Abstract
Antibodies targeting IL-23 ameliorate clinical symptoms of inflammatory bowel disease. Paradoxically, IL-17 neutralization exacerbates colitis. In this issue, Lee et al. and Maxwell et al. reveal a protective function of IL-17 through maintenance of intestinal barrier integrity, helping to explain this dichotomy (Lee et al., 2015; Maxwell et al., 2015).
A decade ago, the Th1/Th2 paradigm was overhauled to incorporate the newly described Th17 cell population. A key discovery leading to this paradigm shift was the recognition that IL-23, a heterodimer that shares the p40 subunit with IL-12, is required for Th17 activity in vivo. The IL-23/IL-17 axis is now known to play a pivotal role in protection against infectious microbes, particularly fungi. However, with yin comes yang, and IL-23 and IL-17 are pathogenic in a number of autoimmune diseases. Even prior to the discovery of Th17 cells, efforts to develop biologic agents targeting these cytokines were underway in the pharmaceutical industry. In recent years the fruits of this labor have begun to be realized, with antibodies against the IL-12/23 p40 subunit (ustekinumab) in increasingly widespread clinical use. Antibodies targeting IL-17A (secukinumab) or the IL-17 receptor (brodalumab) were remarkably effective in psoriasis trials, and secukinumab was recently approved for use in moderate to severe plaque psoriasis (Sanford and McKeage, 2015).
Inflammatory bowel disease (IBD) is associated with exacerbated inflammatory responses in the gut. Polymorphisms in genes encoding components of the Th17 pathway, such as IL23R and STAT3, are associated with IBD based on genome wide association studies (GWAS). Indeed, elevated levels of both IL-23 and IL-17 are detected in IBD in humans as well as in mouse models of colitis. Moreover, ustekinumab or anti-IL-23 Abs (MEDI2070) alleviated symptoms in patients with Crohn’s disease (CD) that is refractory to anti-TNF therapy. Therefore, targeting IL-23 in IBD appears to be an effective therapeutic strategy.
In surprising contrast, clinical trials of anti-IL-17A or anti-IL-17RA antibodies in CD showed no improvement or even exacerbation of disease (Hueber et al., 2012). Although disappointing clinically, these findings were consistent with many (but not all) mouse models of colitis. For example, studies using anti-IL-17A treatment or Il17a−/− mice in dextran sodium sulfate (DSS)-induced intestinal injury or a CD4+T cell transfer model of colitis have demonstrated exacerbated symptoms (O'Connor et al., 2009; Ogawa et al., 2004; Song et al., 2015). On the other hand, blockade of IL-17A reduced pathology in an IL-10-deficient colitogenic setting (Yen et al., 2006). Broadly speaking, the fidelity of mouse models with respect to human IBD remains controversial.
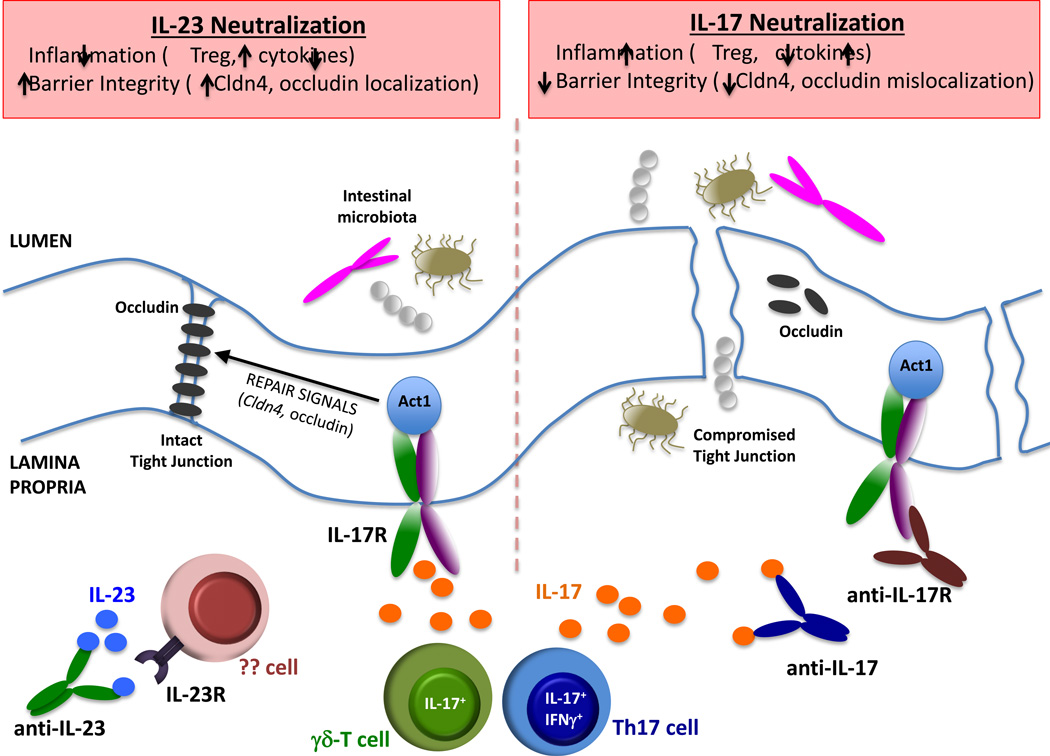
In this issue of Immunity, Lee et al. and Maxwell et al. address this dichotomy between IL-23 and IL-17A in mediating pathogenesis of IBD. Both groups came to a similar conclusion that the dominant function of IL-17 in this setting is to preserve the intestinal epithelial barrier, despite its potential to drive pathogenic inflammation as it does in other autoimmune conditions. Lee et al. using the DSS model of colitis showed that the expression of genes associated with epithelial tight junction integrity was not altered in Il17a−/− mice. Rather, they found that the increased gut permeability in Il17a−/− mice was associated with abnormal localization of the tight junction protein