Abstract
Background
Leptin and adiponectin are secreted from adipose tissue and exert opposing effects on C-reactive protein (CRP) levels and insulin resistance. As hypertrophic adipocytes secrete more leptin and less adiponectin, the leptin-to adiponectin ratio (LAR) has been proposed as a useful measure of insulin resistance and vascular risk. We investigated whether LAR may serve as a better predictor than either leptin or adiponectin alone for low-grade inflammation and insulin resistance independent of obesity in a non-diabetic Taiwanese population.
Methods
This study included 568 non-diabetic Taiwanese individuals (297 men, 271 women). CRP, leptin and adiponectin were measured using enzyme-linked immunosorbent assay. The degree of insulin resistance was determined using the homeostasis model assessment of insulin resistance (HOMA-IR).
Results
In the receiver operator characteristic analysis, the area under the curve of LAR in predicting individuals with elevated CRP and insulin resistance was significantly greater than that for either leptin (p = 0.0035 vs. elevated CRP, p < 0.0001 vs. insulin resistance) or adiponectin alone (p = 0.0131 vs. elevated CRP, p = 0.0006 vs. insulin resistance), suggesting that LAR might be a better predictor of individuals with low grade inflammation and insulin resistance. In the multivariable analysis adjusted for age, gender, smoking status, and components of metabolic syndrome, LAR was still strongly associated with levels of CRP (p < 0.001, all participants; p = 0.002, nonobese individuals; p < 0.001, obese individuals) and HOMA-IR index (p < 0.001, all participants, obese and nonobese individuals).
Conclusions
The LAR is related to low grade inflammation and insulin resistance independent of obesity in non-diabetic Taiwanese, and the strength of associations between LAR with CRP and HOMA-IR are greater than the association with leptin or adiponectin alone.
Keywords: Adiponectin, C-reactive protein, Insulin resistance, Leptin, Obesity
INTRODUCTION
Obesity is the most common nutritional disorder, and it has become a veritable epidemic in industrialized countries. It is well known that obesity is associated with increased cardiovascular morbidity, mortality1 and metabolic derangements including insulin resistance, dyslipidemia, and hypertension.2-4 Although the mechanisms linking obesity and cardiovascular disease are not completely understood, recent evidence indicates that inflammation and insulin resistance may play important roles.
C-reactive protein (CRP), a marker of systemic inflammation, is an easily measured inflammatory marker that has proven to be a strong predictor of cardiac events in patients with5 and without6 preexisting cardiovascular disease. CRP activity is stimulated by other cytokines, especially interleukin-6 (IL-6), which originates mainly from abdominal adipose tissue.7 Several studies have shown a strong association between CRP and obesity.8,9 Insulin resistance is also an independent risk factor for cardiovascular events in the normal population and in patients with preexisting cardiovascular disease.10,11 There is growing evidence that insulin resistance is also associated with abdominal obesity.12,13
It has been recognized in recent years that adipose tissue is an important endocrine organ, secreting several bioactive molecules, termed adipokines, which regulate whole-body metabolism and the immune response. Leptin and adiponectin, the two best-characterized adipokines, respond in a reciprocal manner to increasing adiposity. Plasma levels of leptin are elevated in obese individuals, and increased levels of leptin have been associated with higher levels of CRP14,15 and are predictive of future cardiovascular events.16 In contrast, plasma adiponectin levels are reduced in obese individuals, and lower levels of adiponectin are associated with higher levels of CRP17-19 and correlate significantly and independently with coronary artery disease.20 Mounting evidence also suggests that hyperleptinemia and hypoadiponectinemia are associated with insulin resistance,21-23 and the leptin-to-adiponectin ratio (LAR) correlates with insulin resistance better than either leptin or adiponectin levels alone.22
Increasing evidence has suggested that levels of adipokines differ among ethnic groups. Although people of Chinese origin have lower abdominal adiposity, their levels of leptin and adiponectin are significantly lower than those of other ethnic groups.24,25 The impact of adiposity on the levels of leptin and adiponectin was also greater in Chinese as compared with the other ethnic group.25 In addition to these findings, insulin resistance and plasma CRP levels are lower in the Chinese population compared with individuals of other ethnic origins.25,26 Therefore, the associations of leptin and adiponectin with CRP and insulin resistance in the Chinese population may differ from those in other ethnic groups.
In the present study, we investigated a group of nondiabetics within the Taiwanese population and hypothesized that the strength of LAR in predicting subjects with higher CRP levels and insulin resistance would be greater than each of the leptin or adiponectin alone. We also hypothesized that the associations of LAR with CRP levels and insulin resistance will be independent of obesity.
METHODS
Study participants
After written informed consent was obtained, the study participants were recruited consecutively during routine health examinations between October 2003 and September 2005 at the Chang Gung Memorial Hospital. They responded to a standardized questionnaire on their medical history and lifestyle characteristics. Individuals greater than 18 years of age were enrolled. The exclusion criteria included a history of myocardial infarction, stroke, or transient ischemic attack, history of cancer, and current renal or liver disease. Furthermore, individuals with diabetes mellitus (defined as fasting blood glucose ≥ 7.0 mmol/L or the regular use of medications for diabetes mellitus) or CRP levels > 10 mg/L were also excluded. A total of 568 individuals (297 men, mean age = 45.1 ± 9.9 years; 271 women, mean age = 46.7 ± 9.6 years) were enrolled and used in the analysis. Blood pressure (BP) was measured with a random-zero sphygmomanometer by trained physicians or nurses. An individual was designated a current smoker if they smoked at least 1 cigarette per day at the time of survey. All participants reported their ethnicity as being of Han-Chinese origin. The study was approved by the Ethics Committee of the Chang-Gung Memorial Hospital.
