Abstract
Background
The pulmonary veins (PVs) and atria are important foci during that period when atrial fibrillation (AF) is generated and maintained. It is well understood that hypertension and diabetes mellitus (DM) are important risk factors for AF. Dipeptidyl peptidase-IV (DPP-4) inhibitors are new agents in the fight against type 2 DM, though they have been found to have several cardiovascular effects. However, it is not clear whether DPP-4 may modulate the electrical and mechanical characteristics in hypertensive atrium or PVs.
Methods
Conventional microelectrodes were used to record the action potentials (APs) in isolated PVs, right atrium (RA), and left atrium (LA) in Wistar-Kyoto rats (WKY) and spontaneously hypertensive rats (SHR) with or without sitagliptin (10 mg/kg) for 4 weeks.
Results
WKY (n = 5), SHR (n = 7), sitagliptin-treated WKY (n = 5) and sitagliptin-treated SHR (n = 7) had similar PV or sinoatrial spontaneous beating rates. However, the sitagliptin-treated WKY had fewer sinoatrial-PV beating rate differences than WKY, SHR or sitagliptin-treated SHR. WKY and SHR had shorter 90% (APD90) of RA AP duration than sitagliptin-treated WKY or sitagliptin-treated SHR. In contrast, WKY had longer LA APD90 than sitagliptin- treated WKY, but SHR and sitagliptin-treated SHR had similar LA APD90. Sitagliptin-treated WKY or sitagliptin- treated SHR had larger (RA-LA) APD90 differences than WKY or SHR, respectively. Moreover, as compared to WKY the post-rest potentiation of contraction was decreased in SHR, sitagliptin-treated WKY, and sitagliptin-treated SHR.
Conclusions
Sitagliptin significantly affects the electromechanical characteristics of PVs and atria, which can be modulated by hypertension.
Keywords: Atrial fibrillation, Atrium, Dipeptidyl peptidase inhibitor-4, Hypertension, Pulmonary vein
INTRODUCTION
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia, which is associated with high risk of morbidity and mortality from stroke, thromboembolism, heart failure and hospitalizations. 1 In prior epidemiological studies, hypertension and diabetes were the important risk factors for the genesis of AF. 2 The cumulative risk of AF was estimated to be 1.42 times higher in hypertensive subjects and 1.4 times higher in diabetic patients as compared with normotensive and non-diabetic patients, respectively. 3 Several physiological mechanisms could underlie a causal relationship between hypertension or diabetes mellitus (DM) and AF. 4,5 Physiologic changes associated with hypertension or diabetes including inflammation, neural remodeling in the atria, increased left atrial (LA) size, higher risk of coronary artery disease and congestive heart failure all contribute to the development of AF. 5,6,7,8 However, the extent of knowledge was limited concerning the electromechanical effects of hypertension on pulmonary veins (PVs) and LA; both PVs and LA are key factors in the process of initiating and maintaining AF. 9,10,11
Dipeptidyl peptidase-4 (DPP-4) inhibitors are more recently developed drugs used to treat patients with type 2 DM. When DPP-4 is inhibited, circulating levels of intact glucagon-like peptide-1 (GLP-1) have been shown to increase, and glucose tolerance improves in many animal models of insulin resistance. 12 The cardiovascular actions of GLP-1 receptor agonists and DPP-4 in-hibitors include cardioprotection (in preclinical animal studies) with or without hyperglycemia and reductions in blood pressure, postprandial lipids, and markers of inflammation and oxidative stress in clinical studies. 13,14,15 However, the effects of DPP-4 inhibitor on electric and mechanical characteristics of PV and atria were unclear. The aim of this study was to investigate whether DPP-4 can modulate the hypertensive effects on atrial and PV electrical and mechanical characteristics.
