing CABG surgery met the inclusion criteria for lipid therapy. As shown in Table 1, patients with non-POAF were more likely to take preoperative lipid therapy compared with patients with POAF (57.14% in the non-POAF vs. 44.44% in the POAF groups, p = 0.049). Though the p-value is borderline in student-t tests, the results of multivariate Cox proportional hazard analyses were broadly consistent (HR: 0.418, 95% CI: 0.190-0.915, p = 0.029). In short, preoperative β-blockers, ACEI/ARB treat-ment, and lipid therapy to prevent POAF were effective in a Taiwanese population.
POAF and impaired renal function
Based on the Kidney Foundation Disease Outcomes Quality Initiative classification, impaired renal function was defined by eGFR of < 60 mL/min/1.73 m2 calculated Modification of Diet in Renal Disease Formula. Previous studies have reported that impaired renal function is strongly and independently associated with the incidence of atrial fibrillation.23,24 However, there are limited data on the comorbidity of impaired renal function and atrial fibrillation in CABG patients. In the present study, the data showed that the POAF group contained a higher proportion of patients with impaired renal function as compared to those with non-AF. We also found eGFR < 60 mL/min/1.73 m2 was an independent risk factor for POAF using the multivariate Cox proportional hazard analyses.
POAF and CRP as well as serum electrolytes
Recently, there has been growing evidence that dysregulation of the oxidant-antioxidant balance, electrical remodeling, and inflammatory factors play a critical role in the pathogenesis of atrial fibrillation.8 The circulating concentration of CRP rises in response to an inflammatory state and plays a central role in the pathophysiology of several disorders, including cardiovascular disease.25 We report that patients who developed POAF had higher serum CRP levels using the univariate analysis.
Serum electrolytes, including potassium, sodium, and calcium, play a significant physiological role in the regulation of the electrical and mechanical action of the heart. Any abnormal concentration of these ions may result in muscle contraction disorders, cardiac arrhythmias, and drug interactions.26,27 In this study, we also investigated the impact of preoperative serum electrolytes on POAF. The results, which are shown in Table 3, revealed that instead of calcium or potassium, serum sodium was an effective predictor for POAF before CPB. Indeed, the serum sodium ranges with standard deviations overlap, and both are considered to fall into the normal ranges of serum sodium; however, the standard deviations for serum sodium in the POAF and non-PAOF groups were different. We also performed student-t tests in both equal variance and non-equal variance assumptions for the POAF group vs. the non-PAOF group. The results were statistically significant. Sodium current underlies the initiation and propagation of action potentials and, by doing so, determines cardiac excitability and conduction velocity of electrical stimuli through the heart.28 The perioperative period may be associated with a marked neurohumoral stress response, significant fluid losses, and varied fluid replacement regimes. Acute changes in serum sodium concentrations are therefore common. The importance of hypo-/hypernatremia in cardiac arrhythmia has been discussed frequently. Vincent et al. demonstrated that abnormal sodium current properties contribute to cardiac electrical and contractile dysfunction.29 However, the impact of sodium on the POAF in patients undergoing CABG has not yet been determined. In short, we demonstrated for the first time that preoperative serum sodium levels can influence the occurrence of POAF in patients undergoing CABG.
POAF impacts the early postoperative complications and survival rates
The study of Villareal et al.30 at the Texas Heart Institute reported an association of POAF with an increased risk for short-term and long-term adverse outcomes in patients undergoing CABG, including an increased risk for stroke, death, and longer and more complicated hospital stays. In this study, the 30-day, 1-year, and 3-year cumulative survival rate and complications of POAF and non-AF patients are presented in Table 6. Compared with the non-POAF group, the POAF group had a significantly lower cumulative survival rate after 30 days, 1 year, and 3 years. In addition, patients with POAF had a higher frequency of perioperative infection (15.1% vs. 2.1%, p < 0.001), postoperative renal failure (12.8% vs. 2.9%, p = 0.002), and reoperation (6.4% vs. 1.4%, p = 0.05). The frequency of postoperative cerebral vascular accident (CVA) was also similar for the 2 groups (1.6% for the POAF group vs. 0.7% in the non-POAF group, p = 0.605). Our results are in agreement with a previous study, which found patients undergoing a first coronary bypass had higher postoperative mortality and early complications.
