Abstract
Background
Oxidative stress plays an important role in the pathophysiology of atrial fibrillation (AF). The hydrogen peroxide (H2O2) mainly underlies the cellular oxidative stress and free radicals. Left atrium (LA) is the most important AF substrate. However, the effects of H2O2 on the action potential (AP) and ionic currents in LA myocytes have not been fully elucidated.
Methods
The whole-cell patch clamp was used to investigate the APs and ionic currents of L-type calcium current (ICa-L), transient outward currents (Ito), ultra-rapid delayed rectifier potassium current (IKur), delayed rectifier potassium currents (IK), inward rectifier potassium current (IK1), and sodium-calcium exchanger (NCX) before and after H2O2 (100 μM) in isolated rabbit LA myocytes.
Results
H2O2 (100 μM) shortened by 50% (from 40 ± 7 to 21 ± 5 ms) and 90% the AP duration (from 95 ± 12 to 74 ± 11 ms) in LA myocytes (n = 9), but did not change the resting membrane potentials. The H2O2 (100 μM) decreased Ito, but increased IKur and IK. H2O2 (100 μM) also reduced the ICa-L and the reverse mode NCX. However, H2O2 (100 μM) did not change IK1.
Conclusions
H2O2 directly modulated the AP morphology and ionic currents in LA myocytes, which may contribute to the genesis of AF in oxidative stress.
Keywords: Action potential, Ionic current, Oxidative stress
INTRODUCTION
Atrial fibrillation (AF) is the most commonly sustained cardiac arrhythmia in clinical conditions that often cause cardiac dysfunction and even mortality.1 However, the mechanisms underlying the arrhythmogenesis of AF are not fully elucidated. The oxidative stress has been shown to play a potential role in the pathophysiology of AF.2-7 It has been proven that AF increases production of superoxide, which may arise from the effects of the NADPH and xanthine oxidases.2 Those patients with chronic AF also have increased oxidative modification proteins in the atrium.3 In addition, anti-oxidant agents have demonstrated their therapeutic potential to treat AF through the prevention of oxidative stress.8,9
The left atrium (LA) plays an important role in the genesis and maintenance of AF.10,11 This indicated that the oxidative stress in AF patients could be highly expressed in LA, which manifests the importance of oxidative stress in the electrophysiological characteristics of the LA. Moreover, oxidative stress has been demonstrated to regulate the ionic current in cardiomyocytes.7 However, the effects of oxidative stress on the action potential (AP) morphology and ionic currents in LA have not been fully elucidated. Therefore, the purpose of this study was to investigate the effects of hydrogen peroxide (H2O2) on the electrophysiological characteristics of LA myocytes.
MATERIALS AND METHODS
Electrophysiological studies in LA myocytes
This investigation conformed to those protocols as established in the Guide for the Care and Use of Laboratory Animals. Rabbits (weight 1-2 kg) were anesthetized with an intraperitoneal injection of sodium pentobarbital (100 mg kg-1). The LA myocytes were enzymatically dissociated via the same procedure described previously.12,13 Tissues of the LA were gently shaken in 5-10 ml of Ca2+-free oxygenated Tyrode’s solution until single cardiomyocytes were obtained. The solution for single cardiomyocytes was gradually changed to normal oxygenated Tyrode’s solution. The cells were then allowed to stabilize in the bath for at least 30 min before administration of H2O2 (100 μM).
The whole-cell patch clamp was performed by using an Axopatch-1D amplifier (Axon Instruments, Foster City, CA, USA). Before the formation of the membrane-pipette seal, tip potentials were zeroed in Tyrode’s solution. Junction potentials between the bath and pipette solution (9 mV) were corrected for AP recordings. The APs and ionic currents were recorded in the current-clamp mode and the voltage-clamp mode, respectively. The APs were elicited through a 1-Hz electrical stimulus in the LA wall. The resting membrane potential (RMP) was measured during the period between the last repolarization and the onset of the subsequent AP. The AP amplitude (APA) was obtained from the RMP to the peak of the AP depolarization. The AP duration at repolarization of 90% and 50% of the amplitude were measured as the APD90 and APD50.
A small hyperpolarizing step from a holding potential of -50 mV to a testing potential of -55 mV for 80 ms was delivered at the beginning of each experiment. The area under the capacitive currents was divided by the applied voltage step to obtain the total cell capacitance. Normally, 60% to 80% series resistance (Rs) was electronically compensated. The micropipettes were filled with a solution containing (in mM/L) CsCl 130, MgCl2 1, Mg2ATP 5, HEPES 10, EGTA 10, NaGTP 0.1, and Na2 phosphocreatine 5, (pH of 7.2 with CsOH) for the L-type calcium current (ICa-L); containing (in mM/L) NaCl 20, CsCl 110, MgCl2 0.4, CaCl2 1.75, TEACl 20, BAPTA 5, glucose 5, Mg2ATP 5, and HEPES 10, (pH of 7.25 with CsOH) for the Na+-Ca2+ exchanger (NCX) current; and containing (in mM/L) KCl 20, K aspartate 110, MgCl2 1, Mg2ATP 5, HEPES 10, EGTA 0.5, LiGTP 0.1, and Na2 phosphocreatine 5, (pH of 7.2 with KOH) for the APs and the potassium currents.
