Abstract
Background
Atrial fibrillation (AF) is increasingly prevalent in society, and can elevate cardiac morbidity and mortality. Psychosis and gender are known to play important roles in the genesis of AF. However, it is not clear whether gender modulates the impact of different psychoses on the occurrences of AF.
Methods
We identified patients suffering from bipolar disorder and schizophrenia, with and without AF, using the Taiwan National Health Insurance nationwide database. The identified patient population consisted of 927,915 subjects (463,050 males and 464,865 females) from 2001 to 2008, which included 2,963 (3.2 ‰) schizophrenia patients (1,650 males and 1,313 females) and 5,112 (5.5 ‰) bipolar-disorder patients (1,934 males and 3,178 females).
Results
The male and female bipolar-disorder patients had higher prevalences of AF than did male (16.5 ‰ vs. 2.4 ‰, p < 0.001) and female (12.9 ‰ vs. 2.3 ‰, p < 0.001) schizophrenia patients. Furthermore, male and female bipolar-disorder patients had higher AF prevalences than did males (8.5 ‰, p < 0.001) and females (7.2 ‰, p < 0.001) in the general population. Schizophrenia patients had lower AF prevalence than the general population in male, but not in female gender. Males had a higher AF prevalence than females. However, male and female bipolar disorder and schizophrenia patients had similar AF prevalences. Those patients with schizophrenia and bipolar-disorder patients with AF were older than those without AF.
Conclusions
Differing risk factors for AF were identified in bipolar-disorder and schizophrenia patients. Compared to the general population, gender may have different impacts on the occurrence of AF in psychosis patients.
Keywords: Atrial fibrillation, Bipolar disorder, Population-based study, Schizophrenia
INTRODUCTION
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia encountered in clinical practice, and it induces cardiac dysfunction and stroke. 1,2,3,4,5 AF can produce psychological distress due to patient perception of potential illness, and high anxiety. 6,7,8,9 Several risk factors, including abnormalities of the autonomic nervous system, inflammation, and the serotonergic pathway, play important roles in the genesis of both AF and psychoses. 10,11,12,13,14,15Moreover, depressive symptoms may predict AF recurrence after cardioversion or cardiac surgery, which suggests that psychosis is associated with the occurrence of AF. 16,17 In addition, AF can occur in schizophrenia patients after medication or electroconvulsive therapy. 18,19 This being said, it is also possible that psychoses may affect the occurrence of AF.
Gender differences have been shown to play important roles in the pathogenesis of psychosis. 20,21 Similarly, the prevalence of AF was significantly higher in men than in women, 22,23,24 which suggests a role of gender in the pathophysiology of AF. However, it is not clear whether gender can modulate the potential role of psychosis in the occurrence of AF. The National Health Insurance (NHI) program has provided medical care to almost all Taiwanese citizens since 1995. 30 Therefore, analyzing the database from the NHI could provide real-world community-based data, and trends over a long period can be evaluated. The purposes of this study were to evaluate whether gender modulates the impacts of different psychoses on the occurrence of AF.
MATERIALS AND METHODS
Study population
This study used nationwide data from the NHI, which provides data on medical expenditures, and comor-bidities. 3,25 The NHI data included sampling information from the 23 million residents of the island’s population, which included 463,423 males and 465,244 females in 2001. Patients with a diagnosis of schizophrenia were identified from the ICD-9 code of 295, and patients with bipolar disorder were identified from the ICD-9 code of 296. AF was identified from the ICD-9 codes of 427.3 and 427.31. Comorbidities were evaluated in these patients from 2001 to 2008. Patients with more than one diagnosis of psychosis were excluded from the study. Those cases with new AF occurrence were identified from the patients with a new diagnosis of AF from 2001 to 2008.
Statistical analysis
Continuous variables were expressed as the mean ± standard deviation (SD), and gender differences were compared using an unpaired Student’s t-test. Two-way analysis of variance (ANOVA) with post-hoc of Student-Newman-Keuls method was used to compare the differences between bipolar-disorder or schizophrenia patients according to the gender or the presence of AF. Categorical variables were reported as frequencies and compared using a Pearson’s chi-squared analysis or Fisher’s exact test. A two-tailed probability p < 0.05 was considered statistically significant. All statistical analyses were performed with SPSS or SigmaStat.