METHODS
Animal and tissue preparations
This investigation was approved by the Institutional Animal Care and Use Committee of Taipei Medical University and complied with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication no. 85-23, revised 1996). The studied rats were separated into four groups: Wistar-Kyoto (WKY) rats, sitagliptin-treated WKY rats, spontaneously hypertensive rats (SHR) and sitagliptin-treated SHR. At 12 weeks of age, WKY and SHR were treated by oral gavage for 4 weeks with 10 mg/kg sitagliptin (Januvia; Merck Sharp and Dohme, Pavia, Italy), a selective DPP4-inhibitor for vesicles. The systolic blood pressure (132 ± 3 vs. 133 ± 4 mmHg) and diastolic blood pressure (60 ± 5 vs. 67 ± 4 mmHg) were similar between WKY with (n = 2) and without (n = 4) sitagliptin treatment. However, the systolic blood pressure (166 ± 5 vs. 224 ± 4 mmHg, p < 0.05) and diastolic blood pressure (89 ± 3 vs. 121 ± 10 mmHg, p < 0.05) were significantly lower in SHR with (n = 4) and without (n = 4) sitagliptin treatment. The body weights were similar among the WKY, SHR, sitgliptin-treated WKY and sitagliptin-treated SHR (310 ± 6 vs. 293 ± 7 vs. 321 ± 2 vs. 320 ± 3 gm). The rats were sacrificed at 16 weeks old after they were anesthetized with an intraperitoneal injection of sodium pentobarbital (100 mg/kg). The heart of each rat was rapidly excised and subsequently dissected. The PVs were separated from the atrium at the level of the LA-PV junction and separated from the lungs at the end of the PV myocardial sleeve in Tyrode’s solution with a composition (in mM) of 137 NaCl, 4 KCl, 15 NaHCO3, 0.5 NaH2PO4, 0.5 mgCl2, 2.7 CaCl2, and 11 dextrose; the pH was adjusted to 7.4 by titration with NaOH. One end of the preparations, consisting of the PVs and LA-PV junction, was pinned with needles to the bottom of a tissue bath. The other end (distal PV) was connected to a Grass FT03C force transducer (Astro-Med Inc, West Warwick, RI, USA) with a silk thread. For atrial experiments, the RA and LA were isolated and prepared as described previously. 16 The adventitial or epicardial side of the preparations faced upwards. The PV, right atrium (RA) and LA tissue strips were superfused at a constant rate (3 ml/min) with Tyrode’s solution saturated with a 97% O2-3% CO2 gas mixture. The temperature was maintained at 37 °C, and the preparations were allowed to equilibrate for 1 h before electrophysiological assessment.
Electrophysiological studies
The transmembrane action potentials (APs) of the PVs, sinoatrial node (SAN), RA and LA anterior wall were recorded using machine-pulled glass capillary microelectrodes filled with 3 M KCl in PVs, RA or LA preparations. The preparations were connected to a WPI model FD223 electrometer under tension with 150 mg as described previously.17 The electrical and mechanical events (contractility and diastolic tension) were simultaneously displayed and recorded on a Gould 4072 oscilloscope and Gould TA11 recorder. The signals were recorded with DC coupling and a 10-kHz low-pass filter cut-off frequency using a data acquisition system. Signals were recorded digitally with 16-bit accuracy at a rate of 125 kHz.
The AP amplitude (APA) was obtained by measuring the difference between the resting membrane potential (RMP) or maximum diastolic potential and the peak of AP depolarization. AP durations at repolarization rates of 90%, 50%, and 20% of the APA were measured as APD90, APD50, and APD20, respectively. The RMP, APA, APD90, APD50, APD20, and contractile forces were measured under 4-Hz pacing of the PVs, RA and LA preparations. Post-rest potentiation of contraction. In cardiac muscle, the force of contraction increases after a transient interruption of drive. 18 The difference of APD90 between RA and LA was presented as ΔAPD90. The increase in contraction amplitude after rest intervals longer than the basic stimulation interval is referred to as post-rest potentiation of contraction (PRPC), which is a measure of the sarcoplasmic reticulum Ca2+-ATPase pumping activity. PRPC was determined after different rest intervals (10, 30, 120 s) in isolated electrical paced LA preparations. The change of PRPC was divided by baseline contractile forces to present as Δ/baseline (%).
Statistical analysis
All quantitative data were expressed as the mean ± SEM. Statistical significance between different groups was determined by unpaired t-test or two-way analysis of variance (ANOVA) with Fisher’s least significant difference for post-hoc test analysis of multiple comparisons as appropriate. A value of p < 0.05 was considered statistically significant.
RESULTS
Effects of sitagliptin on Sinoatrial and PV spontaneous beating or contractility
As shown in Figure 1, WKY, SHR, sitagliptin-treated WKY and sitagliptin-treated SHR had similar PV spontaneous beating rates. WKY, SHR, sitagliptin-treated WKY and sitagliptin-treated SHR also had similar spontaneous activities in RA, which was over-driven by sinoatrial automaticity. However, sitagliptin-treated WKY had less sinoatrial-PV beating rate differences (0.5 ± 0.2 vs. 1.9 ± 0.2 vs. 1.2 ± 0.4 vs. 1.3 ± 0.5 Hz, p < 0.05) than WKY, SHR or sitagliptin-treated SHR.
Figure 1.