The ICa-L was measured as an inward current during depolarization from a holding potential of -50 mV to testing potentials ranging from -40 to +60 mV in 10-mV steps for 300 ms at a frequency of 0.1 Hz by means of a perforated patch clamp. The NaCl and KCl in the external solution were replaced by TEACl and CsCl, respectively.
The transient outward potassium current (Ito) was studied with a double-pulse protocol. A 30-ms pre-pulse from -80 to -40 mV was used to inactivate the sodium channels, followed by a 300-ms test pulse to +60 mV in 10-mV steps at a frequency of 0.1 Hz. CdCl2 (200 μ M) that was added to the bath solution to inhibit ICa-L. Ito was measured as the difference between the peak outward current and steady state current. The ultra-rapid delayed rectifier potassium current (IKur) was studied with a double-pulse protocol, consisting of a 100-ms depolarizing pre-pulse to +40 mV from a holding potential of -50 mV, followed by 150-ms voltage steps from -40 to +60 mV in 10 mV increments at room temperature to provide an adequate temporal resolution. The IKur was measured as 4-aminopyridine (1 mM) sensitive currents. The delayed rectified outward potassium current (IK) was measured from the peak outward current at the end of 1 s of the depolarization from -40 to +60 in 10-mV steps at a frequency of 0.1 Hz during the infusion of CdCl2 (200 μ M) and 4-aminophyridine (2 mM) in the bath solution. The inward rectifier potassium current (IK1) was activated from -40 mV to test potentials ranging from -20 to -120 mV in 10-mV steps for 1s at a frequency of 0.1 Hz, under the infusion of CdCl2 (200 μ M) and 4-aminopyridine (2 mM) in the bath solution. The amplitudes of the IK1 were measured as 1 mM barium-sensitive currents.
The NCX current was elicited by depolarizing pulses between -100 to +100 mV from a holding potential of -40 mV for 300 ms at a frequency of 0.1 Hz. The am-plitudes of the NCX current were measured as 10 mM nickel-sensitive currents.13 The external solution (in mM) consisted of NaCl 140, CaCl2 2, MgCl2 1, HEPES 5 and glucose 10 with a pH of 7.4, and contained strophanthidin (10 μM), nitrendipine (10 μM) and niflumic acid (100 μM).
Statistical analysis
All continuous data are expressed as the mean ± SEM. A paired t-test was used to compare the effects of electric activity of LA myocytes before and after H2O2. A p-value lower than 0.05 was considered statistically significant.
RESULTS
Effects of H2O2 on the morphology of AP in LA myocytes
As shown in Figure 1, H2O2 (100 μM) significantly shorten APD50 by 47%, and APD90 by 22% in LA myocytes (n = 9) both in the 1 Hz and 2 Hz pacing modes. However, H2O2 (100 μM) did not change the RMP and APA.
Figure 1.
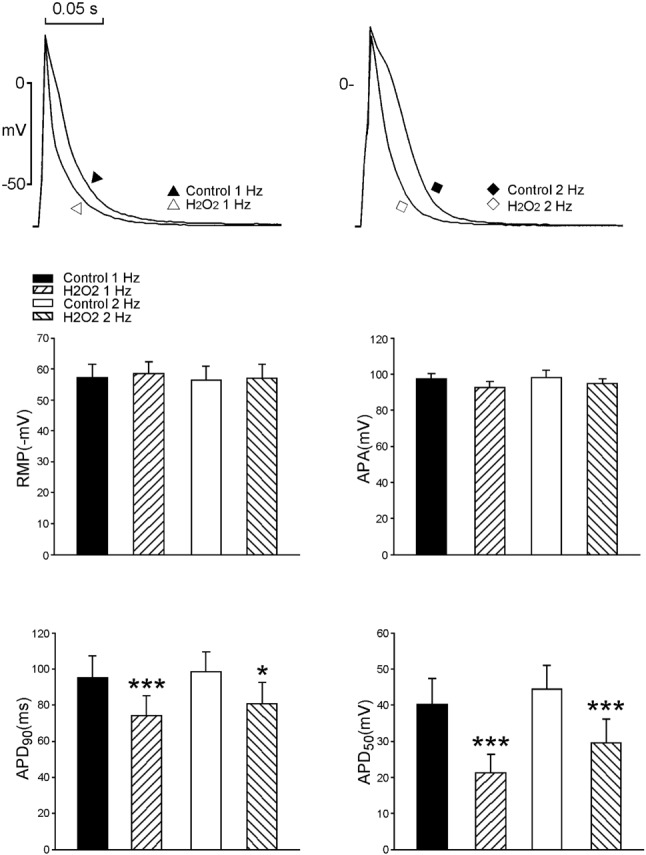
Effects of H2O2 (100 μM) on the action potential characteristics in left atrium (LA) myocytes. Examples and average data (n = 9) showing the shortening of 50% and 90% of AP duration after H2O2 in LA myocytes. * p < 0.05, *** p < 0.005 versus those before H2O2.