RESULTS
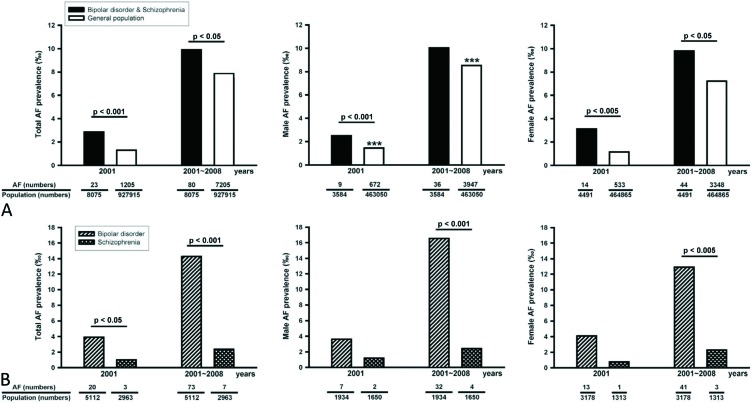
In 2001, there were 2963 (3.2‰) schizophrenia patients (1650 males and 1313 females) and 5112 (5.5‰) bipolar-disorder patients (1934 males and 3178 females) among 927,915 residents, which were sampled from the total NHI Taiwanese residents. As shown in Figure 1A, total, male, and female schizophrenia or bipolar-disorder patients had greater prevalences of AF than did total, male, and female general population in 2001. In 2001, males had a higher AF prevalence than did females. However, male and female schizophrenia or bipolar-disorder patients had similar prevalences of AF in 2001. In addition, total bipolar-disorder patients had more AF than total schizophrenia patients in 2001. However, bipolar-disorder and schizophrenia patients had similar AF prevalences in respect to gender in 2001 (Figure 1B). As shown in Table 1, bipolar-disorder patients were older and included more females than schizophrenia patients or the general population (age of 32 ± 20 years, p < 0.001). Bipolar-disorder patients were associated with greater prevalences of thyroid dysfunction, diabetes, lipid disorder, hypertension, ischemia heart disease, heart failure, cerebrovascular disease, peripheral disease, chronic lung disease, and neoplasms than schizophrenia patients. Compared to the prevalence of AF in the total general population, the total schizophrenia or bipolar-disorder patients had a higher prevalence of AF during the 2001 ˜ 2008 period (Figure 1A). Similarly, female schizophrenia or bipolar-disorder patients had a higher prevalence of AF than females in the general population during the study period. However, male schizophrenia or bipolar-disorder patients and males in the general population had similar AF prevalences during the 2001-2008 period. In addition, males had a higher prevalence of AF than females during the 8-year period. As shown in Figure 1B, total bipolar-disorder patients in 2001 or from 2001-2008 had higher prevalences of AF than did total schizophrenia patients or total general population in 2001 (p < 0.001) or during 2001-2008 (p < 0.001). Similarly, male or female bipolar-disorder patients during 2001-2008 had higher prevalences of AF than male or female schizophrenia patients during 2001-2008 and male (p < 0.001) or female (p < 0.001) population during 2001-2008. However, male or female bipolar-disorder patients and schizophrenia patients had a similar prevalence of AF in 2001. In contrast, total and male schizophrenia patients had lower AF prevalences than the total (p < 0.001) and male (p < 0.001) general population during 2001-2008. However, female schizophrenia patients and females in the general population exhibited an insignificant difference (p = 0.052) in AF prevalences during 2001-2008. In addition, bipolar-disorder and schizophrenia patients of both genders had similar AF prevalences during the 2001-2008 period.
Figure 1.
Prevalences of atrial fibrillation (AF) in the general population and psychosis patients in 2001 and from 2001 to 2008. (A) Male, female, and total prevalences of AF in the general population and in bipolar-disorder and schizophrenia patients. (B) Male, female, and total prevalences of AF in bipolar-disorder and schizophrenia patients. *** p < 0.005 male versus female in the general population in the same period.