occludin. They further showed that conditional deletion of Act1 (the common IL-17R signaling adaptor) in intestinal epithelial cells increased gut permeability during active colitis. These data are in good agreement with a recent report by Youcun Qian’s group, who demonstrated that IL-17A stimulates gut epithelial cell proliferation and healing through a cooperative signaling pathway with the FGFR (Song et al., 2015). Maxwell et al. took an interesting approach to the same question by exploiting a less common colitis mouse model that accurately reflects the human response to biologic therapy. This group did not find an exacerbating effect of anti-IL-17A therapy in DSS colitis, so instead they tested anti-cytokine agents in mice that lack the multidrug resistance gene Abcb1a and are infected with Helicobacter bilis. In this setting, they found that blockade of IL-23 improved disease, whereas blockade of IL-17A or IL-17RA worsened symptoms, which was also associated with reduced intestinal barrier maintenance.
These papers highlight a facet of IL-17 function that has been underappreciated. That is, IL-17 is best known as an inducer of neutrophil-promoting cytokines and chemokines, such as IL-6, G-CSF and CXCL1. IL-17 also activates expression of antimicrobial peptides (AMPs), including β-defensins, lipocalins and S100A8/9 (also known as calprotectin). Consistently, Maxwell et al. showed that expression of β-defensin 1 and S100A8 were reduced following anti-IL-17RA treatment. However, a surprise finding was that neutralization of IL-17RA or IL-17 led to enhanced expression of cytokines and chemokines. A similar increase in pro-inflammatory factors during colitis in Il17a−/− mice was recently reported (Song et al., 2015). Although intriguing, these are not the first studies to suggest roles for IL-17 in wound repair and epithelial maintenance. The connection of IL-17 to occludin and other genes associated with epithelial integrity has been noted in barrier integrity associated with the cornea as well as for IL-17C in intestinal inflammation (Chen et al., 2011; Reynolds et al., 2012).
The data presented here add to a growing body of literature showing that in the gut IL-17 promotes homeostasis and tissue repair rather than driving pathogenic inflammation as it does in psoriasis. This begs the obvious question of what dictates this balance. An attractive candidate for such a regulator is IL-23. In this regard, production of IL-17A by γδ-T cells, the primary producers of gut-protective IL-17A in the DSS model used by Lee et al., was independent of IL-23, implying that blockade of IL-23 would not impair IL-17 production from innate lymphocytes. Maxwell et al. found that IL-17A+IFNγ+ CD4+ T cells were associated with exacerbation of colitis in their model system (Maxwell et al., 2015). These “double-producing” T cells have been implicated in several disease models, and notably are elevated in the inflamed mucosa of IBD patients. Moreover, IL-17A+IFNγ+ CD4+ T cells are IL-23-dependent, regarded as a distinctive trait of pathogenic Th17 cells (McGeachy et al., 2007). Tregs are also associated with protection against colitis. Indeed, colitis symptoms upon antibody treatment correlated closely with changes in Foxp3+ Treg cells (Lee et al., 2015). Recently, it was proposed that Th17 and Treg cells cooperate in the gut to promote repair of the damaged epithelial barrier during colitis (Song et al., 2015). The two studies published in this issue support this emerging theme.