Effects of H2O2 on ionic currents in LA myocytes
Figure 2 shows the effects of H2O2 on the ICa-L in LA myocytes. H2O2 (100 μM) significantly decreased ICa-L, whereas the peak ICa-L (elicited from -40 to +20 mV) was reduced by H2O2 (100 μM) to an extent of 30% (n = 16).
Figure 2.
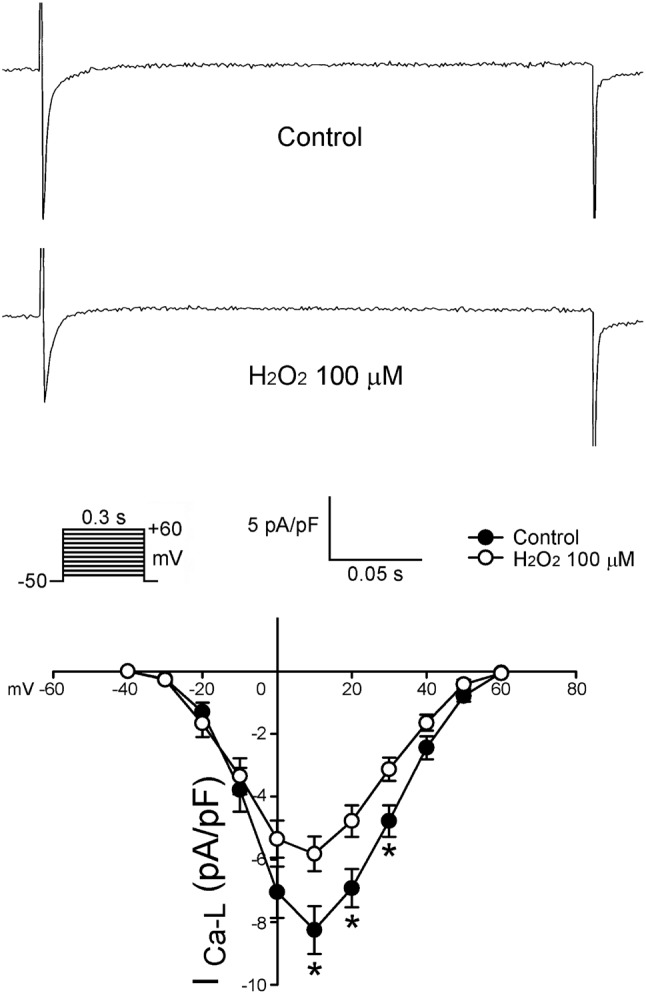
Effects of H2O2 (100 μM) on the ICa-L in LA myocytes. Examples of tracing and I-V relationship of the ICa-L before and after the administration of H2O2 (100 μM) in LA myocytes (n = 16). * p < 0.05 versus those before H2O2.
Figure 3 shows the tracings and I-V relationship of H2O2 on the Ito in LA myocytes. H2O2 (100 μ M) significantly decreased Ito, whereas the peak Ito currents (elicited from -40 to +60 mV) were reduced by H2O2 (100 μ M) to an extent of 45% (n = 24). As shown in Figure 4, the current densities of IKur (elicited from -20 to +60 mV) were also increased to an extent of 74% after the administration of H2O2 (n = 12). Besides, the current densities of IK (elicited from -40 to +60 mV) were increased after the administration of H2O2 by 28% (n = 25, Figure 5). However, H2O2 (100 μ M) did not change the IK1 (Figure 6).
Figure 3.
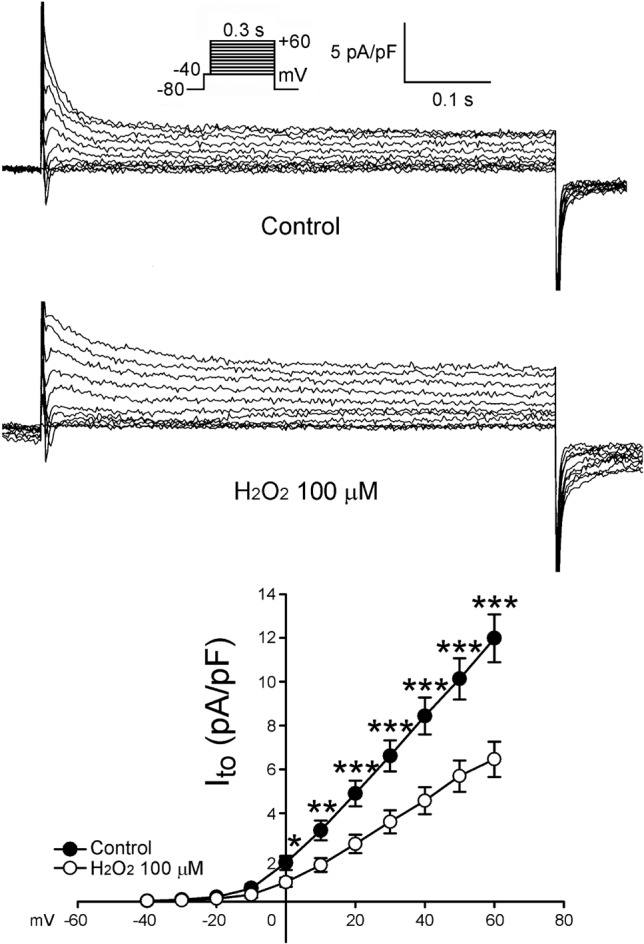
Effects of H2O2 (100 μM) Ito in the atrial myocytes. Examples of the tracings of Ito and I-V relationship of Ito (n = 24). * p < 0.05, ** p < 0.01, *** p < 0.005 versus the control atrial myocytes. The insets of the current traces show the various clamp protocols.