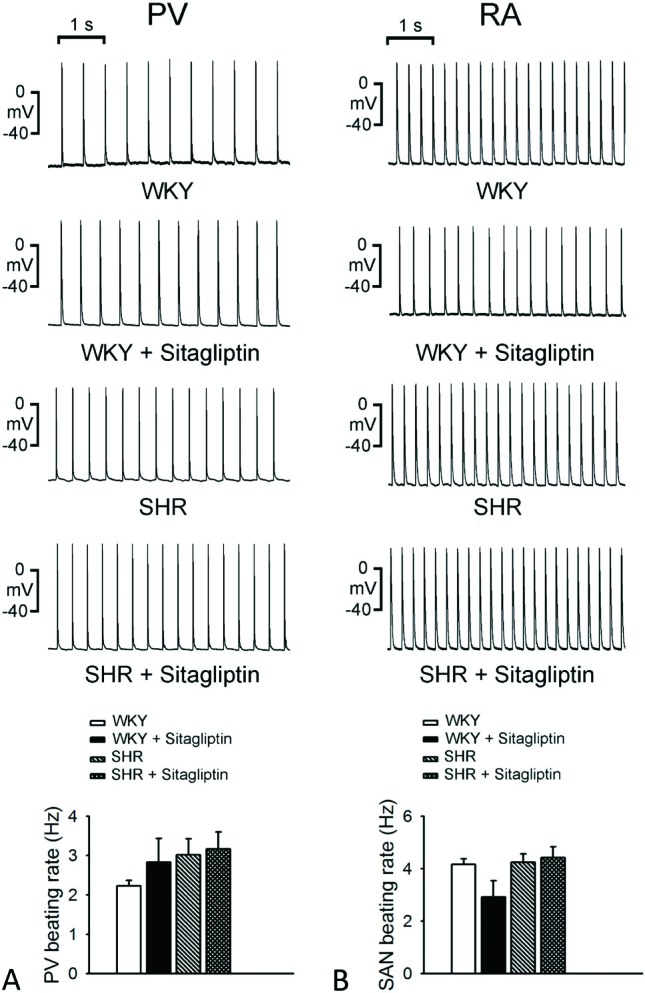
Effects of sitagliptin on the spontaneous beating rate of pulmonary vein (PV) and right atrium (RA) in Wistar-Kyoto rats (WKY) and spontaneously hypertensive rats (SHR). An example and average data (n = 7) show that the WKY and SHR PVs had slower spontaneous beating rate than WKY and SHR RA, respectively (p < 0.05). The treatment of sitagliptin did not significantly change the PV or RA beating rates of WKY and SHR, but has the trend to increase the automaticity of PV. Thus, the spontaneous beating rates of PV are no longer significantly different from that of RA in WKY and SHR with the treatment of sitagliptin.
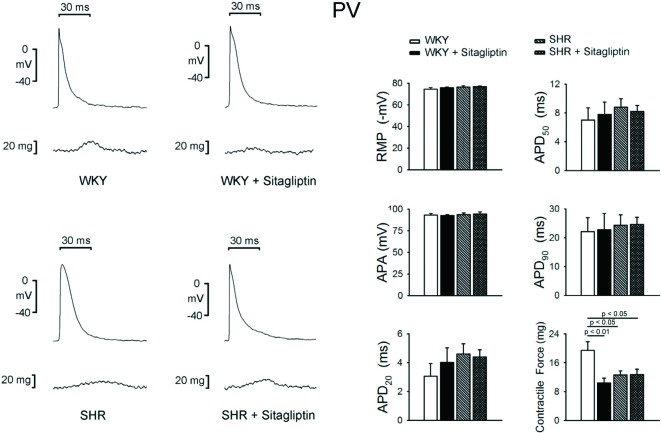
WKY and SHR with or without sitagliptin treatment PVs had similar RMP, APA, APD20, APD50, and APD90, as shown in Figure 2. The SHR PVs exhibited smaller contractile force than WKY PVs. Sitagliptin-treated WKY had significantly smaller contractile forces than WKY PVs. In contrast, SHR and sitagliptin-treated SHR PVs had similar PV contractile forces.
Figure 2.
Electromechanical effects of sitagliptin on pulmonary vein (PV). An example and average data (n = 7) show that the resting membrane potential (RMP), action potential amplitude (APA), action potential duration (APD) of PVs are not different between WKY and SHR with or without sitagliptin treatment. The SHR V had smaller contractile force than WKY PV. Treatment of sitagliptin significantly reduced the contractile forces of WKY PV, but did not change the contractile forces of SHR PV.
Sitagliptin on electromechanical RA and LA electromechanical properties
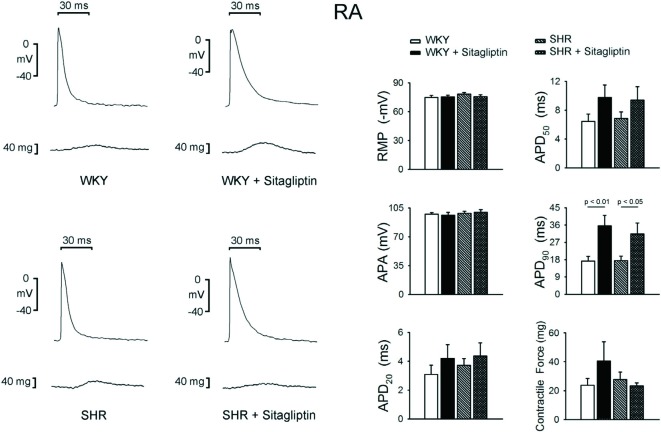
WKY, SHR, sitagliptin-treated WKY and sitagliptin-treated SHR had similar contractile forces, RMP, APA, APD20 and APD50 in RA (Figure 3). However, WKY had shorter RA APD90 than sitagliptin-treated WKY, and SHRalso had shorter RA APD90 than sitagliptin-treated SHR.