Limitations
There were several limitations in this study that should be noted. Firstly, the sample size for this study was relatively small. Second, this was a single-center study, so the data must be interpreted with caution due to the limited generalizability of the findings. Another limitation of this study is that the data were obtained from a single racial and ethnic group.
CONCLUSIONS
The findings of this study are consistent with the recent literature. Patients with isolated CABG who have been administered β-blockers, ACEI/ARBs treatment and lipid therapy before CABG experienced reduced POAF, while those with impaired renal function and higher serum sodium levels before CABG were predisposed to POAF in a Taiwanese population. In addition, the occurrence of POAF following CABG identified a subset of patients with increased early complications and a reduced survival probability following CABG.
Acknowledgments
This study was supported by the grants from the Deh-Tzer Study Group for Human Medical Research Foundation (B1021081), Taipei, Taiwan, and Republic of China to YTT.
REFERENCES
- 1.Levy D, Kannel WB. Postoperative atrial fibrillation and mortality: do the risks merit changes in clinical practice? J Am Coll Cardiol. 2004;43:749–751. doi: 10.1016/j.jacc.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 3.Mirhosseini SJ, Ali-Hassan-Sayegh S, Forouzannia SK. What is the exact predictive role of preoperative white blood cell count for new-onset atrial fibrillation following open heart surgery? Saudi J Anaesth. 2013;7:40–42. doi: 10.4103/1658-354X.109807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Echahidi N, Pibarot P, O’Hara G, Mathieu P. Mechanisms,prevention,and treatment of atrial fibrillation after cardiac surgery. J Am Coll Cardiol. 2008;51:793–801. doi: 10.1016/j.jacc.2007.10.043. [DOI] [PubMed] [Google Scholar]
- 5.Mayson SE, Greenspon AJ, Adams S, et al. The changing face of postoperative atrial fibrillation prevention: a review of current medical therapy. Cardiol Rev. 2007;15:231–241. doi: 10.1097/CRD.0b013e31813e62bb. [DOI] [PubMed] [Google Scholar]
- 6.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsai YT, Lin FY, Lai CH, et al. On-pump beating-heart coronary artery bypass provides efficacious short- and long-term outcomes in hemodialysis patients. Nephrol Dial Transplant. 2012;27:2059–2065. doi: 10.1093/ndt/gfr536. [DOI] [PubMed] [Google Scholar]
- 8.Elahi MM, Flatman S, Matata BM. Tracing the origins of postoperative atrial fibrillation: the concept of oxidative stress-mediated myocardial injury phenomenon. Eur J Cardiovasc Prev Rehabil. 2008;15:735–741. doi: 10.1097/HJR.0b013e328317f38a. [DOI] [PubMed] [Google Scholar]
- 9.Costanzo S, di Niro V, Di Castelnuovo A, et al. Prevention of postoperative atrial fibrillation in open heart surgery patients by preoperative supplementation of n-3 polyunsaturated fatty acids: an updated meta-analysis. J Thorac Cardiovasc Surg. 2013;146:906–911. doi: 10.1016/j.jtcvs.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 10.El-Chami MF, Kilgo PD, Elfstrom KM, et al. Prediction of new onset atrial fibrillation after cardiac revascularization surgery. Am J Cardiol. 2012;110:649–654. doi: 10.1016/j.amjcard.2012.04.048. [DOI] [PubMed] [Google Scholar]
- 11.Mathew JP, Fontes ML, Tudor IC, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291:1720–1729. doi: 10.1001/jama.291.14.1720. [DOI] [PubMed] [Google Scholar]
- 12.Sedrakyan A, Wu AW, Parashar A, et al. Off-pump surgery is associated with reduced occurrence of stroke and other morbidity as compared with traditional coronary artery bypass grafting: a meta-analysis of systematically reviewed trials. Stroke. 2006; 37:2759–2769. doi: 10.1161/01.STR.0000245081.52877.f2. [DOI] [PubMed] [Google Scholar]
- 13.Salamon T, Michler RE, Knott KM, Brown DA. Off-pump coronary artery bypass grafting does not decrease the incidence of atrial fibrillation. Ann Thorac Surg. 2003;75:505–507. doi: 10.1016/s0003-4975(02)04305-9. [DOI] [PubMed] [Google Scholar]