Laboratory measurements
Venous blood (15 mL) was collected from the study subjects the morning after an overnight (8-12 hour) fast. All measurements were performed in a central laboratory. Glucose level was determined enzymatically using the hexokinase method. Total cholesterol and triglyceride (TG) concentrations were measured by automatic enzymatic colorimetry. High-density lipoprotein cholesterol (HDL-C) levels were measured enzymatically following phosphotungsten/magnesium precipitation. For individuals with TG levels ≤ 400 mg/dL, low-density lipoprotein cholesterol (LDL-C) levels were calculated using the Friedewald formula. For those patients with TG levels > 400 mg/dL, serum LDL-C levels were detected with commercial reagents using a standard protocol. Serum insulin levels were measured by immunoradiometric assay (BioSource, Nivelles, Belgium), with intra- and inter-assay coefficients of variation of 5.3% and 9.5%, respectively. Insulin resistance was estimated by the homeostasis model assessment of insulin resistance (HOMA-IR) index using the following formula: HOMA-IR = fasting serum insulin (μ U/mL) × fasting plasma glucose (mmol/L)/22.5. CRP levels and IL-6 were measured using a high-sensitivity enzyme-linked immunosorbent assay (ELISA) developed in-house and performed in the sandwich format. All in-house kits were compared with commercial ELISA kits and exhibited good-to-excellent correlations.27 The ultrasensitive ELISA allowed detection of low serum levels of CRP and approached that of the commercial ultrasensitive assay for high sensitive CRP on a Behring Nephelometer Analyzer II, with a correlation coefficient greater than 0.9 and slopes close to 1.0.27 Serum leptin and adiponectin levels were measured by an in-house sandwich enzyme immunoassay. Our kits compared well with the commercial kits for leptin (Biovendor, Heidelberg, Germany) and adiponectin (R&D Systems, Minneapolis, MN, USA), with correlation coefficients of 0.99 and 0.98, respectively. The within-day precision and day-to-day precision were 5.1% and 4.0% for leptin and 7.7% and 7.0% for adiponectin, respectively.
Anthropometric measurements
Anthropometrics were obtained with the participants in light clothing, no footwear, and after 12 hours of fasting. Body weight was measured to the nearest kilogram using a digital scale, and height was measured to the nearest centimeter in the standing position using a wall standiometer. Body mass index (BMI) was computed as the ratio of weight to the square of height (kg/m2). Waist circumference (WC) was measured to the nearest centimeter at the midpoint between the lower limit of the rib cage and the iliac crest. Obesity was defined as BMI ≥ 25 kg/m2 according to the Asian criteria.28
Statistic analysis
The clinical characteristics of the participants are expressed as means ± standard deviation (SD) and percentages, except when the distribution was strongly skewed, in which case the median and interquartile ranges are given. Pearson’s correlation analysis was performed to determine the correlations of CRP and HOMA-IR with leptin, adiponectin and LAR, as well as metabolic syndrome-related factors. HOMA-IR, CRP, TG, IL-6, leptin, adiponectin, and LAR were logarithmically transformed prior to statistical analysis to adhere to an assumption of normality. To compare the relative diagnostic strengths of leptin, adiponectin, and LAR in detecting individuals with elevated CRP levels and insulin resistance, receiver operator characteristic (ROC) curves were plotted; the area under curves (AUC) for leptin, adiponectin, and LAR were compared by a nonparametric approach. The relationships between CRP and HOMA-IR with metabolic syndrome-related factors, IL-6, and different adipokines (i.e. leptin, adiponectin and LAR) were further investigated using multiple linear regression analysis, while controlling for other variables including age, gender, and smoking status. Values of p < 0.05 from a two-sided test were considered statistically significant, and all statistical analyses were conducted with the SPSS statistical package for Windows version 19.0 (SPSS, Chicago, IL, USA).
RESULTS
The general characteristics of the participants
Table 1 shows the anthropometric data for participants stratified by obese status and gender. No statistically significant differences between the nonobese and obese groups were observed for age, cholesterol level, or LDL-C level, but analyses revealed statistically significant differences for many variables. It was determined that higher percentages of current smokers (p < 0.001) were obese. Additionally, obese individuals exhibited elevated values for systolic BP (p < 0.001), diastolic BP (p < 0.001), TG (p < 0.001), fasting glucose (p < 0.001), fasting insulin (p < 0.001), HOMA-IR (p < 0.001), BMI (p < 0.001), WC (p < 0.001), waist-to-hip ratio (p < 0.001), CRP levels (p < 0.001), IL-6 levels (p = 0.02), leptin (p < 0.001), and LAR (p < 0.001) compared to nonobese participants. In contrast, HDL-C (p < 0.001) and adiponectin (p < 0.001) levels were significantly lower in the obese group.
Table 1. Baseline characteristics of study individuals according to obesity status and gender.
