Abstract
Background
Pre-infarction angina reduces myocardial infarct size by preventing the myocardium from being subjected to ischemia reperfusion (I/R) injury. Ischemic preconditioning is the proposed mechanism for this effect. Sphingosine 1 phosphate (S1P) activates ischemic preconditioning pathways and may play a role in the presence of cardioprotective effects of pre-infarction angina. Therefore, we evaluated the relationship between pre-infarction angina and serum S1P levels.
Methods
Between May 2011 and January 2012, 79 patients with acute myocardial infarction were included in the study. In addition to taking routine medical histories, all of the patients were questioned as to whether or not they had pre-infarction angina. We determined patients serum levels of S1P at admission and discharge, and peak creatine kinase MB and troponin levels were also measured in the pre-infarction angina positive and negative groups.
Results
Of the 79 patients included in the study, 36 had pre-infarction angina and 43 had not. Baseline characteristics were similar between the groups. The median level of serum S1P in patients with pre-infarction angina was significantly higher than in those without pre-infarction angina both at admission and discharge [0.54 (0.14-1.35) vs. 0.26 (0.12-0.62) p = 0.014/0.51 (0.20-1.81) vs. 0.30 (0.13-0.68) p = 0.010]. Serum high sensitive troponin levels were significantly lower in patients with pre-infarction angina [0.97 (0.39-3.07) vs. 2.56 (0.9-6.51) p = 0.034]. Serum S1P levels both at admission and discharge tended to be higher in patients with more angina episodes, but the differences between these subgroups were not statistically significant.
Conclusions
Patients who experienced pre-infarction angina had higher serum S1P levels than patients without pre-infarction angina. This study supported our hypothesis that the cardioprotective effects of pre-infarction angina may in part be mediated by S1P.
Keywords: Ischemic preconditioning, Pre-infarction angina, Sphingosine 1 phosphate
INTRODUCTION
Revascularization reduces myocardial injury dramatically in the setting of an acute myocardial infarction. However, restoration of blood flow may paradoxically result in further damage to the myocardium.1 This phenomenon, known as ischemia reperfusion (I/R) injury, leads to a continuity of cellular death by activating the cellular apoptotic pathways.
As general knowledge in the medical community about I/R injury has increased, many studies have carefully reviewed the mechanism of myocardial injury and attempted to slow down or stop the pathways which lead to cell death. Since first described by Murry et al.,2 ischemic preconditioning has become the most viable approach to reduce I/R injury. Theoretically, ischemic preconditioning can be defined as transient, sublethal ischemic episodes rendering the myocardium more resistant to a sustained, lethal ischemic period. Adenosine,3,4 bradykinin5 and opioid agonists6 all seem to be associated with myocardial preconditioning.
Sphingosine 1 phosphate (S1P) is an active meta-bolite of sphingolipid metabolism, and has functions in intracellular calcium mobilization, cytoskeletal organization, angiogenesis, cellular differentiation and survival.7 The source of circulating S1P is red blood cells, platelets, leukocytes, and endothelial cells.8-10 S1P binds to plasma proteins, particularly to high-density lipoprotein (HDL) in circulation.11 It has been shown that exogenous or endogenous S1P serves as an important cardioprotectant.12,13
Pre-infarction angina can be used as a surrogate marker of ischemic preconditioning. Napoli et al. showed that new angina preceding acute myocardial infarction is associated with lower cardiac marker levels and better contractile recovery.14
S1P is an important cardioprotective molecule which activates preconditioning pathways. The cardioprotective effects of pre-infarction angina may be mediated by endogenous release of S1P. The relationship between pre-infarction angina and serum S1P levels remains largely unknown. So we aimed to evaluate the relationship between pre-infarction angina and serum S1P levels.