Figure 3.
Electromechanical effects of sitagliptin on right atrium (RA). An example and average data (n = 7) show that the RMP, APA, APD20, APD50 and contractile forces of RA are not different between WKY and SHR with or without sitagliptin treatment. The treatment of sitagliptin increased the APD90 of WKY RA and increased the APD90 of SHR RA.
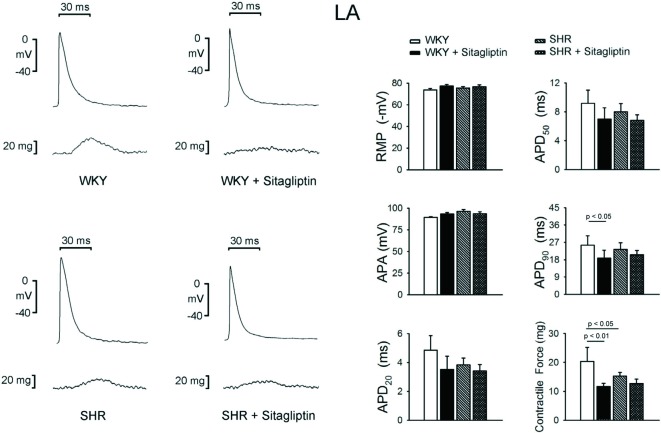
As shown in Figure 4, sitagliptin-treated WKY and sitagliptin-treated SHR had similar RMP, APA, APD20 and APD50 in LA. Contrarily, WKY had longer LA APD90 than sitagliptin-treated WKY, but SHR and sitagliptin-treated SHR had similar LA APD90. Moreover, the SHR and sitagliptin-treated WKY had lesser LA contractility than WKY.
Figure 4.
Electromechanical effects of sitagliptin on left atrium (LA). An example and average data (n = 7) show that the RMP, APA, APD20, APD50 of LA are not different between WKY and SHR with or without sitagliptin treatment. The treatment of sitagliptin decreased the APD90 of WKY LA, but did not change the APD90 of SHR LA. The treatment of sitagliptin decreased the contractile forces of WKY LA, but did not change the contractile forces of SHR LA.
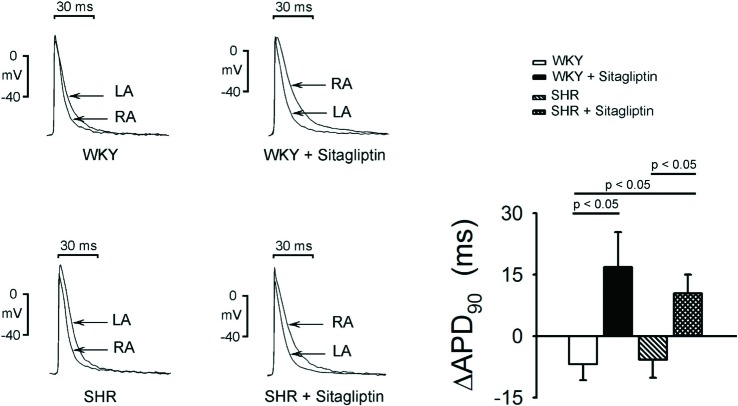
WKY RA had shorter APD90 than WKY LA, as shown in Figure 5. In contrast, sitagliptin-treated WKY RA had longer APD90 than sitagliptin-treated WKY LA. Thus, in SHR, RA and LA had similar APD90. However, sitagliptin-treated SHR RA had longer APD90 than sitagliptin-treated SHR LA. Accordingly, sitagliptin-treated WKY had larger (RA-LA) APD90 differences than WKY, and sitagliptin-treated SHR had larger (RA-LA) APD90 differences than WKY or SHR.
Figure 5.
Effects of sitagliptin on interatrial conducting dispersion. An example and average data (n = 7) show that the difference of APD90 between RA and LA was presented as RA-LA DAPD90. The baseline APD90 of RA was significantly shorter than LA in WKY rats. The treatment of sitagliptin significantly prolonged the APD90 of RA and shortened the APD90 of LA. The APD90 of RA became significantly longer than that of LA after the treatment of sitagliptin in WKY rats. The baseline APD90 of RA was not significantly different from LA in SHR. But the treatment of sitagliptin significantly prolonged the APD90 of RA. Therefore, the APD90 of RA became significantly longer than that of LA after the treatment of sitagliptin in SHR.
Post-rest potentiation of LA contraction
As shown in Figure 6, the percentile increment of WKY PRPC after a 30 or 120 s pause was significantly larger than that after just a 10 s pause in WKY LA. But a 120 s pause did not increase PRPC more than that after a 30 s pause. Sitagliptin-treated WKY had diminished after 10, 30 and 120 s pause PRPC, which was smaller than those in WKY. In addition, there were similar 10, 30 and 120 s pause PRPC in sitagliptin-treated WKY.
Figure 6.