| Total (n = 568) | Nonobese (n = 350) | Obese (n = 218) | Women (n = 271) | Men (n = 297) | |
| Age (years) | 45.9 ± 9.8 | 45.3 ± 10.0 | 46.8 ± 9.6 | 46.7 ± 9.8 | 45.1 ± 9.9† |
| Smokers (%) | 144 (25.4%) | 72 (20.6%) | 72 (33.0%)# | 14 (5.2%) | 130 (43.8%)‡ |
| SBP (mmHg) | 114.8 ± 17.5 | 112.2 ± 17.2 | 119.0 ± 17.1# | 113.7 ± 19.0 | 115.8 ± 15.9 |
| DBP (mmHg) | 76.0 ± 10.6 | 74.2 ± 10.3 | 78.9 ± 10.4# | 73.8 ± 10.5 | 78.0 ± 10.3‡ |
| CHOL (mmol/L) | 5.13 ± 0.91 | 5.08 ± 0.91 | 5.22 ± 0.92 | 5.09 ± 0.93 | 5.17 ± 0.90 |
| TG (mmol/L) | 1.27 (0.86-1.84) | 1.06 (0.77-1.68) | 1.58 (1.12-2.09) # | 1.02 (0.75-1.51) | 1.53 (1.04-2.23) ‡ |
| HDL-C (mmol/L) | 1.44 ± 0.37 | 1.51 ± 0.40 | 1.33 ± 0.30# | 1.60 ± 0.37 | 1.29 ± 0.31‡ |
| LDL-C (mmol/L) | 2.99 ± 0.83 | 2.94 ± 0.82 | 3.07 ± 0.83 | 2.93 ± 0.82 | 3.04 ± 0.83 |
| FG (mmol/L) | 5.17 ± 0.48 | 5.09 ± 0.45 | 5.33 ± 0.49# | 5.06 ± 0.44 | 5.27 ± 0.48‡ |
| FI (mU/mL) | 7.96 (6.14-10.82) | 7.19 (5.57-9.23) | 10.19 (7.36-13.39) # | 7.51 (5.86-10.26) | 8.48 (6.57-11.51) † |
| HOMA-IR | 1.81 (1.39-2.51) | 1.63 (1.21-2.16) | 2.37 (1.69-3.20) # | 1.70 (1.28-2.36) | 2.00 (1.53-2.66) ‡ |
| BMI (kg/m2) | 24.2 ± 3.4 | 22.1 ± 1.9 | 27.6 ± 2.4# | 23.4 ± 3.6 | 24.9 ± 3.1‡ |
| WC (cm) | 84.8 ± 9.4 | 80.1 ± 7.0 | 92.4 ± 7.6# | 81.5 ± 10.0 | 87.9 ± 7.5‡ |
| WHR | 0.87 ± 0.06 | 0.85 ± 0.06 | 0.89 ± 0.05# | 0.85 ± 0.07 | 0.88 ± 0.05‡ |
| CRP (mg/L) | 0.59 (0.25-1.18) | 0.43 (0.20-0.95) | 0.81 (0.43-1.65) # | 0.53 (0.24-1.17) | 0.64 (0.30-1.21) |
| IL-6 (pg/L) | 2.19 (1.20-3.91) | 2.00 (1.10-3.69) | 2.41 (1.40-4.50)* | 2.30 (1.40-4.10) | 2.00 (1.20-3.80) |
| Leptin (mg/L) | 15.0 (8.2-26.0) | 12.5 (6.4-21.5) | 18.4 (12.0-32.2) # | 24.8 (16.3-33.1) | 9.5 (5.3-14.5) ‡ |
| ADPN (mg/L) | 6.10 (3.71-9.26) | 6.91 (4.36-10.64) | 4.82 (3.23-7.08) # | 7.87 (5.42-11.54) | 4.45 (3.07-6.86) ‡ |
| LAR | 2.69 (1.28-4.64) | 1.95 (0.84-3.38) | 3.94 (2.36-7.66) # | 3.09 (1.73-5.46) | 2.15 (1.07-3.86) ‡ |
Data are presented as mean ± SD, number (percentage), or median (inter-quartile range) as appropriate. Data with skewed distribution (triglyceride level, insulin level, HOMA-IR, CRP level, IL-6 level, leptin level, adiponectin level, and LAR) were compared among these quartiles after log transformation.
* p < 0.05 versus nonobese individuals; # p < 0.001 versus nonobese individuals; † p < 0.05 versus female individuals; ‡ p< 0.001 versus female individuals.
ADPN, adiponectin; BMI, body mass index; CHOL, cholesterol; CRP, C-reactive protein; DBP, diastolic blood pressure; FG, fasting glucose; FI, fasting insulin; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance; IL-6, interleukin-6; LAR, leptin-to-adiponectin ratio; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure; TG, triglycerides; WC, waist circumference; WHR, waist-hip ratio.
Similarly, male participants were older and had a higher percentage of current smokers (p < 0.001). Male individuals also demonstrated higher values for diastolic BP (p < 0.001), TG (p < 0.001), fasting glucose (p < 0.001), fasting insulin (p < 0.001), HOMA-IR (p < 0.001), BMI (p < 0.001), WC (p < 0.001) and waist-to-hip ratio (p < 0.001), as well as lower value for HDL-C (p < 0.001), as compared with female participants. Additionally, male individuals had significant lower values for leptin (p < 0.001), adiponectin (p < 0.001) and LAR (p < 0.001) than female groups.
Correlations of CRP and HOMA-IR with components of metabolic syndrome and adipokines
The results of Pearson’s correlation analysis of CRP levels and HOMA-IR index with biological measurements while controlling for age, gender and smoking status are shown in Table 2. TG, BMI, leptin and LAR were all positively correlated with CRP levels and HOMA-IR index in nonobese and obese individuals. In contrast, CRP levels and HOMA-IR index were negatively correlated with HDL-C and adiponectin in both groups. IL-6 was positively correlated with CRP levels in both groups, but no correlation with HOMA-IR was found.
Table 2. Pearson′s correlation coefficients of CRP and HOMA-IR with components of metabolic syndrome and adipokines, stratified by obesity status.
| All subjects (n = 568) | Nonobese (n = 350) | Obese (n = 218) | ||||||
| CRP | HOMA-IR | CRP | HOMA-IR | CRP | HOMA-IR | |||
| SBP | 0.175# | 0.250# | 0.103 | 0.196* | 0.181* | 0.211* | ||
| DBP | 0.164# | 0.226# | 0.093 | 0.104 | 0.159* | 0.263# | ||
| TG | 0.262# | 0.317# | 0.220# | 0.338# | 0.199* | 0.141* | ||
| HDL-C | -0.253# | -0.303# | -0.220# | -0.270# | -0.160* | -0.246# | ||
| BMI | 0.322# | 0.470# | 0.211# | 0.312# | 0.187* | 0.316# | ||
| IL-6 | 0.305# | 0.063 | 0.262# | 0.02 | 0.330# | 0.035 | ||
| Leptin | 0.365# | 0.659# | 0.260# | 0.579# | 0.363# | 0.666# | ||
| ADPN | -0.265# | -0.340# | -0.245# | -0.317# | -0.148* | -0.226# | ||
| LAR | 0.355# | 0.567# | 0.279# | 0.488# | 0.314# | 0.542# |
Adjusted for age, gender and smoking status. Abbreviations are as presented in Table 1. Data with skewed distribution (TG, HOMA-IR, CRP, leptin, ADPN, LAR, and IL-6) were logarithmically transformed before statistical testing to meet the assumption of normal distributions.