METHODS
Between May 2011 and January 2012, 79 patients with acute myocardial infarction were enrolled into the study. Patients who were diagnosed with unstable angina whose cardiac markers did not rise and who had a history of previous myocardial infarction, surgical or percutaneous revascularization and malignancy were also excluded from the study. The presence, onset, duration and number of the pre-infarction angina episodes were recorded while taking anamnesis by asking questions like: ‘what is your complaint that was the basis of your admission to emergency services? When did your chest pains start, what was the duration of your pain, have you ever had any pain like this one before, and was this the first pain that you ever had like this? Did you have similar complaints previously during the last several days? If you had any pain previously what was the duration of your pain, when did it start, what was the minimum and maximum durations of your pains, and how many times did you have pain like this?’ to each patient. Serum samples were collected for S1P analysis upon admission to the coronary care unit and again before discharge. Thirty minutes after blood samples were obtained, the samples were centrifuged at 6000 rpm, and serum was removed and frozen at -80° Celcius. Measurement of S1P was made with S1P Elisa Kits (Echelon Biosciences, Utah, USA). All samples were processed simultaneously. High sensitive troponin T (ROCHE Diagnostics) and creatine kinase MB (CKMB) levels were processed every six hours until peak levels of these cardiac markers were noted. Fasting blood glucose, blood urea nitrogen (BUN), creatinine, total cholesterol, low-density lipoprotein (LDL), HDL, triglyceride (TG), glycated hemoglobin (HbA1c) were also recorded. Additionally, an echocardiographic examination was performed on all patients before discharge.
All patients underwent conventional coronary angiography. The treatment of patients (primary percutaneous coronary intervention, fibrinolytic treatment and medical treatment) was performed according to current scientific knowledge. Coronary collateral circulation provides a cardioprotective effect other than ischemic preconditioning pathways. Therefore, patients who had collaterals at infarct-related artery territory were excluded from S1P analysis.
Statistical analysis
The results were expressed as mean ± standard deviation, median-interquartile range and percentile values. All assumptions done before statistical analysis and suitable parametric or non parametric tests were chosen according to assumptions. Comparisons of the continuous variables were done with the unpaired Student’s t-test and the Mann-Whitney U test. The chi-square test was used to assess the significance of the difference between dichotomous variables. The correlation of the S1P levels with the cardiac markers were analyzed with the use of Spearman’s Rho correlation. The Kruskal-Wallis H test was used to evaluate the significance between more than two independent variables. The SPSS (Statistical Package for the Social Sciences Program) for Windows version 17.0 was used for statistical analysis.
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, and was approved by the local ethics committee. Informed consent was also obtained from all participants.
RESULTS
A total of 86 patient serum samples at admission and discharge were collected for S1P analysis, but 7 patients (four with pre-infarction angina) were removed from analysis due to coronary collateral circulation at the infarct-related artery territory. Overall, 79 patients were included in the study. Of these 79 patients, 54 (68.3%) had ST-segment elevation myocardial infarction (STEMI), and 25 (31.7%) had non-STEMI (NSTEMI). Baseline characteristics of the patients are given in Table 1.
Table 1. Baseline characteristics of study patients.
| Overall(n = 79) | Pre-infarction angina positive | Pre-infarction angina negative | ||