Figure 4.
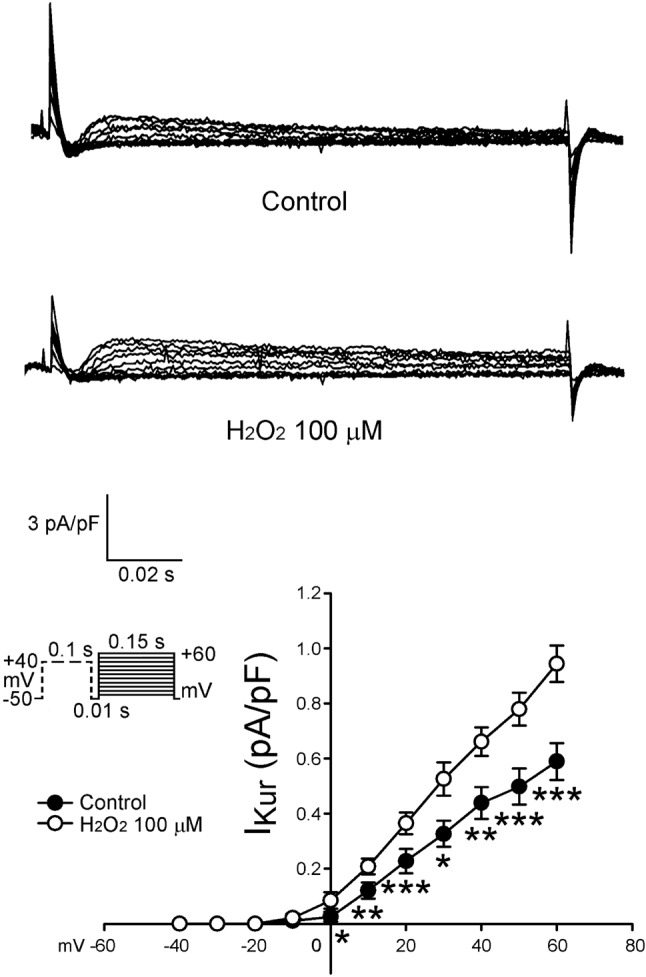
Tracings and I-V relationship of IKur in the atrial myocytes. Examples of tracing before and after the administration of H2O2 (100 μM) IKur in atrial myocytes. The insets of the current traces show the various clamp protocols. The I-V relationship of IKur of control (n = 12) and H2O2-treated (n = 12) in atrial myocytes. * p < 0.05, ** p < 0.01, *** p < 0.005 versus the control atrial myocytes.
Figure 5.
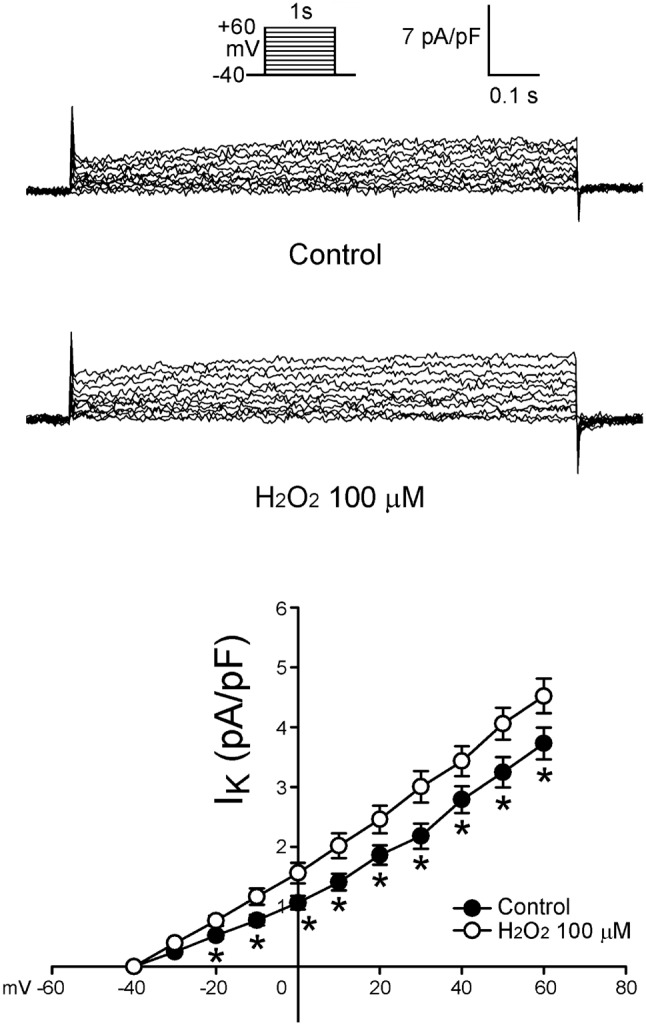
Tracings and I-V relationship of IK in the atrial myocytes. Examples of tracing before and after the administration of H2O2 (100 μM) IK in atrial myocytes. The insets of the current traces show the various clamp protocols. The I-V relationship of IK of control (n = 25) and H2O2-treated (n = 25) in atrial myocytes. * p < 0.05 versus the control atrial myocytes.