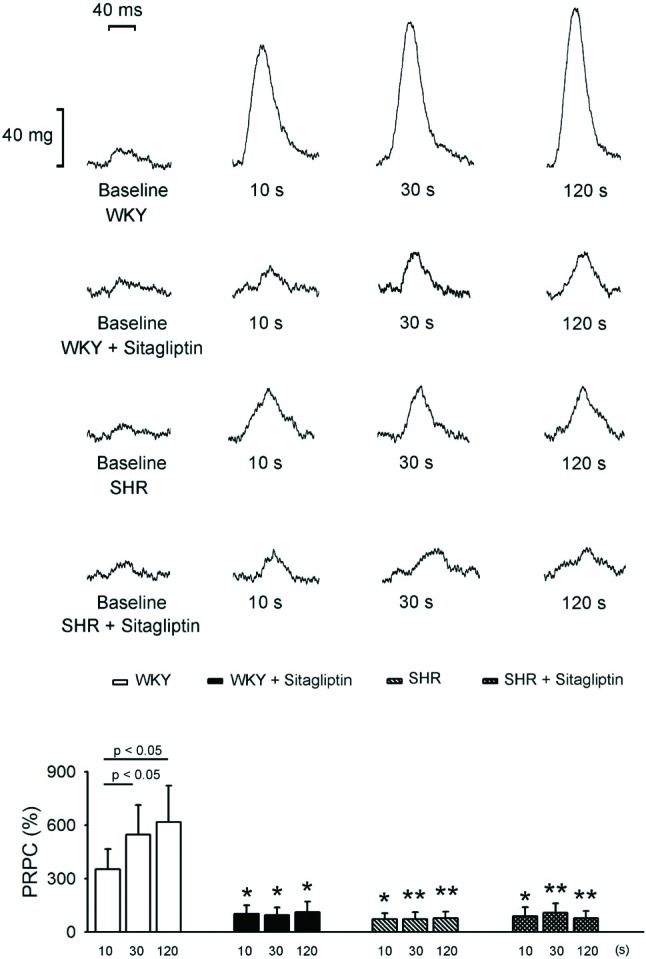
Effects of sitagliptin on post-rest potentiation contraction (PRPC) of left atrium (LA). An example and average data (n = 7) show that the increment of PRPC was divided by baseline contractile forces to present as D/baseline (%). In WKY, the percentile increment of PRPC after 30 or 120 s pause was significantly larger than that after 10 s pause. But the PRPC did not increase further after 120 s pause than that after 30 s pause. Treatment with sitagliptin significantly diminished the PRPC of WKY rats with 10, 30 and 120 s pause. In SHR, the PRPC was significantly smaller than that of WKY. The treatment of sitagliptin cannot significantly change the PRPC of SHR with 10, 30 and 120 s pause. * p < 0.05, ** p < 0.01 vs. the control (WKY).
As compared to WKY, SHR had smaller 10, 30 and 120 s pause PRPC. Moreover, SHR and sitagliptin-treated SHR had similar respective 10, 30 and 120 s pause PRPC. In addition, there were similar 10, 30 and 120 s pause PRPC in SHR or sitagliptin-treated SHR.
DISCUSSION
DPP-4 inhibitors are newly available drugs approved for the treatment of type 2 DM mainly by improving meal-stimulated insulin secretion by pancreatic β-cells, which is accomplished by sparing the hormone glucagon-like peptide-1 (GLP-1) from degradation by the enzyme DPP-4. 19 GLP-1 receptors have been reported to be widely expressed in the heart and vasculature with specific localization in vascular endothelium/smooth muscle, endocardium and cardiomyocytes, suggesting that GLP-1 may play an important role in the cardiovascular system. 12,13,14,15 Experimental data from animal and human studies indicate that GLP-1 has inotropic and vasodilatory effects, increased myocardial glucose uptake, improvement of endothelial function, reduction in infarct size, as well as potential anti-inflammatory and antiatherogenic actions. 20,21 Recent work from several different laboratories has indicated that DPP4 inhibitor or glucagon-like peptide-1 (GLP-1) receptor agonists exert wide-ranging cardiovascular effects, such as modulation of heart rate, blood pressure, vascular tone and myocardial contractility. 22,23 However, the modulation of PV automaticity had not been previously elucidated. In the present study, we found that sitagliptin could modulate the electric and mechanical characteristics of PVs and both atria. Previous studies have shown that DPP4 inhibitor or GLP-1 receptor agonist could induce an endothelial-dependent vasorelaxation via endothelial nitric oxide (NO) synthase activation. 24,25 A systemic NO-induced vasodilatation results in lower blood pressure accompanied by reflex tachycardia. 26,27 In this study, we also found that sitagliptin reduced the blood pressure in SHR, similar to those in previous experiments. 28,29 Nonetheless, the reflex tachycardia resulting from systemic vasodilatory effect of sitagliptin was not found in our study. It is known that hypertension can induce PV pressure overloading and structural remodeling with resulting contractile impairment of PV. 30,31 The treatment of sitagliptin reduced the contractile forces of PV, and would be the vasodilatory effect of NO produced by NOS activation or alteration of intracellular calcium (Ca2+) regulation. Moreover, sitagliptin-treated WKY had less sinoatrial-PV beating rate differences than WKY, SHR or sitagliptin-treated SHR, which suggests that hypertension may modulate the DPP-4 effects in PV and sinoatrial node activity.