Table 1. Comparison of comorbidities between bipolar-disorder and schizophrenia patients with atrial fibrillation (AF) .
| Total | Male | Female | |||||||
| (ICD-9 codes) | Schizophrenia | Bipolar disorder | p value | Schizophrenia | Bipolar disorder | p value | Schizophrenia | Bipolar disorder | p value |
| No (%) of patients | 2963 | 5112 | 1650 (55.7) | 1934 (37.8) | < 0.001 | 1313 (44.3) | 3178 (62.2) | < 0.001 | |
| Age (years) | 39 ± 14 | 46 ± 17 | < 0.001 | 38 ± 13 | 47 ± 18 | < 0.001 | 41 ± 15 | 45 ± 17 | < 0.001 |
| Number of coexisting conditions (n, %) | |||||||||
| Disorders of the thyroid gland (240-246) | 166 (5.6) | 907 (17.7) | < 0.001 | 51 (3.1) | 169 (8.7) | < 0.001 | 115 (8.8) | 738 (23.2) | < 0.001 |
| Diabetes mellitus (250) | 593 (20.0) | 1339 (26.2) | < 0.001 | 292 (17.7) | 463 (23.9) | < 0.001 | 301 (22.9) | 876 (27.6) | < 0.001 |
| Disorders of lipid metabolism (272) | 685 (23.1) | 1901 (37.2) | < 0.001 | 359 (21.8) | 714 (36.9) | < 0.001 | 326 (24.8) | 1187 (37.4) | < 0.001 |
| Hypertensive disease (401-405) | 741 (25.0) | 2218 (43.4) | < 0.001 | 383 (23.2) | 878 (45.4) | < 0.001 | 358 (27.3) | 1340 (42.2) | < 0.001 |
| Ischemic heart disease (410-414) | 351 (11.9) | 1468 (28.7) | < 0.001 | 184 (11.2) | 572 (29.6) | < 0.001 | 167 (12.7) | 896 (28.2) | < 0.001 |
| Acute myocardial infarction (410) | 16 (0.5) | 48 (0.9) | NS | 12 (0.7) | 27 (1.4) | NS | 4 (0.3) | 21 (0.7) | NS |
| Heart failure (428) | 113 (3.8) | 431 (8.4) | < 0.001 | 52 (3.2) | 155 (8.0) | < 0.001 | 61 (4.7) | 276 (8.7) | < 0.001 |
| Cerebrovascular disease (430-438) | 271 (9.2) | 1119 (21.9) | < 0.001 | 131 (7.9) | 446 (23.1) | < 0.001 | 140 (10.7) | 673 (21.2) | < 0.001 |
| Occlusion and stenosis of precerebral arteries (433) | 11 (0.4) | 63(1.2) | < 0.001 | 4 (0.2) | 33 (1.7) | < 0.001 | 7 (0.5) | 30 (0.9) | NS |
| Occlusion of cerebral arteries (434) | 104 (3.5) | 485 (9.5) | < 0.001 | 48 (2.9) | 219 (11.3) | < 0.001 | 56 (4.3) | 266 (8.4) | < 0.001 |
| Transient cerebral ischemia (435) | 31 (1.1) | 273 (5.3) | < 0.001 | 16 (1.0) | 115 (6.0) | < 0.001 | 15 (1.1) | 158 (5.0) | < 0.001 |
| Peripheral arterial disease (443, 444) | 63 (2.1) | 309 (6.0) | < 0.001 | 34 (2.1) | 102 (5.3) | < 0.001 | 29 (2.2) | 207 (6.5) | < 0.001 |
| Chronic lung disease (490-496, 500-508) | 893 (30.1) | 2166 (42.4) | < 0.001 | 499 (30.2) | 848 (43.9) | < 0.001 | 394 (30.0) | 1318 (41.5) | < 0.001 |
| Neoplasms (140-239) | 715 (24.1) | 2330 (45.6) | < 0.001 | 312 (18.9) | 659 (34.1) | < 0.001 | 403 (30.7) | 1671 (52.6) | < 0.001 |
| Chronic kidney disease | 47 (1.6) | 221 (4.3) | < 0.001 | 22 (1.3) | 99 (5.1) | < 0.001 | 25 (1.9) | 122 (3.8) | < 0.001 |
NS, non-significant.