* p < 0.05; # p < 0.001.
ADPN, adiponectin; BMI, body mass index; DBP, diastolic blood pressure; HDL-C, high-density lipoprotein cholesterol; IL-6, interleukin-6; LAR, leptin-to-adiponectin ratio; SBP, systolic blood pressure; TG, triglycerides.
ROC curves of leptin, adiponectin, and LAR for the detection of high CRP levels and insulin resistance
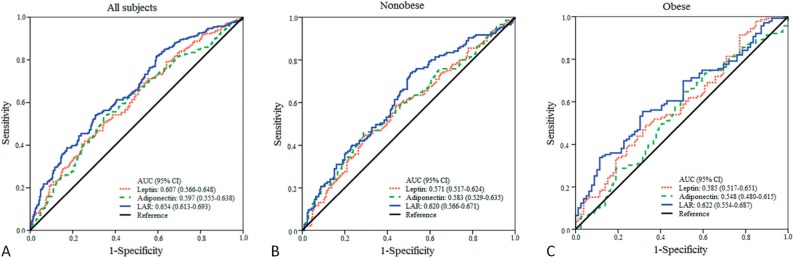
Figure 1 shows the ROC curves for leptin, adiponectin, and LAR used to detect individuals with elevated CRP levels. The median value of CRP levels (i.e. 0.585 mg/L) was used as a cutoff value and participants with CRP levels greater than the median value were defined as high CRP levels. Among all participants, the AUC for LAR in detecting individuals with elevated CRP levels was significantly greater than those for leptin or adiponectin (p = 0.0035, LAR vs. leptin; p = 0.0131, LAR vs. adiponectin; Figure 1A). When participants were stratified according to BMI, the AUC for LAR was greater than that for leptin in nonobese individuals (p = 0.0406, Figure 1B). There was also a trend that the AUC for LAR was greater than that for adiponectin in the obese group (p = 0.0909, Figure 1C).
Figure 1.
ROC curves for individuals with high CRP levels (i.e. CRP levels > 0.585 mg/L). (A) The AUC for LAR is significantly higher than those for leptin (p = 0.0035) and adiponectin (p = 0.0131). (B) In nonobese individuals, the AUC for LAR is significantly higher than that for leptin (p = 0.0406). (C) In obese individuals, there was a trend that the AUC for LAR is higher than that for adiponectin (p = 0.0909). AUC, area under curves; CI, confidence interval; CRP, C-reactive protein; LAR, leptin-to-adiponectin ratio.
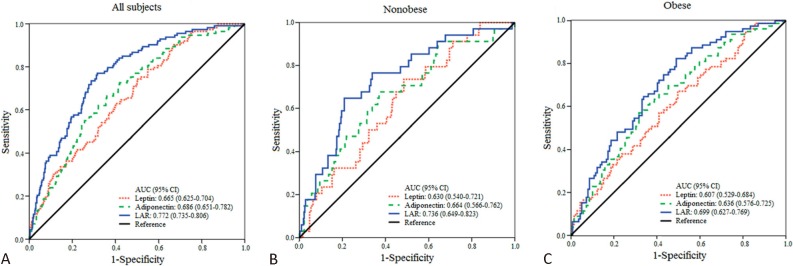
Figure 2 depicts the ROC curves for leptin, adiponectin, and LAR for detecting individuals with insulin resistance. Individuals with an HOMA-IR index greater than the 80th percentile (i.e. HOMA-IR index > 2.74) were categorized as insulin-resistant. The selection of the 80th percentile as the cut-off point for HOMA-IR was in agreement with the Bruneck study,29 a population-based prospective study of Italian patients on the prevalence of insulin resistance in metabolic disorders. The AUC for LAR in detecting insulin-resistant individuals was significantly greater than that for either leptin (p < 0.0001) or adiponectin (p = 0.0006) in detecting individuals with insulin resistance (Figure 2A). The AUC for LAR was also greater than those for leptin in both nonobese (p = 0.0121; Figure 2B) and obese individuals (p = 0.0032; Figure 2C). Although the AUC for LAR was greater than those for adiponectin in both groups, the differences were not statistically significant.
Figure 2.
ROC curves for individuals with insulin resistance (i.e. HOMA-IR index > 2.74). (A) The AUC for LAR is significantly higher than those for leptin (p < 0.0001) and adiponectin (p = 0.0006). (B) In nonobese individuals, the AUC for LAR is significantly higher than that for leptin (p = 0.0121). (C) In obese individuals, the AUC for LAR is also significantly higher than that for leptin (p = 0.032). AUC, area under curves; CI, confidence interval; HOMA-IR, homeostasis model assessment of insulin resistance; LAR, leptin-to-adiponectin ratio.
Multiple linear regression analysis of the association between CRP and factors of interests
Multiple linear regression analysis with levels of CRP as the dependent variables and factors associated with metabolic syndromes, IL-6 and different adipokines (i.e. leptin, adiponectin and LAR) as the independent variables are presented in Table 3. When leptin was entered into the analysis (model 1), IL-6 and leptin were positively correlated with CRP levels in both nonobese and obese groups, while TG was positively correlated with CRP levels only in the nonobese group. When adiponectin was entered into the analysis (model 2), a positive correlation of IL-6 with CRP levels and a negative correlation of adiponectin with CRP levels were found in both nonobese and obese groups. TG was positively correlated with CRP levels only in the nonobese group. When LAR was entered instead of leptin or adiponectin (model 3), the IL-6 and LAR were still strongly correlated with CRP levels in both nonobese and obese groups. However, the association of TG with CRP levels was attenuated.