Despite the epidemiological recognition that hypertension is an independent risk factor for AF, a few stu-dies have examined the atrial remodeling involving the structural and electrophysiological consequences of hypertension.32 These previous studies demonstrated that hypertension is associated with diverse changes of effective refractory period (ERP) and arrhythmia inducibility. 33 All of these hypertension-induced electrophysiological alterations were more evident in the LA myocardium. In this study, we found different properties of LA from RA and diverse consequences of atrial remodeling induced by hypertension. Moreover, SHR PV had smaller contractility that WKY PVs. Previous studies have shown that hypertension may induced LA and PV myocardial stretch and dysfunction. 32,33 Although this study did not present significant atrial electrical differences between SHR and WKY, the hypertension possibly attenuated the LA electrical and mechanical responses to treatment of sitagliptin. Increased LA fibrosis and electrical preconditioning induced by hypertension, including abnormal cellular Ca2+ handling, should reduce the effect of sitagliptin. 34
The treatment of sitagliptin significantly increased the RA APD90 in WKY and SHR, and also increased the contractile force of RA in WKY. Sitagliptin had been demonstrated to increase the intracellular calcium which might cause the prolongation of APD and increased contractile force of WKY RA. Nonetheless, the treatment of sitagliptin in SHR prolonged the APD, but did not increase the contractile force significantly. The structural remodeling of hypertensive atrium appeared to attenuate the contractile force enhancement by DPP4 inhibitor. On the contrary, the treatment of sitagliptin reduced both the APD90 and contractile force of WKY LA. Although the mechanism for the discrepant responses to DPP4 inhibitor between RA and LA is not clear, the structural and electrophysiological difference between RA and LA may possess dissimilar response to DPP4 inhibitor. We found that SHR had significantly smaller PV contractile forces and LA PRPC than WKY. Previous studies had demonstrated the abnormality of Ca2+ handling which included the SR Ca2+ uptake and sarco-lemmal Na+/Ca2+ exchanger activity in hypertension. 33,34 Treatment of sitagliptin also significantly reduced the LA PRPC in WKY. The treatment of sitagliptin seems to attenuate calcium reuptake by SR Ca2+-ATPase pumping activity which reduced the calcium store of SR, thence reduced PRPC.
In this study, we observed a discrepant electrophysiological and mechanical effect of sitagliptin on RA and LA, with and without hypertension, and conducted the interatrial conduction dispersion. Furthermore, the shortened LA APD90 predisposed the reentrance and maintenance of atrial arrhythmogenesis. Moreover, this study found that sitagliptin may differentially change the cardiac electrophysiology between the RA and LA. Additionally, the diverse effects on APD between the RA and LA will increase dispersions of the interatrial refractoriness and conduction to facilitate the maintenance of AF. However, the mechanism for the different effects of sitagliptin on the RA and LA is still not clear. Intracellular Ca2+ homeostasis regulated by Ca2+ handling proteins, which control cardiomyocyte function, including sarcoplasmic reticulum Ca2+-ATPase pump and Na+/Ca2+ exchanger, may be implicated with the differential effect of sitalgiptin on RA and LA cardiomyocytes. 35 In a previous study, hypertension per se could induce atrial enlargement and dysfunction with structural abnormalities characterized by increased interstitial fibrosis and inflammatory infiltrates. 36 DPP4 inhibitors contain several beneficial effects in cardiovascular disease reported in previous animal and human experiments. 4 Therefore, it is not clear whether the increased conduction heterogeneity between RA and LA caused by sitagliptin in WKY and SHR may have arrhythmogenetic potentials.
This study should be interpreted with caution due to the potential limitations. We did not study the direct effects of DPP-4 inhibitor by adding the drugs to the isolated tissues, since this study attempted to mimic clinical situations. It is not clear whether our findings were caused by the direct effects of sitagliptin. In addition, we didn’t perform western blot or study patch clamp and calcium image from isolated single myocytes. Thus, the underlying ionic and molecular mechanisms for the effects of sitagliptin were not fully elucidated. Moreover, this study only evaluated the effects of sitagliptin in 12 week-old (adult) SHR or WKY rats. We can’t exclude the possibilities that sitagliptin may have different effects in rats from another age group or other models of hypertension. Finally, the single dose of sitagliptin used in the present study also limited the opportunity to examine the dose-dependent effect of DPP-4 inhibition.
CONCLUSION
Sitagliptin significantly affects the electromechanical characteristics of PVs and atria, which can be modulated by hypertension.