- 14.Sakamoto A, Hamasaki T, Kitakaze M. Perioperative landiolol administration reduces atrial fibrillation after cardiac surgery: a meta-analysis of randomized controlled trials. Adv Ther. 2014;31:440–450. doi: 10.1007/s12325-014-0116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan MF, Wendel CS, Movahed MR. Prevention of post-coronary artery bypass grafting (CABG) atrial fibrillation: efficacy of prophylactic beta-blockers in the modern era: a meta-analysis of latest randomized controlled trials. Ann Noninvasive Electrocardiol. 2013;18:58–68. doi: 10.1111/anec.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumagai K, Nakashima H, Urata H, et al. Effects of angiotensin II type 1 receptor antagonist on electrical and structural remodeling in atrial fibrillation. J Am Coll Cardiol. 2003;41:2197–2204. doi: 10.1016/s0735-1097(03)00464-9. [DOI] [PubMed] [Google Scholar]
- 17.Nakashima H, Kumagai K, Urata H, et al. Angiotensin II antagonist prevents electrical remodeling in atrial fibrillation. Circulation. 2000;101:2612–2617. doi: 10.1161/01.cir.101.22.2612. [DOI] [PubMed] [Google Scholar]
- 18.Shiroshita-Takeshita A, Brundel BJ, Burstein B, et al. Effects of simvastatin on the development of the atrial fibrillation substrate in dogs with congestive heart failure. Cardiovasc Res. 2007;74:75–84. doi: 10.1016/j.cardiores.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Kumagai K, Nakashima H, Saku K. The HMG-CoA reductase inhibitor atorvastatin prevents atrial fibrillation by inhibiting inflammation in a canine sterile pericarditis model. Cardiovasc Res. 2004;62:105–111. doi: 10.1016/j.cardiores.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 20.Brinkman W, Herbert MA, O’Brien S, et al. Preoperative beta-blocker use in coronary artery bypass grafting surgery: national database analysis. JAMA Intern Med. 2014;174:1320–1327. doi: 10.1001/jamainternmed.2014.2356. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Ma L. Effect of preoperative angiotensin-converting enzyme inhibitor on the outcome of coronary artery bypass graft surgery. Eur J Cardiothorac. 2014 doi: 10.1093/ejcts/ezu298. [DOI] [PubMed] [Google Scholar]
- 22.White CM, Kluger J, Lertsburapa K, et al. Effect of preoperative angiotensin converting enzyme inhibitor or angiotensin receptor blocker use on the frequency of atrial fibrillation after cardiac surgery: a cohort study from the atrial fibrillation suppression trials II and III. Eur J Cardiothorac Surg. 2007;31:817–820. doi: 10.1016/j.ejcts.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Sandhu RK, Kurth T, Conen D, et al. Relation of renal function to risk for incident atrial fibrillation in women. Am J Cardiol. 2012;109:538–542. doi: 10.1016/j.amjcard.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baber U, Howard VJ, Halperin JL, et al. Association of chronic kidney disease with atrial fibrillation among adults in the United States: REasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Circ Arrhythm Electrophysiol. 2011;4:26–32. doi: 10.1161/CIRCEP.110.957100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scrivo R, Vasile M, Bartosiewicz I, Valesini G. Inflammation as “common soil” of the multifactorial diseases. Autoimmun Rev. 2011;10:369–374. doi: 10.1016/j.autrev.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Fijorek K, Puskulluoglu M, Tomaszewska D, et al. Serum potassium,sodium and calcium levels in healthy individuals - literature review and data analysis. Folia Med Cracov. 2014;54:53–70. [PubMed] [Google Scholar]
- 27.Amin AS, Asghari-Roodsari A, Tan HL. Cardiac sodium channelopathies. Pflugers Arch. 2010;460:223–237. doi: 10.1007/s00424-009-0761-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balser JR. The cardiac sodium channel: gating function and molecular pharmacology. J Mol Cell Cardiol. 2001;33:599–613. doi: 10.1006/jmcc.2000.1346. [DOI] [PubMed] [Google Scholar]
- 29.Algalarrondo V, Wahbi K, Sebag F, et al. Abnormal sodium current properties contribute to cardiac electrical and contractile dysfunction in a mouse model of myotonic dystrophy type 1. Neuromuscul Disord. 2015;25:308–320. doi: 10.1016/j.nmd.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 30.Villareal RP, Hariharan R, Liu BC, et al. Postoperative atrial fibrillation and mortality after coronary artery bypass surgery. J Am Coll Cardiol. 2004;43:742–748. doi: 10.1016/j.jacc.2003.11.023. [DOI] [PubMed] [Google Scholar]