Table 3. Multiple linear regression analysis of the relationship between CRP, components of metabolic syndrome, IL-6 and adipokines.
| Model 1 | Model 2 | Model 3 | |||||||
| Nonobese | Variables | β (se) | p | Variables | β (se) | p | Variables | β (se) | |
| Systolic BP | 0.001 (0.002) | 0.784 | Systolic BP | 0.001 (0.002) | 0.656 | Systolic BP | < 0.001 (0.002) | 0.865 | |
| Diastolic BP | 0.001 (0.004) | 0.713 | Diastolic BP | 0.001 (0.004) | 0.791 | Diastolic BP | 0.001 (0.004) | 0.692 | |
| Triglyceride | 0.263 (0.121) | 0.031 | Triglyceride | 0.269 (0.120) | 0.026 | Triglyceride | 0.225 (0.122) | 0.065 | |
| HDL-cholesterol | -0.002 (0.002) | 0.218 | HDL-cholesterol | -0.001 (0.002) | 0.635 | HDL-cholesterol | -0.001 (0.002) | 0.607 | |
| IL-6 | 0.263 (0.059) | < 0.001 | IL-6 | 0.265 (0.059) | < 0.001 | IL-6 | 0.261 (0.059) | < 0.001 | |
| Leptin | 0.190 (0.089) | 0.035 | Adiponectin | -0.256 (0.104) | 0.014 | LAR | 0.198 (0.064) | 0.002 | |
| Obese | Model 1 | Model 2 | Model 3 | ||||||
| Variables | β (se) | p | Variables | β (se) | p | Variables | β (se) | p | |
| Systolic BP | 0.003 (0.003) | 0.267 | Systolic BP | 0.003 (0.003) | 0.245 | Systolic BP | 0.003 (0.003) | 0.276 | |
| Diastolic BP | < 0.001 (0.004) | 0.678 | Diastolic BP | 0.001 (0.004) | 0.716 | Diastolic BP | < 0.001 (0.004) | 0.973 | |
| Triglyceride | 0.242 (0.136) | 0.077 | Triglyceride | 0.216 (0.138) | 0.12 | Triglyceride | 0.198 (0.135) | 0.144 | |
| HDL-cholesterol | -0.002 (0.003) | 0.443 | HDL-cholesterol | -0.001 (0.003) | 0.659 | HDL-cholesterol | -0.001 (0.003) | 0.815 | |
| IL-6 | 0.299 (0.072) | < 0.001 | IL-6 | 0.343 (0.071) | < 0.001 | IL-6 | 0.310 (0.070) | < 0.001 | |
| Leptin | 0.325 (0.119) | 0.007 | Adiponectin | -0.259 (0.124) | 0.038 | LAR | 0.310 (0.087) | < 0.001 |
* Adjusted for age, gender and smoking status.
Model 1: systolic BP, diastolic BP, triglyceride, HDL-cholesterol, IL-6 and leptin as the independent variables; Model 2: systolic BP, diastolic BP, triglyceride, HDL-cholesterol, IL-6 and adiponectin as the independent variables; Model 3: systolic BP, diastolic BP, triglyceride, HDL-cholesterol, IL-6 and LAR as the independent variables.
Abbreviations are presented as in Table 1.
Data with skew distribution (CRP, triglyceride, IL-6, leptin, adiponectin and LAR) are logarithmically transformed before statistical testing to meet the assumption of normal distributions.
The dependent variable is log CRP.
BP, blood pressure; HDL, high-density lipoprotein; IL-6, interleukin-6; LAR, leptin-to-adiponectin ratio.
Multiple linear regression analysis of the association between HOMA-IR and factors of interest
The results of multiple linear regression models with the HOMA-IR index as the dependent variable and the factors associated with metabolic syndromes, IL-6 and the levels of different adipokines as the independent variables are presented in Table 4. When leptin was entered into the analysis (model 1), the leptin was positively correlated with HOMA-IR index in both nonobese and obese groups. When adiponectin was entered into the analysis (model 2), the adiponectin was negatively correlated with HOMA-IR index in both the nonobese and obese groups. In both model 1 and model 2, TG in the nonobese group and diastolic BP in the obese group were positively correlated with HOMA-IR index, while HDL-C was negatively correlated with HOMA-IR in the obese group. When LAR was entered in the analysis instead of leptin or adiponectin levels (model 3), the LAR was still strongly correlated with the HOMA-IR index. TG in the nonobese group and diastolic BP in the obese group were still positively correlated with HOMA-IR index, but the negative association of HDL-C with HOMA-IR index was attenuated.
Table 4. Multiple linear regression analysis of the relationship between HOMA-IR, components of metabolic syndrome, IL-6 and adipokines.
| Nonobese | Model 1 | Model 2 | Model 3 | ||||||
| Variables | β (se) | p | Variables | β (se) | p | Variables | β (se) | p | |
| Systolic BP | 0.002 (0.001) | 0.041 | Systolic BP | 0.002 (0.001) | 0.008 | Systolic BP | 0.002 (0.001) | 0.038 | |
| Diastolic BP | -0.002 (0.001) | 0.196 | Diastolic BP | -0.002 (0.001) | 0.116 | Diastolic BP | -0.002 (0.001) | 0.175 | |
| Triglyceride | 0.131 (0.042) | 0.002 | Triglyceride | 0.166 (0.044) | < 0.001 | Triglyceride | 0.119 (0.043) | 0.005 | |
| HDL-cholesterol | -0.001 (0.001) | 0.177 | HDL-cholesterol | -0.001 (0.001) | 0.505 | HDL-cholesterol | < 0.001 (0.001) | 1 | |
| IL-6 | -0.013 (0.021) | 0.526 | IL-6 | -0.010 (0.022) | 0.693 | IL-6 | -0.003 (0.021) | 0.525 | |
| Leptin | 0.199 (0.031) | < 0.001 | Adiponectin | -0.111 (0.038) | 0.004 | LAR | 0.147 (0.022) | < 0.001 | |
| Obese | Model 1 | Model 2 | Model 3 | ||||||
| Variables | β (se) | p | Variables | β (se) | p | Variables | β (se) | p | |
| Systolic BP | -0.001 (0.001) | 0.519 | Systolic BP | -0.001 (0.001) | 0.692 | Systolic BP | -0.001 (0.001) | 0.495 | |
| Diastolic BP | 0.003 (0.002) | 0.05 | Diastolic BP | 0.005 (0.002) | 0.009 | Diastolic BP | 0.004 (0.002) | 0.029 | |
| Triglyceride | -0.005 (0.001) | 0.93 | Triglyceride | -0.017 (0.065) | 0.797 | Triglyceride | -0.045 (0.058) | 0.439 | |
| HDL-cholesterol | -0.003 (0.001) | 0.004 | HDL-cholesterol | -0.003 (0.001) | 0.023 | HDL-cholesterol | -0.002 (0.001) | 0.078 | |
| IL-6 | -0.047 (0.031) | 0.128 | IL-6 | 0.001 (0.033) | 0.98 | IL-6 | -0.028 (0.030) | 0.345 | |
| Leptin | 0.384 (0.051) | < 0.001 | Adiponectin | -0.171 (0.059) | 0.004 | LAR | 0.297 (0.037) | < 0.001 |
* Adjusted for age, gender and smoking status.