Figure 6.
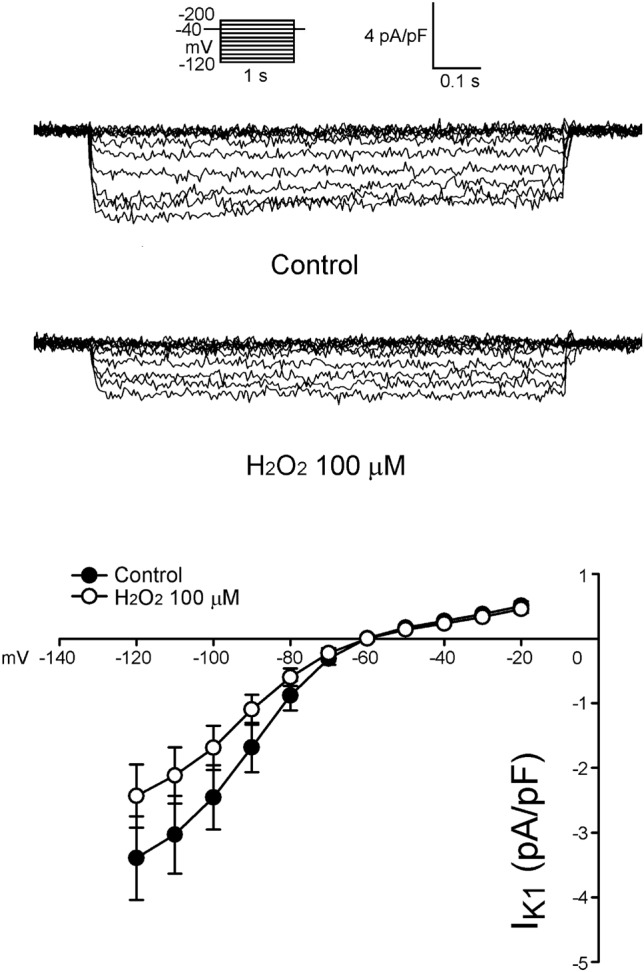
Tracings and I-V relationship of IK1 in the atrial myocytes. Examples of tracing before and after the administration of H2O2 (100 μM) IK1 in atrial myocytes. The insets of the current traces show the various clamp protocols. The control (n = 13) and H2O2-treated (n = 13) myocytes have a similar current density of the IK1.
Furthermore, as shown in Figure 7, H2O2 (100 μ M) decreased the reverse mode of nickel-sensitive NCX currents in LA myocytes. The peak reverse mode nickel-sensitive NCX currents (elicited from -40 to 60 mV) were reduced by H2O2 (100 μM) to a significant extent of 34% (n = 22). However, H2O2 (100 μM) did not change the forward mode of nickel-sensitive NCX currents in LA myocytes.
Figure 7.
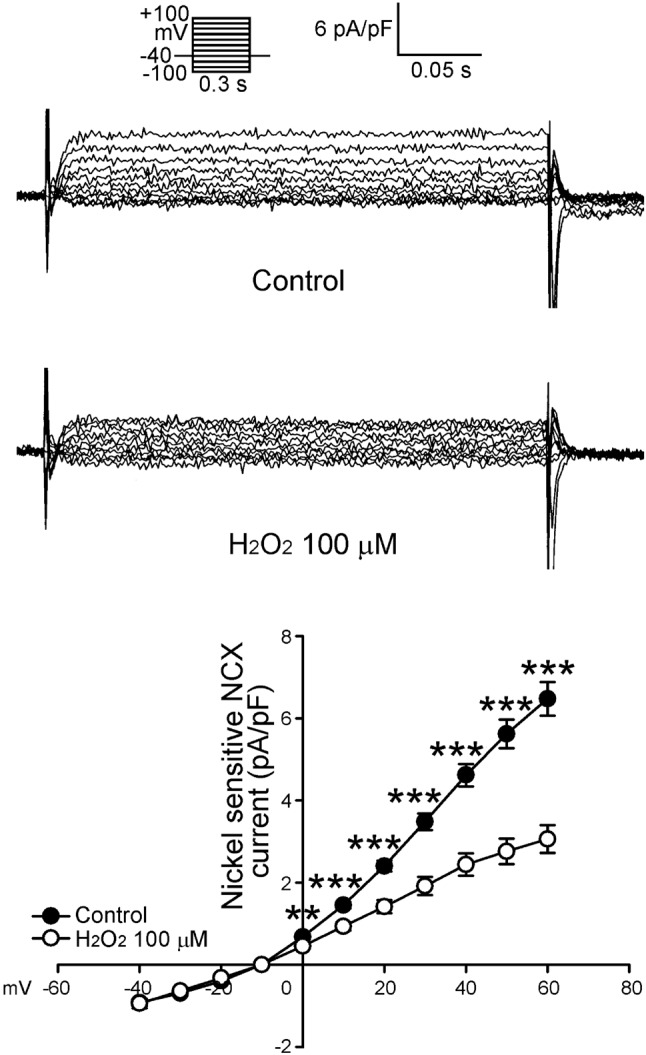
Tracings and I-V relationship of Na+-Ca2+ exchanger (NCX) in the atrial myocytes. Examples of tracing before and after the administration of H2O2 (100 μM) NCX in atrial myocytes. The insets of the current traces show the various clamp protocols. The I-V relationship of NCX of control (n = 22) and H2O2-treated (n = 22) in reverse mode of atrial myocytes. ** p < 0.01, *** p < 0.005 versus the control atrial myocytes.