REFERENCES
- 1.Krahn AD, Manfreda J, Tate RB, et al. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med . 1995;98:476–484. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- 2.Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population based-estimates. Am J Cardiol. 1998;82:2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 3.Verdecchia P, Reboldi G, Gattobigio R, et al. Atrial fibrillation in hypertension: predictors and outcome. Hypertension . 2003;41:218–223. doi: 10.1161/01.hyp.0000052830.02773.e4. [DOI] [PubMed] [Google Scholar]
- 4.Healey JS, Connolly SJ. Atrial fibrillation: hypertension as a causative agent, risk factor for complications, and potential therapeutic target. Am J Cardiol. 2003;91(suppl):9G–14G. doi: 10.1016/s0002-9149(03)00227-3. [DOI] [PubMed] [Google Scholar]
- 5.Dubin S, Glazer NL, Smith NL, et al. Diabetes mellitus, glycemic control, and risk of atrial fibrillation. J Gen Intern Med. 2010;25:853–858. doi: 10.1007/s11606-010-1340-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lip GY, Varughese GI. Diabetes mellitus and atrial fibrillation: perspectives on epidemiological and pathophysiological links. Int J Cardiol . 2005;105:319–321. doi: 10.1016/j.ijcard.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Rutter MK, Parise H, Benjamin EJ, et al. Impact of glucose intolerance and insulin resistance on cardiac structure and function: sex-related difference in the Framingham Heart Study. Circulation . 2003;107:448–454. doi: 10.1161/01.cir.0000045671.62860.98. [DOI] [PubMed] [Google Scholar]
- 8.Otake H, Suzuki H, Honda T, Maruyama Y. Influences of autonomic nervous system on atrial arrhythmogenic substrates and the incidence of atrial fibrillation in diabetes heart. Int Heart J. 2009;50:627–641. doi: 10.1536/ihj.50.627. [DOI] [PubMed] [Google Scholar]
- 9.Chen YJ, Chen SA. Electrophysiology of pulmonary veins. J Cardiovasc Electrophysiol. 2006;17:220–224. doi: 10.1111/j.1540-8167.2005.00317.x. [DOI] [PubMed] [Google Scholar]
- 10.Chen YJ, Chen SA, Chang MS, et al. Arrhythmogenic activity of cardiac muscle in pulmonary veins of the dog: implication for the genesis of atrial fibrillation. Cardiovasc Res. 2000;48:265–273. doi: 10.1016/s0008-6363(00)00179-6. [DOI] [PubMed] [Google Scholar]
- 11.Chen YJ, Chen YC, Chen SA, et al. Cardiac cellular electrophysiology, voltage clamp, and patch clamp. Acta Cardiol Sin. 2009;25:59–63. [Google Scholar]
- 12.Kristensen J, Mortensen UM, Schmidt M, et al. Lack of cardioprotection from subcutaneously and preischemic administered liraglutide in a closed chest porcine ischemia reperfusion model. BMC Cardiovasc Disord . 2009;9:31–31. doi: 10.1186/1471-2261-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forst T, Weber MM, Pfützner A, et al. Cardiovascular benefits of GLP-1-based therapies in patients with diabetes mellitus type 2: effects on endothelial and vascular dysfunction beyond glycemic control. Exp Diabetes Res. 2012;2012:635472–635472. doi: 10.1155/2012/635472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thrainsdottir I, Malmberg K, Olsson A, et al. Initial experience with GLP-1 treatment on metabolic control and myocardial function in patients with type 2 diabetes mellitus and heart failure. Diab Vasc Dis Res. 2004;1:40–43. doi: 10.3132/dvdr.2004.005. [DOI] [PubMed] [Google Scholar]
- 15.Mistry GC, Maes AL, Lasseter KC, et al. Effect of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on blood pressure in nondiabetic patients with mild to moderate hypertension. J Clin Pharmacol. 2008;48:592–598. doi: 10.1177/0091270008316885. [DOI] [PubMed] [Google Scholar]
- 16.Lin YK, Chen YC, Cheng CC, et al. Interactions of aging and hydrogen peroxide on pulmonary vein electrical activity: implications in the pathophysiology of atrial fibrillation. Acta Cardiol Sin. 2011;27:109–114. [Google Scholar]
- 17.Chang CJ, Chen YC, Kao YH, et al. Dabigatran and thrombin modulate electrophysiological characteristics of pulmonary vein and left atrium. Circ Arrhythm Electrophysiol. 2012;5:1176–1183. doi: 10.1161/CIRCEP.112.971556. [DOI] [PubMed] [Google Scholar]
- 18.Bers DM, Bassani RA, Bassani JW, et al. Paradoxical twitch potentiation after rest in cardiac muscle: increased fractional release of SR calcium. J Mol Cell Cardiol . 1993;25:1047–1057. doi: 10.1006/jmcc.1993.1117. [DOI] [PubMed] [Google Scholar]