Model 1: systolic BP, diastolic BP, triglyceride, HDL-cholesterol, IL-6 and leptin as the independent variables; Model 2: systolic BP, diastolic BP, triglyceride, HDL-cholesterol, IL-6 and adiponectin as the independent variables; Model 3: systolic BP, diastolic BP, triglyceride, HDL-cholesterol, IL-6 and LAR as the independent variables.
Abbreviations are presented as in Table 1.
Data with skew distribution (HOMA-IR, triglyceride, IL-6, leptin, adiponectin and LAR) are logarithmically transformed before statistical testing to meet the assumption of normal distributions.
The dependent variable is log HOMA-IR.
BP, blood pressure; HDL, high-density lipoprotein; IL-6, interleukin-6; LAR, leptin-to-adiponectin ratio.
DISCUSSION
In this cross-sectional study in a nondiabetic Taiwanese population, CRP levels and HOMA-IR index were found to be positively associated with LAR and leptin and negatively associated with adiponectin in both nonobese and obese individuals. Importantly, ROC analysis showed the diagnostic strength of LAR in detecting individuals with elevated CRP levels and insulin resistance was significantly better than either leptin or adiponectin alone. These findings indicate that LAR is a more powerful marker for predicting individuals with elevated CRP levels and insulin-resistant than either leptin or adiponectin alone. Furthermore, the correlation of LAR with CRP levels and HOMA-IR index remained highly significant in the multiple linear regression analysis for nonobese and obese individuals after adjusting for age, gender, and smoking status. These results indicate that LAR may serve as a useful obesity-independent indicator of low grade inflammation and insulin resistance in the nondiabetic Taiwanese population.
It is well-known that adipose tissue is more than a simple, inert storage depot for lipid. It is also an important endocrine organ secreting a number of bioactive peptides, collectively called adipokines, which mediate metabolic and cardiovascular complications associated with obesity. Leptin and adiponectin respond in a reciprocal manner to increasing adiposity and are the two best-characterized adipokines. Accumulating data suggested that leptin had proinflammatory properties,30 whereas adiponectin was considered an anti-inflammatory cytokine.31 The independent association between leptin and CRP levels in healthy humans was first described by Shamsuzzaman et al.14 Subsequently, Ble et al.15 found that leptin levels were directly associated with CRP levels independent of IL-6 and other cytokines, supporting the hypothesis that leptin directly stimulated the production of acute-phase proteins in the liver. This finding was consistent with a previous study demonstrating the presence of leptin receptors in human hepatocytes.32 In contrast, a growing body of evidence suggests an inverse relationship between adiponectin and CRP levels.17-19 Adiponectin has been shown to counteract the proinflammatory effects of tumor necrosis factor-α (TNF-α) in vascular cells in vitro.33 Thus, adiponectin may indirectly inhibit IL-6 and CRP expression through its ability to inhibit TNF-α. Our findings are consistent with the original studies showing leptin to be positively correlated and adiponectin negatively correlated with plasma CRP levels. Furthermore, we demonstrated a significant, not previously reported association between LAR and plasma CRP levels independent of IL-6 and obesity. Furthermore, we also showed LAR to have a better diagnostic strength for detecting individuals with elevated CRP levels than either leptin or adiponectin alone.
Mounting evidence also points toward strong associations of leptin and adiponectin with insulin resistance. Leptin is thought to convey information about nutritional status to the brainstem via hypothalamic pathways, thereby increasing the brain’s motor and autonomic responses to satisfy signals, leading to smaller individual meals, reduced cumulative food intake, and a lower body weight.34 In vitro and in vivo studies suggest that leptin increases glucose uptake and metabolism in skeletal muscle via a 5′-AMP-activated protein kinase-(AMPK) dependent pathway.35 Although leptin has been reported to be a “good” hormone because it improves insulin resistance, obese individuals tend to have unusually high circulating levels of leptin as a result of leptin resistance.36 This explains the positive correlation between hyperleptinemia and insulin resistance. In contrast, adiponectin improves insulin sensitivity by enhancing glucose utilization and fatty-acid oxidation through the AMPK pathway in muscle and liver,37 and an inverse relationship between adiponectin and insulin resistance has been reported in nondiabetic individuals.23 In this study we demonstrated a positive correlation for leptin and an inverse correlation for adiponectin with the HOMA-IR index, consistent with previous reports.23,38 Since the HOMA-IR index increases with hyperleptinemia and hypoadiponectinemia, it is plausible that LAR may be a more powerful predictor of insulin resistance. In line with this hypothesis, Jung et al. found that the adiponectin-to-leptin ratio correlated significantly to HOMA-IR in Korean male subjects.21 Finucane et al.22 also confirmed that the LAR in nondiabetic white adults was at least as strongly associated with the gold standard measure of insulin resistance (clamp M/I value) as currently used methods such as fasting insulin or HOMA-derived insulin sensitivity. However, these studies did not compare the association between HOMA-IR and LAR to associations with leptin and adiponectin, presenting only associations with LAR. Interestingly, in the Multi-Ethnic Study of Atherosclerosis (MESA) study,25 Rasmussen-Torvik et al. demonstrated that the association of leptin, adiponectin and LAR with HOMA-IR were similar across all ethnic groups, including a Chinese population. In this study, the author only included a small Chinese population sample size (219 Chinese participants) and did not formally compare the association of LAR, leptin and adiponectin with HOMA-IR using the ROC curve analysis. In the present study, we determined that LAR was positively correlated with insulin resistance in a nondiabetic Taiwanese population independent of obesity. The diagnostic strength of LAR in detecting insulin-resistant individuals was better than either leptin or adiponectin alone according to the ROC curve analysis.