The data in these complementary studies, as well as those from Song et al., help explain the clinical conundrum that IL-23 blockade is an effective treatment strategy for IBD, whereas targeting IL-17 appears to be harmful. Some key questions of course remain. What regulates IL-17 expression in γδ-T cells, if not IL-23? One likely candidate is IL-1β, which induces IL-17 γδ-T cells in mice. Why are murine colitis models so variable? The contribution of the intestinal microbiota is likely to be an important facet of the intestinal response, and the interplay between IL-17 signaling and the microbiota needs to be explored. IL-17RA deficiency humans is associated with chronic opportunistic Candida albicans infections, yet there is no apparent dissemination of C. albicans in patients treated with anti-IL-17 biologic drugs, at least so far. It is conceivable that IL-17 signaling may differ on intestinal epithelia compared to other epithelial surfaces. It is also possible that these data raise issues regarding potential adverse effects of anti-IL-17A therapy in diseases where IBD exhibits significant overlap (e.g., celiac disease and ankylosing spondylitis). Lastly, IL-22 is another Th17-derived cytokine that is more commonly viewed as having a protective role in epithelial tissue. Clearly in these settings IL-22 and IL-17 are not redundant, but is their function in any way cooperative (as has been suggested in psoriasis models)?
Most of the constituent elements reported in these papers were already known, such as the conflict between IL-23 and IL-17 in colitis, the potential of IL-17 to drive epithelial repair, and the idea that γδ-T cells can be major sources of IL-17. Nonetheless, these reports elegantly put these concepts together in a comprehensive picture that is highly relevant from a clinical standpoint. In other words, if there’s something strange and you don’t feel good, who ya gonna call? IL-23, not IL-17, might be the real Gut-Buster.
If there’s something strange
And you don’t feel good
Who ya gonna call?
GUT BUSTERS
Is interleukin-one
Churning in your tum?
Who ya gonna call?
GUT BUSTERS
I ain’t afraid of no poop
I ain’t afraid of no pee
Is it IL-17
Making you feel green?
Who ya gonna call?
GUT BUSTERS
No, it’s 23
Causing agony
Who ya gonna call?
GUT BUSTERS
I ain’t afraid of E coli
I ain’t afraid of Salmonella
Lemme tell ya something
IL-17 makes me feel good!
Figure.
Acknowledgments
SLG was supported by NIH grants AI107825 and DE082250. We thank J. Richardson for helpful suggestions.
References
- Chen Y, Yang P, Li F, Kijlstra A. The effects of Th17 cytokines on the inflammatory mediator production and barrier function of ARPE-19 cells. PLoS One. 2011;6:e18139. doi: 10.1371/journal.pone.0018139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueber W, Sands BE, Lewitzky S, Vandemeulebroecke M, Reinisch W, Higgins PD, Wehkamp J, Feagan BG, Yao MD, Karczewski M, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn's disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693–1700. doi: 10.1136/gutjnl-2011-301668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Tato C, Joyce-Shaikh B, Gulan F, Cayatte C, Chen Y, Blumenschein W, Judo M, Ayanoglu G, McClanahan T, et al. Interleukin-23-dependent IL-17 production regulates intestinal epithelial permeability. Immunity. 2015 doi: 10.1016/j.immuni.2015.09.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell J, Zhang Y, Brown W, Smith C, Byrne F, Florino M, Stevens E, Bigler J, Davis J, Rottman J, et al. Differential roles for interleukin-23 and interleukin-17 in intestinal immunoregulation. Immunity. 2015 doi: 10.1016/j.immuni.2015.08.019. in press. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nature Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- O'Connor W, Jr, Kamanaka M, Booth CJ, Town T, Nakae S, Iwakura Y, Kolls JK, Flavell RA. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nature Immunol. 2009;10:603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa A, Andoh A, Araki Y, Bamba T, Fujiyama Y. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin Immunol. 2004;110:55–62. doi: 10.1016/j.clim.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Reynolds JM, Martinez GJ, Nallaparaju KC, Chang SH, Wang YH, Dong C. Cutting edge: regulation of intestinal inflammation and barrier function by IL-17C. J Immunol. 2012;189:4226–4230. doi: 10.4049/jimmunol.1103014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford M, McKeage K. Secukinumab: first global approval. Drugs. 2015;75:329–338. doi: 10.1007/s40265-015-0359-0. [DOI] [PubMed] [Google Scholar]
- Song X, Dai D, He X, Zhu S, Yao Y, Gao H, Wang J, Qu F, Qiu J, Wang H, et al. Growth Factor FGF2 Cooperates with Interleukin-17 to Repair Intestinal Epithelial Damage. Immunity. 2015;43:488–501. doi: 10.1016/j.immuni.2015.06.024. [DOI] [PubMed] [Google Scholar]
- Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]