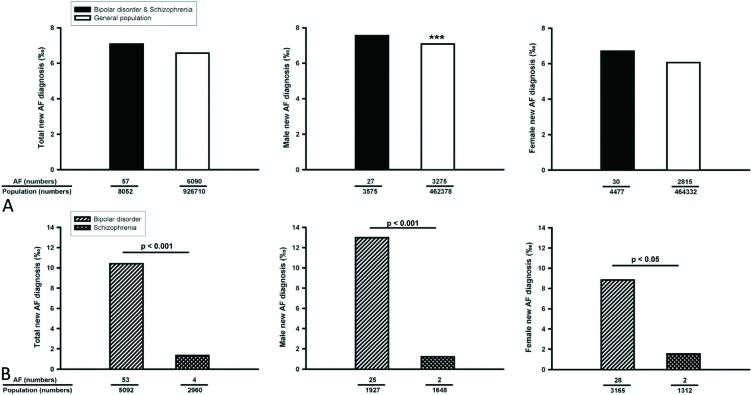
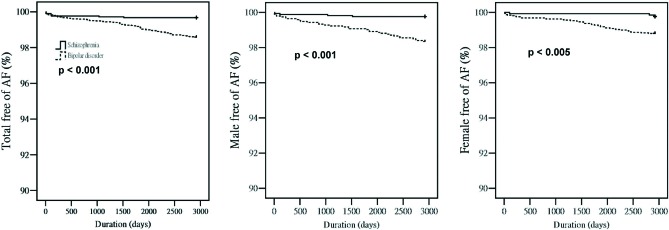
From 2001 to 2008, there were similar new AF occurrences among the total, male, and female general population and total bipolar-disorder or schizophrenia patients (Figure 2A). However, males in the general population had more new AF occurrences than did females in the general population (p < 0.001). In contrast, male and female bipolar-disorder or schizophrenia patients had similar new occurrences of AF during this period. As shown in Figure 2B, total, male, and female bipolar-disorder patients had more new AF occurrences than did total, male, and female schizophrenia patients (Figure 2B). In addition, total and male bipolar-disorder patients had more new AF occurrences than did total (p < 0.005) and male (p < 0.005) general population. However, female bipolar-disorder and females in the general population had similar incidences of new AF occurrences (p = 0.058). In contrast, male and total schizophrenia patients had fewer new AF occurrences than the male (p < 0.01) and total (p < 0.001) general population. However, female schizophrenia patients and females in the general population had similar incidences of new AF occurrences (p = 0.052). Moreover, male and female bipolar-disorder and schizophrenia patients had similar incidences of new AF occurrences from 2001 to 2008. Moreover, the Kaplan-Meier analysis also showed different occurrences of AF between bipolar-disorder and schizophrenia patients in males, females, and in both sexes together (Figure 3).
Figure 2.
New diagnoses of atrial fibrillation (AF) in the general population and in psychosis patients from 2001 to 2008. (A) Male, female, and total new diagnoses of AF in the general population and in bipolar-disorder and schizophrenia patients. (B) Male, female, and total new diagnoses of AF in bipolar-disorder and schizophrenia patients. *** p < 0.005 males versus females in the general population.
Figure 3.
Kaplan-Meier analysis of new diagnoses of atrial fibrillation (AF) in bipolar-disorder and schizophrenia patients.
Table 2 summarizes comorbidities of schizophrenia and bipolar-disorder patients with and without AF. We found that bipolar-disorder patients with AF had higher incidences of thyroid disorders, lipid metabolism disorders, and cerebrovascular diseases than schizophrenia patients with AF. Moreover, the multivariate analysis of bipolar-disorder and schizophrenia patients found that age [odds ratio (OR) = 1.02, p = 0.003], thyroid disease (OR = 3.07, p < 0.001), hypertension (OR = 2.85, p = 0.014), ischemic heart disease (OR = 2.72, p = 0.002), heart failure (OR = 3.75, p < 0.001), occlusion and stenosis of precerebral arteries (OR = 2.39, p = 0.034), and chronic kidney disease (OR = 2.21, p = 0.008) were independent risk factors for the genesis of AF.