| (n = 36) | (n = 43) | p | ||
| Age (years) | 55.1 ± 10 | 55.8 ± 11.2 | 54.4 ± 9.4 | 0.549 |
| Gender (Male), n (%) | 66 (83) | 29 (80) | 37 (86) | 0.512 |
| Hypertension, n (%) | 37 (46) | 18 (50) | 19 (44) | 0.606 |
| Diabetes, n (%) | 31 (39) | 14 (38) | 17 (39) | 0.953 |
| Hyperlipidemia, n (%) | 24 (30) | 9 (25) | 15 (34) | 0.341 |
| Smoking, n (%) | 54 (68) | 26 (72) | 28 (65) | 0.499 |
| Family history, n (%) | 18 (22) | 8 (22) | 10 (23) | 0.913 |
| MI type (STEMI), n (%) | 54 (68) | 23 (63) | 31 (72) | 0.435 |
| Time of arrival to CCU (minutes) (median-IQR) | 155 (60-392) | 180 (60-480) | 150 (60-360) | 0.846 |
| Fasting blood glucose (mg/dl) (mean ± SD) | 138 ± 62 | 141 ± 49 | 136 ± 73 | 0.76 |
| BUN (mg/dl) (mean ± SD) | 18 ± 10 | 18 ± 10 | 19 ± 11 | 0.652 |
| Creatinine (mg/dl) (mean ± SD) | 0.8 ± 0.2 | 0.8 ± 0.3 | 0.8 ± 0.1 | 0.718 |
| HbA1c (%) (mean ± SD) | 7.3 ± 1.7 | 7.1 ± 1.8 | 7.4 ± 1.7 | 0.638 |
| Total cholesterol (mg/dl) (mean ± SD) | 194 ± 42 | 197 ± 44 | 191 ± 40 | 0.524 |
| HDL (mg/dl) (mean ± SD) | 39 ± 9 | 39 ± 8 | 38 ± 8 | 0.543 |
| LDL (mg/dl) (mean ± SD) | 126 ± 34 | 128 ± 36 | 124 ± 31 | 0.639 |
| TG (mg/dl) (mean ± SD) | 148 ± 94 | 139 ± 78 | 155 ± 106 | 0.471 |
| ECG changes, n(%) | 0.79 | |||
| Anterior | 35 (44) | 17 (47) | 18 (41) | |
| Treatment modality at discharge, n (%) | 0.449 | |||
| PCI | 54 (68.3) | 23 (63.8) | 31 (72) | |
| CABG | 13 (16.4) | 8 (22.2) | 5 (11.6) | |
| Medical follow | 12 (15.1) | 5 (13.8) | 7 (16.2) | |
| Culprit lesion; n (%) | 0.45 | |||
| LAD | 37 (46.8) | 16 (44.4) | 21 (48.8) | |
| RCA | 25 (31.6) | 10 (27.7) | 15 (34.8) | |
| CX | 17 (21.5) | 10 (27.7) | 7 (16.2) | |
| Number of diseased vessels, n (%) | 0.277 | |||
| Single vessel | 39 (49.3) | 17 (47.2) | 22 (51.1) | |
| Two vessel | 26 (32.9) | 10 (27.7) | 16 (37.2) | |
| Three vessel | 14 (17.7) | 9 (25) | 5 (11.6) |
BUN, blood urine nitrogen; CABG, coronary artery bypass grafting; CCU, coronary care unit; CX, circumflex; ECG, electrocardiography; HDL, high density lipoprotein; IQR, interquartile range 25-75; LAD, left anterior descending; LDL, low density lipoprotein; MI, myocardial infarction; PCI, percutaneous coronary intervention; RCA, right coronary artery; SD, standard deviation; STEMI, ST-segment elevation myocardial infarction; TG, triglyceride.
Blood samples were collected an median of 155 (from 60-392) minutes after the onset of the chest pain. Thirty six of the 79 patients had pre-infarction angina. The median duration of pain in these patients was 7 (4.25-18.75) days and median number of pain episodes was 4 (2-6) times.
Serum S1P, CKMB, high sensitive troponin levels and ejection fractions of the patients are given in Table 2. The median level of admittance serum S1P in patients with pre-infarction angina was significantly higher than in those patients without pre-infarction angina. Median level of serum S1P at the discharge was also significantly higher in patients with pre-infarction angina compared to the patients without pre-infarction angina. The peak level of high sensitive troponin was significantly lower in patients with pre-infarction angina. However, the peak level of CKMB and left ventricular ejection fraction were similar between the groups.
Table 2. Serum S1P, CKMB, high sensitive troponin levels and ejection fractions of two groups.
| Pre-infarction angina positive | Pre-infarction angina negative | ||
| (n = 36) | (n = 43) | p | |
| Admission S1P μM (median-IQR) | 0.54 (0.14-1.35) | 0.26 (0.12-0.62) | 0.014 |
| Discharge S1P μM (median-IQR) | 0.51 (0.20-1.81) | 0.30 (0.13-0.68) | 0.01 |
| Peak CKMB U/L (median-IQR) | 60.5 (34.7-130.7) | 87.0 (44.0-145.0) | 0.635 |
| Peak high sensitive troponin ng/dl (median-IQR) | 0.97 (0.39-3.07) | 2.56 (0.9-6.51) | 0.034 |
| EF % (median-IQR) | 50 (39-53) | 47 (40-50) | 0.956 |
CKMB, creatine kinase MB, EF, ejection fraction; IQR; interquartile range 25-75; S1P, sphingosine 1 phosphate.