- 19.Ahren B, Holst JJ, Martensson H, Balkan B. Improved glucose tolerance and insulin secretion by inhibition of dipeptidyl peptidase IV in mice. Eur J Pharmacol . 2000;404:239–245. doi: 10.1016/s0014-2999(00)00600-2. [DOI] [PubMed] [Google Scholar]
- 20.Neumiller JJ, Wood L, Campbell RK. Dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes mellitus. Pharmacotherapy . 2010;30:463–484. doi: 10.1592/phco.30.5.463. [DOI] [PubMed] [Google Scholar]
- 21.Goyal S, Kumar S, Bijjem KV, Singh M. Role of glucagon-like peptide-1 in vascular endothelial dysfunction. Indian J Exp Biol . 2010;48:61–69. [PubMed] [Google Scholar]
- 22.Okerson T, Yan P, Stonehouse A, Brodows R. Effects of exenatide on systolic blood pressure in subjects with type 2 diabetes. Am J Hypertens. 2010;23:334–339. doi: 10.1038/ajh.2009.245. [DOI] [PubMed] [Google Scholar]
- 23.Gill A, Hoogwerf BJ, Burger J, et al. Effect of exenatide on heart rate and blood pressure in subjects with type 2 diabetes mellitus: a double-blind, placebo-controlled, randomized pilot study. Cardiovasc Diabetol . 2010;9:6–6. doi: 10.1186/1475-2840-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golpon HA, Puechner A, Welte T, et al. asorelaxant effect of glucagon-like peptide-(7-36) amide and amylin on the pulmonary circulation of the rat. Regul Pept. 2001;102:81–86. doi: 10.1016/s0167-0115(01)00300-7. [DOI] [PubMed] [Google Scholar]
- 25.Poornima I, Brown SB, Bhashyam S, et al. Chronic glucagon-like peptide-1 infusion sustains left ventricular systolic function and prolongs survival in the spontaneously hypertensive, heart failure-prone rat. Circ Heart Fail. 2008;1:153–160. doi: 10.1161/CIRCHEARTFAILURE.108.766402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamamoto H, Lee CE, Marcus JN, et al. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest. 2002;110:43–52. doi: 10.1172/JCI15595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marney A, Kunchakarra S, Byrne L, Brown NJ. Interactive hemodynamic effects of dipeptidyl peptidase-IV inhibition and angiotensin-converting enzyme inhibition in humans. Hypertension . 2010;56:728–733. doi: 10.1161/HYPERTENSIONAHA.110.156554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pacheco BP, Crajoinas RO, Couto GK, et al. Dipeptidyl peptidase IV inhibition attenuates blood pressure rising in young spontaneously hypertensive rats. J Hypertens . 2011;29:520–528. doi: 10.1097/HJH.0b013e328341939d. [DOI] [PubMed] [Google Scholar]
- 29.Lee TI, Kao YH, Chen YC, et al. The dipeptidyl peptidase-4 inhibitor sitagliptin modulates calcium dysregulation, inflammation, and PPARs in hypertensive cardiomyocytes. Int J Cardiol. 2013;15,168:5390–5395. doi: 10.1016/j.ijcard.2013.08.051. [DOI] [PubMed] [Google Scholar]
- 30.Huser J, Blatter LA, Lipsius SL. Intracellular Ca2+ release contributes to automaticity in cat atrial pacemaker cells. J Physiol. 2000;524:415–422. doi: 10.1111/j.1469-7793.2000.00415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diaz ME, Graham HK, O’Neill SC, et al. The control of sarcoplasmic reticulum Ca content in cardiac muscle. Cell Calcium. 2005;38:391–396. doi: 10.1016/j.ceca.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 32.Lau DH, Mackenzie L, Kelly DJ, et al. Hypertension and atrial fibrillation: evidence of progressive atrial remodeling with electrostructural correlate in a conscious chronically instrumented ovine model. Heart Rhythm. 2010;7:1281–1290. doi: 10.1016/j.hrthm.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 33.Kistler PM, Sanders P, Dodic M, et al. Atrial electrical and structural abnormalities in an ovine model of chronic blood pressure elevation after prenatal corticosteroid exposure: implications for development of atrial fibrillation. Eur Heart J. 2006;27:3045–3056. doi: 10.1093/eurheartj/ehl360. [DOI] [PubMed] [Google Scholar]
- 34.Lee TI, Chen YC, Kao YH, et al. Rosiglitazone induces arrhythmogenesis in diabetic hypertensive rats with calcium handling alteration. Int J Cardiol. 2013;165:299–307. doi: 10.1016/j.ijcard.2011.08.072. [DOI] [PubMed] [Google Scholar]
- 35.Deacon CF, Marx N. Potential cardiovascular effects on incretin-based therapies. Expert Rev Cardiovasc Ther. 2012;10:337–351. doi: 10.1586/erc.12.5. [DOI] [PubMed] [Google Scholar]
- 36.Lau DH, Mackenzie L, Rajendram A, et al. Characterization of cardiac remodeling in a large animal “one-kidney, one-clip” hypertensive model. Blood Press. 2010;19:119–125. doi: 10.3109/08037050903576767. [DOI] [PubMed] [Google Scholar]