Table 2. Comparison of comorbidities between bipolar-disorder and schizophrenia patients with atrial fibrillation (AF) .
| With ICD-9 codes | Schizophrenia | Bipolar disorder | ||||
| With ICD-9 codes | With AF | Without AF | p value | With AF | Without AF | p value |
| No. of patients | 7 | 2956 | 73 | 5039 | ||
| Age (years) | 60 ± 19 | 39 ± 14 | < 0.001 | 66 ± 12 | 46 ± 17* | < 0.001 |
| Male gender, n (%) | 4 (57) | 1646 (56) | NS | 32 (44) | 1902 (38)* | NS |
| Number of coexisting conditions (n, %) | ||||||
| Disorders of the thyroid gland (240-246) | 0 (0) | 166 (5.6) | NS | 24 (32.9)* | 883 (17.5)* | < 0.001 |
| Diabetes mellitus (250) | 2 (28.6) | 591 (20.0) | NS | 36 (49.3) | 1303 (25.9)* | < 0.001 |
| Disorders of lipid metabolism (272) | 1 (14.3) | 684 (23.1) | NS | 42 (57.5)* | 1859 (36.9)* | < 0.001 |
| Hypertensive disease (401-405) | 5 (71.4) | 736 (24.9) | NS | 67 (91.8) | 2151 (42.7)* | < 0.001 |
| Ischemic heart disease (410-414) | 5 (71.4) | 346 (11.7) | NS | 59 (80.8) | 1409 (28.0)* | < 0.001 |
| Acute myocardial infarction (410) | 0 (0) | 16 (0.5) | NS | 4 (5.5) | 44 (0.9)* | < 0.001 |
| Heart failure | 3 (42.9) | 110 (3.7) | < 0.001 | 38 (52.1) | 393 (7.8)* | < 0.001 |
| Cerebrovascular disease (430-438) | 1 (14.3) | 270 (9.1) | NS | 51 (69.9)* | 1068 (21.2)* | < 0.001 |
| Occlusion and stenosis of precerebral arteries (433) | 0 (0) | 11 (0.4) | NS | 6 (8.2) | 57 (1.1)* | < 0.001 |
| Occlusion of cerebral arteries (434) | 1 (14.3) | 103 (3.5) | NS | 28 (38.4) | 457 (9.1)* | < 0.001 |
| Transient cerebral ischemia (435) | 0 (0) | 31 (1.1) | NS | 12 (16.4) | 261 (5.2)* | < 0.001 |
| Peripheral arterial disease (443, 444) | 0 (0) | 63 (2.1) | NS | 8 (11.0) | 301 (6.0)* | NS |
| chronic lung disease (490-496, 500-508) | 5 (71.4) | 888 (30.0) | NS | 59 (80.8) | 2107 (41.8)* | < 0.001 |
| Neoplasms (140-239) | 1 (14.3) | 714 (24.2) | NS | 34 (46.6) | 2296 (45.6)* | NS |
| Chronic kidney disease | 2 (28.6) | 45 (1.5) | NS | 11 (15.1) | 210 (4.2)* | < 0.001 |
NS, non-significant.
* p < 0.05 versus schizophrenia patients with or without AF.
DISCUSSION
In this study, we found for the first time that different psychoses were associated with different incidences of AF. Moreover, our study indicated an increased prevalence of AF in bipolar-disorder patients. During the follow-up in 2001-2008, bipolar-disorder patients had 1.5-fold more new diagnoses of AF compared to the general population. Bipolar disorder may increase inflammation and serotonin dysfunction, which are also important factors contributing to the genesis of AF. 10 These findings were consistent with the observation that depression may increase the occurrence of AF after cardioversion or cardiac surgery. 16,17 Depression may increase AF symptoms, which might spur patients to seek medical treatment. This possibility may also increase the incidence of AF in bipolar-disorder patients. In addition, older age and additional comorbidities in bipolar-disorder patients may also play a role in the higher occurrences of AF.
In this study, we found a low incidence of AF in schizophrenia patients compared to bipolar-disorder patients and the general population. During this study follow-up period, only 18% of schizophrenia patients had newly diagnosed AF compared to the general population. The reasons are uncertain, but it is possible that schizophrenia patients were younger and associated with fewer comorbidities than bipolar-disorder patients. Besides, different pathophysiological mechanisms between schizophrenia and bipolar disorder may play a role in the different incidences of AF. However, we cannot exclude the possibilities that schizophrenia patients may have fewer AF symptoms or poor communication which results in a low incidence of AF.