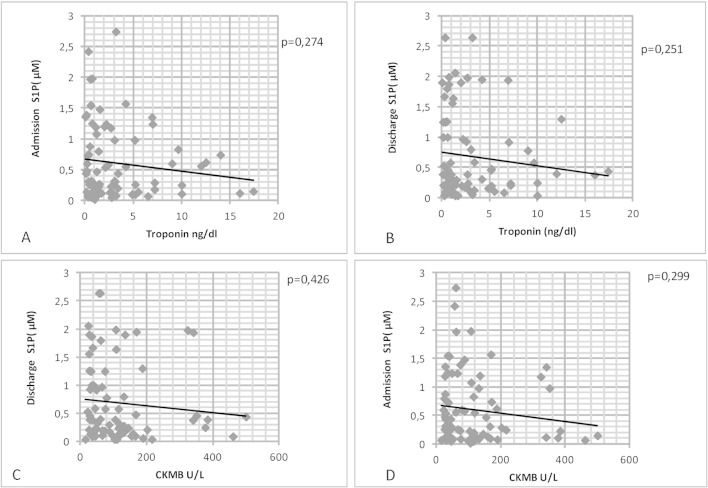
We examined whether there was any relationship between serum S1P levels and cardiac markers. Although there was an inverse association between CKMB and high sensitive troponin levels with both admission and discharge S1P levels, these correlations were not statistically significant (Figure 1).
Figure 1.
A and B show the relationship between S1P and high sensitive troponin levels. C and D show the relationship between S1P and CKMB levels. Spearman’s Rho respectively: -0.084, -0.119, -0.168, -0.160 S1P, sphingosine 1 phosphate. CKMB, creatine kinase MB.
We also examined whether there was any relationship between angina episode frequency and serum S1P levels. We determined that median serum S1P level at the admission was 0.26 (0.12-0.62) μM in patients without pre-infarction angina, 0.40 (0.13-0.79) μM in patients who experienced one or two angina episodes, 0.74 (0.19-1.39) μM in patients who had three or more angina episodes. S1P levels upon discharge were 0.30 (0.13-0.68) μM, 0.31 (0.16-0.82) μM, and 1.00 (0.22-1.89) μM, respectively. Serum S1P levels both at admission and discharge tended to be higher with more angina episodes, but this difference did not achieve statistical significance.
DISCUSSION
In the present study, we showed that patients who experienced pre-infarction angina had significantly higher serum S1P levels than patients who had not. This finding supports our hypothesis that cardioprotective effects of pre-infarction angina may partially be mediated by S1P.
The role of S1P in ischemic preconditioning, and its cardioprotective effects have been demonstrated in animal studies. It is known that endogenous mediators activate some intracellular kinases in the process of preconditioning. These kinases open mitochondrial KATP channels and prevent the opening of the mitochondrial permeability transposition pores. The net effect of this process is stabilization of mitochondria and eventual cellular survival. In an experimental study, the negative effects of severe hypoxia on myocardial cell viability were prevented in rat myocytes after preincubating with S1P, resulting in survival equivalent to normoxemic state. This cardioprotective effect of S1P was abolished with a mitochondrial KATP channel antagonist. This study demonstrated that exogenous S1P reduces the myocardial infarct size in the hypoxemic state and this effect involves signaling mechanisms requiring mitochondrial KATP channel opening.12 Vessey et al. showed that S1P is an important cardioprotective molecule both in preconditioning and postconditioning. Its effect was blocked with G protein cell receptor antagonist and inhibition of phosphatidil inositol 3 kinase.15 It has been demonstrated that not only exogenous but also endogenous release of S1P provides important cardioprotection.13 Our findings are consistent with that data suggesting patients with pre-infarction angina had higher serum S1P levels and lower cardiac troponin levels as an indicator of smaller infarct size.
There have been human studies evaluating S1P levels in patients with coronary artery disease. Deutschman et al. evaluated serum S1P levels in 308 patients undergoing coronary angiography for any indications. They found that S1P is a strong predictor for both occurrence and severity of coronary artery disease.16 In another study Sattler et al. evaluated S1P and HDL levels in patients with MI, stable coronary artery disease (CAD) and control groups. They found that plasma S1P levels are lower in stable CAD patients than in controls. They also found that non HDL bound S1P is significantly higher in myocardial infarction (MI) and stable CAD patients than controls, and that non HDL bound S1P is correlated with the severity of coronary atherosclerosis.17 Knapp et al. evaluated S1P levels between control patients and acute MI patients. S1P levels were significantly lower in acute MI patients.18 All of these studies demonstrate that serum S1P levels are significantly lower in acute MI patients than in control groups and this finding can be concluded as these low S1P levels can diminish the expected cardioprotection. But none of these studies provide any insight as to the role of S1P in human preconditioning because they were not designed to demonstrate the role of S1P in preconditioning. Our study demonstrated that S1P levels are increasing with sublethal ischemia-reperfusion cycles (pre-infarction angina positive group). This finding can support the role of S1P in human preconditioning.