DISCUSSION
Oxidative stress plays a vital role in the pathophysiology of a wide range of cardiovascular diseases, including AF.6,7,14 H2O2 may mediate the pathological process of the ischemia, heart failure, inflammation, and renin-angiotensin or adrenergic activations, which are recognized to increase the occurrence of AF as well.7,15,16 Oxidative stress not only can induce atrial remodeling, but also may alter atrial electrophysiology.15,17 Additionally, several studies have investigated the electrophysiological variation after the exposure of free radicals and oxidative stress to result in a reduction of the resting membrane potential, action potential amplitude and an augmentation of the automaticity as similar as our study.18,19 Since H2O2 shortened the AP duration to a great extent in the cardiomyocytes of the LA, it could expand the dispersion of the AP duration in the substrate of LA to facilitate the genesis of reentry circuits, which is one of the arrhythmogenic mechanisms.15,20 Although, the concentration (100 μM) of H2O2 used in this study had been assumed to be clinically relevant,15 which demonstrated a generally pathophysiological phenomenon in the atrial electrophysiology, the single dose of H2O2 limited the opportunity to examine its dose-dependent effects.
In the study, we found that H2O2 at a pathophysiological concentration significantly modified the function of the potassium and Ca2+ regulatory channels in left atrial myocytes. Our data showed that oxidative stress induced a dominantly decreased level in ICa-L and Ito but not in IK1. The mechanism of reduced potassium currents by oxidative stress is unclear and inconsistent. It is possibly influenced by inadequate buffering of the calcium channels and restricting the cell membrane of cardiomyocytes.16,21 The Ito in this study was significantly diminished over 40% after the administration of H2O2. This result resembled what in some studies is related to chronic human AF and rapid pacing heart failure model.22-24 Moreover, the increased density of IK current could be the main character to downgrade the level of APD50 and APD90 in our study. On the other hand, Liu et al. reported that the direct inhibition of the IKur could prolong the action potential period to prevent atrial fibrillation.25 It explained the effect of increased current densities of IKur to reduce the action potential period in our study and possibly induce atrial arrhythmia in the oxidative stress of atrial myocytes.
Previous study has shown that oxidative stress could induce a Ca2+-overload by H2O2 that disorganized the regulation protein of calcium channel or lipid oxidation of the cell membrane.26 That is consistent with our results of reducing ICa-L and NCX current density. In addition, H2O2 enhanced the intracellular Ca2+ release by increasing the opening probability of ryanodine receptors.27,28 Those effects may demonstrate the arrhythmogenic effects of H2O2, but previous studies reported that the reactive oxygen species (ROS) revealed controversial in ICa-L and the mechanism was unidentified.29-31 Nonetheless, the manifestation of the LA myocytes in this study received a high level of ROS could mimic acute oxidant cardiac injury, which was similar with those in the study from Fearon et al.29 Therefore, it was reasonable to assume that oxidative stress has a high arrhythmogenic potential for inducing AF through enhancing the atrial automaticity and the triggered activity with Ca2+ overload. Moreover, higher expression of the intracellular Ca2+ overload and Ca2+ release by the influence of H2O2 may increase the contractility of atrial myocytes in our previous findings.15,32 In chronic AF, sustained oxidative stress created the conditions that favor reduced ICa-L entry and increased ICa-L efflux via forward-mode operation of the NCX by strengthening the manifestation of the NCX protein and attenuating the performance of the ICa-L channel.22,23 However, Qin et al. reported that H2O2-mediated oxidative stress could impair calcium handling of the cardiomyocytes through decreasing sarcoplasmic reticulum Ca2+-ATPase (SERCA) activity.33 In our study, oxidative stress reduced the currents at the reverse-mode of the NCX and ICa-L, but remained Ca2+ overloaded probably owing to the increased calcium in the cytoplasm through the opening of ryanodine receptors and decreased SERCA activity by H2O2.
Anti-oxidants have been indicated to have an anti-arrhythmic potential in previous research indicating that ascorbic acid might decrease the occurrence of post-operative AF and modify the electrical remodeling in rapid atrial pacing model. Hence, the comprehensive cognition of oxidative stress induced by sepsis, heart failure, and the activation of the renin-angiotensin or adrenergic systems in the pathogenesis of AF indeed may provide the possible therapy for this obsessive arrhythmia. Furthermore, our study brought out a newly probable mechanism of oxidative stress in the electrophysiological characteristics of atrial myocytes to trigger AF.
CONCLUSIONS
H2O2 directly modulated the AP morphology and ionic currents in LA myocytes, which may contribute to the genesis of AF in oxidative stress.