Because of the greater impact of adiposity on the levels of leptin and adiponectin in the Chinese population, it is important to investigate whether the associations of these adipokines with CRP levels and HOMA-IR index are independent of obesity. In the present study, the power of LAR to predict individuals with elevated CRP levels and insulin resistance differed between the nonobese and obese groups. In the nonobese group, LAR was significantly more powerful than leptin in predicting individuals with elevated CRP levels and insulin resistance, but there was no difference in the AUCs for LAR and adiponectin in both ROC analyses. In the obese group, there was a trend that LAR was more powerful than adiponectin in predicting individuals with elevated CRP levels, and LAR was significantly more powerful than leptin in predicting insulin-resistant individuals. Nevertheless, multiple regression analysis identified LAR to be significantly associated with CRP levels and HOMA-IR index in both the nonobese and obese groups. Interaction analysis revealed no impact of obesity on the association of LAR with CRP levels and HOMA-IR index (p = 0.733 for CRP levels, p = 0.476 for HOMA-IR index; data not shown), indicating the associations of LAR with CRP levels and HOMA-IR index were independent of obesity.
Previous studies have identified increased leptin levels and decreased adiponectin levels as independent risk factors for coronary artery disease.16,20 A growing body of evidence also suggests that LAR may serve as an atherogenic index in obese type 2 diabetic patients39 and is a powerful independent predictor of intima media thickness in healthy individuals.40 In support of this evidence, we demonstrated that LAR is a better independent predictor of low-grade inflammation and insulin resistance, which partially explains the missing link between adiposity and atherosclerosis in the adipo-vascular axis.41
There are some possible limitations of our study that merit mention. First, because of the cross-sectional study design, our results did not establish causality. A long-term follow-up study of the same population of individuals will highlight any causal relationship between LAR with low grade inflammation and insulin resistance. Second, some reporting bias might have been introduced because certain information in the medical records, such as underlying cardiovascular disease, treatment with lipid-lowering agents, and smoking status were obtained via a standardized questionnaire. The long-term follow-up study mentioned above will enable us to test this hypothesis. Third, statin treatment had been reported to decrease plasma level of adiponectin significantly while exerting neutral effects on insulin sensitivity in nondiabetic, hypercholesterolemic patients.42 Statin was also well-known as an antiinflammatory agent. Individuals taking lipid-lowering agents were not excluded from our study, which might influence the plasma levels of adiponectin and CRP and affect the association of LAR with CRP and HOMA-IR in the multivariate analysis. However, the number of these individuals was relatively small (5 in nonobese group and 4 in the obese group). Furthermore, the associations of LAR with CRP and HOMA-IR remained significant in all participants (CRP, p < 0.001; HOMA-IR, p < 0.001), nonobese (CRP, p = 0.003; HOMA-IR, p < 0.001) and obese group (CRP, p < 0.001; HOMA-IR, p < 0.001) after excluding individuals who were taking lipid-lowering agents. Fourth, we used the HOMA-IR to assess insulin resistance rather than the euglycemic-hyperinsulinemic clamp method, which is thought to be the best way to measure insulin resistance. Nevertheless, the use of the euglycemic-hyperinsulinemic clamp method in clinical practice is limited due to its invasiveness and high cost. HOMA-IR is the simplest index of insulin resistance, particularly in epidemiologic studies. Moreover, HOMA-IR has been shown to be strongly correlated with the euglycemic-hyperinsulinemic clamp method in various clinical trials. Thus, HOMA-IR can be considered to be a reliable marker of insulin resistance. Finally, all of the participants in the present study were of Han-Chinese origin. Whether our findings can be extrapolated to other populations will require further assessment.
CONCLUSIONS
In conclusion, our study has shown that LAR is related to low-grade inflammation and insulin resistance in a nondiabetic Taiwanese population, independent of obesity or other metabolic risk factors for cardiovascular disease, such as blood pressure or hyperlipidemia. The diagnostic strength of LAR in detecting individuals with higher CRP levels and insulin resistance is greater than each of the leptin or adiponectin alone. Taking into consideration that inflammation and insulin resistance are implicated in the development of atherosclerosis, the LAR may also serve as a risk marker for cardiovascular disease in nondiabetic Taiwanese individuals.
Acknowledgments
This study was supported by a grant from Buddhist Tzu Chi General Hospital, Taipei Branch (TCRD-TPE-100-23) to H. H. Chou and a grant from Buddhist Tzu Chi General Hospital, Taipei Branch (TCRD-TPE-99-07) to Y. L. Ko.
Conflicts of interest
None to declare.