We evaluated the relationship between serum S1P levels and CKMB/troponin levels. We found that serum CKMB and troponin levels decrease with the increase of serum S1P levels, but this negative correlation is not statistically significant. It has been shown that S1P has reduced infarct size in an animal study.19 Our result supports these findings, and the relatively lower number of patients included in the study may be the cause for the lack of statistical significance.
With this study, we found that serum S1P levels are increasing as the number of angina episodes increases. Patients who have three or more angina episodes have serum S1P levels two times higher than patients who have not pre-infarction angina. In addition, S1P levels tend to be higher in patients with three or more angina episodes compared to those patients with one or two angina episodes. Ischemic preconditioning pathways seem to be more active with increased sublethal ischemia reperfusion cycles. Again, however, the low number of patients included in the study seems to be the cause for the lack of statistical significance.
We found that serum S1P levels are significantly higher in patients with pre-infarction angina both upon admission to and discharge from the hospital. The aim of admission and discharge S1P measurement was to explore the effects of S1P both at early and late preconditioning phase. The first serum samples were obtained with an average of 323 minutes after onset of infarction symptoms. Egom et al. examined the effect of preconditioning to serum sphingoid base levels in elective PCI patients.20 They examined long chain sphingoid base levels by taking blood samples before the index ischemia, at the first minute of ischemia, the fifth minute of ischemia and the twelfth hour of ischemia. They found significant increase of long chain sphingoid base levels in both coronary sinus and peripheral venous blood samples from the beginning of first minute and the significant high S1P levels continued at twelfth hour also. This study supports that sphingolipids are functionally active both at the early and late phase of preconditioning.
Limitations
One of the most important limitations of our study is the subjective nature of pre-infarction angina. If we had more accurate methods by which to assess preconditioning, we might obtain more accurate results. But in clinical practice, there is no other parameter which can be used as an indicator of ischemic preconditioning except pre-infarction angina in patients presenting with acute MI. Lack of silent ischemia detection and evaluation is another limitation. In patients without pre-infarction angina, silent ischemia may be present and this may activate the mechanism of ischemic preconditioning. This could explain the high levels of S1P in some patients without pre-infarction angina. S1P enzyme-linked immunosorbent assay kits which we used can detect only albumin-bound S1P; HDL-bound S1P cannot be detected. HDL is the most important carrier of S1P, and albumin is generally considered the second. So we cannot detect a significant proportion of S1P. This is another limitation of our study. But as we discussed above, non HDL bound S1P levels were found to be higher in acute MI patients than in the control group. Finally, a low total patient number is another important limitation of our study. We think that some of the circumstances which may reduce the significance of our findings are due to low patient number.
CONCLUSIONS
The present study showed that there is a relationship between pre-infarction angina and serum S1P levels in patients with STEMI and NSTEMI. Our findings suggest that S1P may have a role in human preconditioning.
Acknowledgments
Grant Support: Gazi University Scientific Research Project Department, 01/2011-113, Ankara, Turkey.
DISCLOSURES
All authors certify that they have no relationship with any pharmaceutical or biomedical device companies or other corporations whose products or services are related to the subject matter of the article, or other conflicts of interest.