Acknowledgments
The current study was supported by grants from National Science Council of Taiwan (NSC99-2314-B-016-034-MY3, NSC99-2628-B-038-011-MY3, NSC100-2628-B-038-001-MY4, NSC100-2325-B-010-005, and NSC100-2314-B-038-027-MY3), Wan Fang Hospital (101-wf-eva-11 and 101-wf-phd-01), Taipei Medical University-Wan Fang Hospital (102swf01), and Cathay General Hospital (CGH-MR-10016).
REFERENCES
- 1.Tsang TS, Gersh BJ. Atrial fibrillation: an old disease, a new epidemic. Am J Med. 2002;113:432–435. doi: 10.1016/s0002-9343(02)01245-7. [DOI] [PubMed] [Google Scholar]
- 2.Dudley SC, Jr., Hoch NE, McCann LA, et al. Atrial fibrillation increases production of superoxide by the left atrium and left atrial appendage: role of the NADPH and xanthine oxidases. Circulation. 2005;112:1266–1273. doi: 10.1161/CIRCULATIONAHA.105.538108. [DOI] [PubMed] [Google Scholar]
- 3.Mihm MJ, Yu F, Carnes CA, et al. Impaired myofibrillar energetics and oxidative injury during human atrial fibrillation. Circulation. 2001;104:174–180. doi: 10.1161/01.cir.104.2.174. [DOI] [PubMed] [Google Scholar]
- 4.Kim YM, Guzik TJ, Zhang YH, et al. A myocardial Nox2 containing NAD(P)H oxidase contributes to oxidative stress in human atrial fibrillation. Circ Res. 2005;97:629–636. doi: 10.1161/01.RES.0000183735.09871.61. [DOI] [PubMed] [Google Scholar]
- 5.Korantzopoulos P, Kolettis TM, Galaris D, Goudevenos JA. The role of oxidative stress in the pathogenesis and perpetuation of atrial fibrillation. Int J Cardiol. 2007;115:135–143. doi: 10.1016/j.ijcard.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 6.Van Wagoner DR. Redox modulation of cardiac electrical activity. J Cardiovasc Electrophysiol. 2001;12:183–184. doi: 10.1046/j.1540-8167.2001.00183.x. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Takeda S, Mochizuki S, et al. Mechanisms of hydrogen peroxide-induced increase in intracellular calcium in cardiomyocytes. J Cardiovasc Pharmacol Ther. 1999;4:41–48. doi: 10.1177/107424849900400107. [DOI] [PubMed] [Google Scholar]
- 8.Suenari K, Chen YC, Kao YH, et al. Eicosapentaenoic acid reduces the pulmonary vein arrhythmias through nitric oxide. Life Sci. 2011;89:129–136. doi: 10.1016/j.lfs.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Virtanen JK, Mursu J, Voutilainen S, Tuomainen TP. Serum long-chain n-3 polyunsaturated fatty acids and risk of hospital diagnosis of atrial fibrillation in men. Circulation. 2009;120:2315–2321. doi: 10.1161/CIRCULATIONAHA.109.852657. [DOI] [PubMed] [Google Scholar]
- 10.Oral H, Scharf C, Chugh A, et al. Catheter ablation for paroxysmal atrial fibrillation: segmental pulmonary vein ostial ablation versus left atrial ablation. Circulation. 2003;108:2355–2360. doi: 10.1161/01.CIR.0000095796.45180.88. [DOI] [PubMed] [Google Scholar]
- 11.Wongcharoen W, Chen YC, Chen YJ, et al. Effects of aging and ouabain on left atrial arrhythmogenicity. J Cardiovasc Electrophysiol. 2007;18:526–531. doi: 10.1111/j.1540-8167.2007.00781.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen YJ, Chen SA, Chen YC, et al. Electrophysiology of single cardiomyocytes isolated from rabbit pulmonary veins: implication in initiation of focal atrial fibrillation. Basic Res Cardiol. 2002;97:26–34. doi: 10.1007/s395-002-8384-6. [DOI] [PubMed] [Google Scholar]
- 13.Chen YC, Chen SA, Chen YJ, et al. T-type calcium current in electrical activity of cardiomyocytes isolated from rabbit pulmonary vein. J Cardiovasc Electrophysiol. 2004;15:567–571. doi: 10.1046/j.1540-8167.2004.03399.x. [DOI] [PubMed] [Google Scholar]
- 14.Carnes CA, Chung MK, Nakayama T, et al. Ascorbate attenuates atrial pacing-induced peroxynitrite formation and electrical remodeling and decreases the incidence of postoperative atrial fibrillation. Circ Res. 2001;89:E32–E38. doi: 10.1161/hh1801.097644. [DOI] [PubMed] [Google Scholar]
- 15.Lin YK, Lin FZ, Chen YC, et al. Oxidative stress on pulmonary vein and left atrium arrhythmogenesis. Circ J. 2010;74:1547–1556. doi: 10.1253/circj.cj-09-0999. [DOI] [PubMed] [Google Scholar]
- 16.Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. J Hypertens. 2000;18:655–673. doi: 10.1097/00004872-200018060-00002. [DOI] [PubMed] [Google Scholar]