Gender plays an important role in the occurrence of AF. In this study, as in other studies, we found that males had a higher prevalence of AF. In addition, during long-term follow-up, the male population had a 1.3-fold higher chance of a new diagnosis of AF than females in the general population. Therefore, our studies confirmed through this nationwide database that gender contributes to the pathophysiology of AF. Similar to previous reports, 20,21 we found that more females were bipolar-disorder patients, and more males were schizophrenia patients. Although males were associated with a higher incidence of AF, this study found that gender in bipolar-disorder and schizophrenia patients was not associated with different AF prevalences or new diagnoses of AF. Therefore, it is possible that bipolar-disorder and schizophrenia females are at rather higher risks of AF, compared to the general population. However, a multivariate analysis showed that only age, thyroid disease, hypertension, ischemic heart disease, heart failure, precerebral arterial stenosis, and chronic kidney disease were independent risk factors for the genesis of AF. Moreover, we found that bipolar-disorder patients with AF had more comorbidities than schizophrenia patients with AF. These findings also suggest potentially different pathophysiological mechanisms in AF occurrences among these patients.
There were some limitations in this study. Available information about patient drug history was limited. It is not clear whether the use of medications will affect the occurrences of AF in bipolar disorder or schizophrenia patients. In addition, we cannot verify the accuracy of the cited ICD-9 codes, which will affect our patient number and comorbidties.
CONCLUSION
In conclusion, the purpose of this study was to observe differing risks of bipolar disorder and schizophrenia on the occurrence of AF. We found that gender differences and variations in treatment strategies may be responsible for these changes.
Acknowledgments
The present work was supported by grants from Taipei Medical University-Wan Fang Hospital (102wf-eva-15 and101wf-eva-02) and the National Science Council, Taiwan (NSC99-2314-B-016-034-MY3 and NSC 99-2628-B-038-011-MY3).
REFERENCES
- 1.Krahn AD, Manfreda J, Tate RB, et al. The natural history of atrial fibrillation: incidence, risk factors, and prognosisAm J Med in the Manitoba follow-up study. Am J Med. 1995;98:476–484. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- 2.Psaty BM, Manolio TA, Kuller LH, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 3.Huang JH, Yang HY, Hsu CY, et al. Gender differences in trend of hospital management for atrial fibrillation: a nationwide population-based analysis. Int J Cardiol. 2011;153:89–94. doi: 10.1016/j.ijcard.2011.08.844. [DOI] [PubMed] [Google Scholar]
- 4.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA . 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 5.Murphy NF, Simpson CR, Jhund PS, et al. A national survey of the prevalence, incidence, primary care burden and treatment of atrial fibrillation in Scotland. Heart. 2007;93:606–612. doi: 10.1136/hrt.2006.107573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gehi AK, Sears S, Goli N, et al. Psychopathology and symptoms of atrial fibrillation: implications for therapy. J Cardiovasc Electrophysiol. 2012;23:473–478. doi: 10.1111/j.1540-8167.2011.02264.x. [DOI] [PubMed] [Google Scholar]
- 7.Dabrowski R, Smolis-Bak E, Kowalik I, et al. Quality of life and depression in patients with different patterns of atrial fibrillation. Kardiol Pol . 2010;68:1133–1139. [PubMed] [Google Scholar]
- 8.Thrall G, Lip GY, Carroll D, et al. Depression, anxiety, and quality of life in patients with atrial fibrillation. Chest. 2007;132:1259–1264. doi: 10.1378/chest.07-0036. [DOI] [PubMed] [Google Scholar]
- 9.Ong L, Cribbie R, Harris L, et al. Psychological correlates of quality of life in atrial fibrillation. Qual Life Res. 2006;15:1323–1333. doi: 10.1007/s11136-006-0029-5. [DOI] [PubMed] [Google Scholar]