REFERENCES
- 1.Kloner RA. Does reperfusion injury exist in humans? J Am Coll Cardiol. 1993;21:537–545. doi: 10.1016/0735-1097(93)90700-b. [DOI] [PubMed] [Google Scholar]
- 2.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia:a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 3.Thornton JD, Liu GS, Olsson RA, Downey JM. Intravenous pretreatment with A1-selective adenosine analogues protects the heart against infarction. Circulation. 1992;85:659–665. doi: 10.1161/01.cir.85.2.659. [DOI] [PubMed] [Google Scholar]
- 4.Toombs CF, McGee S, Johnston WE, Vinten-Johansen J. Myocardial protective effects of adenosine. Infarct size reduction with pretreatment and continued receptor stimulation during ischemia. Circulation. 1992;86:986–994. doi: 10.1161/01.cir.86.3.986. [DOI] [PubMed] [Google Scholar]
- 5.Wall TM, Sheehy R, Hartman JC. Role of bradykinin in myocardial preconditioning. J Pharmacol Exp Ther. 1994;270:681–689. [PubMed] [Google Scholar]
- 6.Schultz JE, Hsu AK, Gross GJ. Morphine mimics the cardioprotective effect of ischemic preconditioning via a glibenclamide-sensitive mechanism in the rat heart. Circ Res. 1996;78:1100–1104. doi: 10.1161/01.res.78.6.1100. [DOI] [PubMed] [Google Scholar]
- 7.Watterson K, Sankala H, Milstien S, Spiegel S. Pleiotropic actions of sphingosine-1-phosphate. Prog Lipid Res. 2003;42:344–357. doi: 10.1016/s0163-7827(03)00015-8. [DOI] [PubMed] [Google Scholar]
- 8.Pappu R, Schwab SR, Cornelissen I, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 9.Venkataraman K, Lee YM, Michaud J, et al. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ Res. 2008;102:669–676. doi: 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yatomi Y, Ozaki Y, Ohmori T, Igarashi Y. Sphingosine 1-phosphate: synthesis and release. Prostaglandins. 2001;64:107–122. doi: 10.1016/s0090-6980(01)00103-4. [DOI] [PubMed] [Google Scholar]
- 11.Murata N, Sato K, Kon J, et al. Interaction of sphingosine 1-phosphate with plasma components,including lipoproteins,regulates the lipid receptor-mediated actions. Biochem J. 2000;352 Pt 3:809–815. [PMC free article] [PubMed] [Google Scholar]
- 12.Karliner JS, Honbo N, Summers K, et al. The lysophospholipids sphingosine-1-phosphate and lysophosphatidic acid enhance survival during hypoxia in neonatal rat cardiac myocytes. J Mol Cell Cardiol. 2001;33:1713–1717. doi: 10.1006/jmcc.2001.1429. [DOI] [PubMed] [Google Scholar]
- 13.Vessey DA, Li L, Honbo N, Karliner JS. Sphingosine 1-phosphate is an important endogenous cardioprotectant released by ischemic pre- and postconditioning. Am J Physiol Heart Circ Physiol. 2009;297:H1429–H1435. doi: 10.1152/ajpheart.00358.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Napoli C, Liguori A, Chiariello M, et al. New-onset angina preceding acute myocardial infarction is associated with improved contractile recovery after thrombolysis. Eur Heart J. 1998;19:411–419. doi: 10.1053/euhj.1997.0748. [DOI] [PubMed] [Google Scholar]
- 15.Vessey DA, Li L, Kelley M, et al. Sphingosine can pre- and post--condition heart and utilizes a different mechanism from sphingosine 1-phosphate. J Biochem Mol Toxicol. 2008;22:113–118. doi: 10.1002/jbt.20227. [DOI] [PubMed] [Google Scholar]
- 16.Deutschman DH, Carstens JS, Klepper RL, et al. Predicting obstructive coronary artery disease with serum sphingosine-1-phosphate. Am Heart J. 2003;146:62–68. doi: 10.1016/S0002-8703(03)00118-2. [DOI] [PubMed] [Google Scholar]
- 17.Sattler KJ, Elbasan S, Keul P, et al. Sphingosine 1-phosphate levels in plasma and HDL are altered in coronary artery disease. Basic Res Cardiol. 2010;105:821–832. doi: 10.1007/s00395-010-0112-5. [DOI] [PubMed] [Google Scholar]
- 18.Knapp M, Baranowski M, Czarnowski D, et al. Plasma sphingosine-1-phosphate concentration is reduced in patients with myocardial infarction. Med Sci Monit. 2009;15:CR490–CR493. [PubMed] [Google Scholar]
- 19.Jin ZQ, Zhou HZ, Zhu P, et al. Cardioprotection mediated by sphingosine-1-phosphate and ganglioside GM-1 in wild-type and PKC epsilon knockout mouse hearts. Am J Physiol Heart Circ Physiol. 2002;282:H1970–H1977. doi: 10.1152/ajpheart.01029.2001. [DOI] [PubMed] [Google Scholar]
- 20.Egom EE, Mohamed TM, Mamas MA, et al. Activation of Pak1/Akt/eNOS signaling following sphingosine-1-phosphate release as part of a mechanism protecting cardiomyocytes against ischemic cell injury. Am J Physiol Heart Circ Physiol. 2011;301:H1487–H1495. doi: 10.1152/ajpheart.01003.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]