- 17.Li SY, Du M, Dolence EK, et al. Aging induces cardiac diastolic dysfunction, oxidative stress, accumulation of advanced glycation endproducts and protein modification. Aging Cell. 2005;4:57–64. doi: 10.1111/j.1474-9728.2005.00146.x. [DOI] [PubMed] [Google Scholar]
- 18.Barrington PL, Meier CF, Jr., Weglicki WB. Abnormal electrical activity induced by free radical generating systems in isolated cardiocytes. J Mol Cell Cardiol. 1988;20:1163–1178. doi: 10.1016/0022-2828(88)90596-2. [DOI] [PubMed] [Google Scholar]
- 19.Pallandi RT, Perry MA, Campbell TJ. Proarrhythmic effects of an oxygen-derived free radical generating system on action potentials recorded from guinea pig ventricular myocardium: a possible cause of reperfusion-induced arrhythmias. Circ Res. 1987;61:50–54. doi: 10.1161/01.res.61.1.50. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka H, Habuchi Y, Suto F, Morikawa J. Effects of hydrogen peroxide on the transient outward current in rabbit atrial myocytes. Clin Exp Pharmacol Physiol. 2001;28:743–747. doi: 10.1046/j.1440-1681.2001.03520.x. [DOI] [PubMed] [Google Scholar]
- 21.Lederer WJ, Niggli E, Hadley RW. Sodium-calcium exchange in excitable cells: fuzzy space. Science. 1990;248:283. doi: 10.1126/science.2326638. [DOI] [PubMed] [Google Scholar]
- 22.Van Wagoner DR, Pond AL, McCarthy PM, et al. Outward K+ current densities and Kv1.5 expression are reduced in chronic human atrial fibrillation. Circ Res. 1997;80:772–781. doi: 10.1161/01.res.80.6.772. [DOI] [PubMed] [Google Scholar]
- 23.Bosch RF, Zeng X, Grammer JB, et al. Ionic mechanisms of electrical remodeling in human atrial fibrillation. Cardiovasc Res. 1999;44:121–131. doi: 10.1016/s0008-6363(99)00178-9. [DOI] [PubMed] [Google Scholar]
- 24.Yue L, Feng J, Gaspo R, et al. Ionic remodeling underlying action potential changes in a canine model of atrial fibrillation. Circ Res. 1997;81:512–525. doi: 10.1161/01.res.81.4.512. [DOI] [PubMed] [Google Scholar]
- 25.Liu H, Jin MW, Xiang JZ, et al. Raloxifene inhibits transient outward and ultra-rapid delayed rectifier potassium currents in human atrial myocytes. Eur J Pharmacol. 2007;563:61–68. doi: 10.1016/j.ejphar.2007.01.072. [DOI] [PubMed] [Google Scholar]
- 26.Chen YJ, Chen YC, Wongcharoen W, et al. Effect of K201, a novel antiarrhythmic drug on calcium handling and arrhythmogenic activity of pulmonary vein cardiomyocytes. Br J Pharmacol. 2008;153:915–925. doi: 10.1038/sj.bjp.0707564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patterson E, Po SS, Scherlag BJ, Lazzara R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm. 2005;2:624–631. doi: 10.1016/j.hrthm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 28.Anzai K, Ogawa K, Kuniyasu A, et al. Effects of hydroxyl radical and sulfhydryl reagents on the open probability of the purified cardiac ryanodine receptor channel incorporated into planar lipid bilayers. Biochem Biophys Res Commun. 1998;249:938–942. doi: 10.1006/bbrc.1998.9244. [DOI] [PubMed] [Google Scholar]
- 29.Fearon IM, Palmer AC, Balmforth AJ, et al. Modulation of recombinant human cardiac L-type Ca2+ channel alpha1C subunits by redox agents and hypoxia. J Physiol. 1999;514:629–637. doi: 10.1111/j.1469-7793.1999.629ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zorn-Pauly K, Schaffer P, Pelzmann B, et al. Oxidized LDL induces ventricular myocyte damage and abnormal electrical activity--role of lipid hydroperoxides. Cardiovasc Res. 2005;66:74–83. doi: 10.1016/j.cardiores.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 31.Viola HM, Arthur PG, Hool LC. Transient exposure to hydrogen peroxide causes an increase in mitochondria-derived superoxide as a result of sustained alteration in L-type Ca2+ channel function in the absence of apoptosis in ventricular myocytes. Circ Res. 2007;100:1036–1044. doi: 10.1161/01.RES.0000263010.19273.48. [DOI] [PubMed] [Google Scholar]
- 32.Lin YK, Lai MS, Chen YC, et al. Hypoxia and reoxygenation modulate the arrhythmogenic activity of the pulmonary vein and atrium. Clin Sci (Lond) 2012;122:121–132. doi: 10.1042/CS20110178. [DOI] [PubMed] [Google Scholar]
- 33.Qin F, Siwik DA, Lancel S, et al. Hydrogen peroxide-mediated SERCA Cysteine 674 oxidation contributes to impaired cardiac myocyte relaxation in senescent mouse heart. J Am Heart Assoc. 2013;2:e000184. doi: 10.1161/JAHA.113.000184. [DOI] [PMC free article] [PubMed] [Google Scholar]