- 10.Yusuf S, Al-Saady N, Camm AJ, et al. 5-hydroxytryptamine and atrial fibrillation: how significant is this piece in the puzzle? J Cardiovasc Electrophysiol. 2003;14:209–214. [PubMed] [Google Scholar]
- 11.Eaker ED, Sullivan LM, Kelly-Hayes M, et al. Tension and anxiety and the prediction of the 10-year incidence of coronary heart disease, atrial fibrillation, and total mortality: the Framingham Offspring Study. Psychosom Med. 2005;67:692–696. doi: 10.1097/01.psy.0000174050.87193.96. [DOI] [PubMed] [Google Scholar]
- 12.Johnson AK, Grippo AJ. Sadness and broken hearts: neurohumoral mechanisms and co-morbidity of ischemic heart disease and psychological depression. J Physiol Pharmacol . 2006;57(Suppl 11):5–29. [PubMed] [Google Scholar]
- 13.Issac TT, Dokainish H, Lakkis NM. Role of inflammation in initiation and perpetuation of atrial fibrillation: a systematic review of the published data. J Am Coll Cardiol. 2007;50:2021–2028. doi: 10.1016/j.jacc.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 14.Chou CC, Chen PS. New concepts in atrial fibrillation: mechanism and remodeling. Med Clin North Am. 2008;92:53–63. doi: 10.1016/j.mcna.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carney RM, Freedland KE, Veith RC. Depression, the autonomic nervous system, and coronary heart disease. Psychosom Med. 2005;67(Suppl 1):S29–S33. doi: 10.1097/01.psy.0000162254.61556.d5. [DOI] [PubMed] [Google Scholar]
- 16.Lange HW, Herrmann-Lingen C. Depressive symptoms predict recurrence of atrial fibrillation after cardioversion. J Psychosom Res. 2007;63:509–513. doi: 10.1016/j.jpsychores.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Tully PJ, Bennetts JS, Baker RA, et al. Anxiety, depression, and stress as risk factors for atrial fibrillation after cardiac surgery. Heart Lung. 2011;40:4–11. doi: 10.1016/j.hrtlng.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Low RA, Jr., Fuller MA, Popli A. Clozapine induced atrial fibrillation. J Clin Psychopharmacol. 1998;18:170–170. doi: 10.1097/00004714-199804000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Kuwahara T, Yoshino A, Horikawa N, Nomura S. Delayed sympathetic hyperactivity following electroconvulsive therapy in patients with catatonic schizophrenia. Nihon Shinkei Seishin Yakurigaku Zasshi. 2009;29:79–83. [PubMed] [Google Scholar]
- 20.Jogia J, Dima D, Frangou S. Sex differences in bipolar disorder: a review of neuroimaging findings and new evidence. Bipolar Disord. 2012;14:461–471. doi: 10.1111/j.1399-5618.2012.01014.x. [DOI] [PubMed] [Google Scholar]
- 21.Kirkbride JB, Errazuriz A, Croudace TJ, et al. Incidence of schizophrenia and other psychoses in England, 1950-2009: a systematic review and meta-analyses. PLoS One. 2012;7:e31660–e31660. doi: 10.1371/journal.pone.0031660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dagres N, Nieuwlaat R, Vardas PE, et al. Gender-related differences in presentation, treatment, and outcome of patients with atrial fibrillation in Europe: a report from the Euro Heart Survey on Atrial Fibrillation. J Am Coll Cardiol. 2007;49:572–577. doi: 10.1016/j.jacc.2006.10.047. [DOI] [PubMed] [Google Scholar]
- 23.Gurevitz OT, Varadachari CJ, Ammash NM, et al. The effect of patient sex on recurrence of atrial fibrillation following successful direct current cardioversion. Am Heart J . 2006;152:155.e9–155.e13. doi: 10.1016/j.ahj.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 24.Michelena HI, Powell BD, Brady PA, et al. Gender in atrial fibrillation: ten years later. Gend Med. 2010;7:206–217. doi: 10.1016/j.genm.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Yang HY, Chiu WC, Huang JH, et al. Analysis of 10-year nationwide population-based data on sex differences in hospitalization for heart failure. Heart Vessels. 2013;28:721–727. doi: 10.1007/s00380-012-0299-5. [DOI] [PubMed] [Google Scholar]