REFERENCES
- 1.Krauss RM, Winston M, Fletcher BJ, Grundy SM. Obesity: impact on cardiovascular disease. Circulation. 1998;98:1472–1476. [PubMed] [Google Scholar]
- 2.Lebovitz HE, Banerji MA. Point:visceral adiposity is causally related to insulin resistance. Diabetes Care. 2005;28:2322–2325. doi: 10.2337/diacare.28.9.2322. [DOI] [PubMed] [Google Scholar]
- 3.Aneja A, El-Atat F, McFarlane SI, Sowers JR. Hypertension and obesity. Recent Prog Horm Res. 2004;59:169–205. doi: 10.1210/rp.59.1.169. [DOI] [PubMed] [Google Scholar]
- 4.Carr MC, Brunzell JD. Abdominal obesity and dyslipidemia in the metabolic syndrome:importance of type 2 diabetes and familial combined hyperlipidemia in coronary artery disease risk. J Clin Endocrinol Metab. 2004;89:2601–2607. doi: 10.1210/jc.2004-0432. [DOI] [PubMed] [Google Scholar]
- 5.Liuzzo G, Biasucci LM, Gallimore JR, et al. The prognostic value of C-reactive protein and serum amyloid a protein in severe unstable angina. N Engl J Med. 1994;331:417–424. doi: 10.1056/NEJM199408183310701. [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM, Buring JE, Shih J, et al. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98:731–733. doi: 10.1161/01.cir.98.8.731. [DOI] [PubMed] [Google Scholar]
- 7.Mohamed-Ali V, Goodrick S, Rawesh A, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 8.Ford ES. Body mass index, diabetes, and C-reactive protein among U.S. adults. Diabetes Care. 1999;22:1971–1977. doi: 10.2337/diacare.22.12.1971. [DOI] [PubMed] [Google Scholar]
- 9.Visser M, Bouter LM, McQuillan GM, et al. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 10.Jeppesen J, Hansen TW, Rasmussen S, et al. Insulin resistance, the metabolic syndrome, and risk of incident cardiovascular disease:a population-based study. J Am Coll Cardiol. 2007;49:2112–2119. doi: 10.1016/j.jacc.2007.01.088. [DOI] [PubMed] [Google Scholar]
- 11.Tenenbaum A, Adler Y, Boyko V, et al. Insulin resistance is associated with increased risk of major cardiovascular events in patients with preexisting coronary artery disease. Am Heart J. 2007;153:559–565. doi: 10.1016/j.ahj.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Despres JP. Abdominal obesity as important component of insulin-resistance syndrome. Nutrition. 1993;9:452–459. [PubMed] [Google Scholar]
- 13.Kohrt WM, Kirwan JP, Staten MA, et al. Insulin resistance in aging is related to abdominal obesity. Diabetes. 1993;42:273–281. [PubMed] [Google Scholar]
- 14.Shamsuzzaman AS, Winnicki M, Wolk R, et al. Independent association between plasma leptin and C-reactive protein in healthy humans. Circulation. 2004;109:2181–2185. doi: 10.1161/01.CIR.0000127960.28627.75. [DOI] [PubMed] [Google Scholar]
- 15.Ble A, Windham BG, Bandinelli S, et al. Relation of plasma leptin to C-reactive protein in older adults (from the Invecchiare nel Chianti study) Am J Cardiol. 2005;96:991–995. doi: 10.1016/j.amjcard.2005.05.058. [DOI] [PubMed] [Google Scholar]
- 16.Wallace AM, McMahon AD, Packard CJ, et al. Plasma leptin and the risk of cardiovascular disease in the west of Scotland coronary prevention study (WOSCOPS) Circulation. 2001;104:3052–3056. doi: 10.1161/hc5001.101061. [DOI] [PubMed] [Google Scholar]
- 17.Ouchi N, Kihara S, Funahashi T, et al. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation. 2003;107:671–674. doi: 10.1161/01.cir.0000055188.83694.b3. [DOI] [PubMed] [Google Scholar]
- 18.Matsubara M, Namioka K, Katayose S. Decreased plasma adiponectin concentrations in women with low-grade C-reactive protein elevation. Eur J Endocrinol. 2003;148:657–662. doi: 10.1530/eje.0.1480657. [DOI] [PubMed] [Google Scholar]
- 19.Matsushita K, Yatsuya H, Tamakoshi K, et al. Inverse association between adiponectin and C-reactive protein in substantially healthy Japanese men. Atherosclerosis. 2006;188:184–189. doi: 10.1016/j.atherosclerosis.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 20.Kumada M, Kihara S, Sumitsuji S, et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–89. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 21.Jung CH, Rhee EJ, Choi JH, et al. The relationship of adiponectin/leptin ratio with homeostasis model assessment insulin resistance index and metabolic syndrome in apparently healthy Korean male adults. Korean Diabetes J. 2010;34:237–243. doi: 10.4093/kdj.2010.34.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finucane FM, Luan J, Wareham NJ, et al. Correlation of the leptin:adiponectin ratio with measures of insulin resistance in non-diabetic individuals. Diabetologia. 2009;52:2345–2349. doi: 10.1007/s00125-009-1508-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsubara M, Katayose S, Maruoka S. Decreased plasma adiponectin concentrations in nondiabetic women with elevated homeostasis model assessment ratios. Eur J Endocrinol. 2003;148:343–350. doi: 10.1530/eje.0.1480343. [DOI] [PubMed] [Google Scholar]
- 24.Mente A, Razak F, Blankenberg S, et al. Ethnic variation in adiponectin and leptin levels and their association with adiposity and insulin resistance. Diabetes Care. 2010;33:1629–1634. doi: 10.2337/dc09-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rasmussen-Torvik LJ, Wassel CL, Ding J, et al. Associations of body mass index and insulin resistance with leptin, adiponectin, and the leptin-to-adiponectin ratio across ethnic groups:the Multi-Ethnic Study of Atherosclerosis (MESA) Ann Epidemiol. 2012;22:705–709. doi: 10.1016/j.annepidem.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anand SS, Razak F, Yi Q, et al. C-reactive protein as a screening test for cardiovascular risk in a multiethnic population. Arterioscler Thromb Vasc Biol. 2004;24:1509–1515. doi: 10.1161/01.ATV.0000135845.95890.4e. [DOI] [PubMed] [Google Scholar]
- 27.Wu TL, Tsao KC, Chang CP, et al. Development of ELISA on microplate for serum C-reactive protein and establishment of age-dependent normal reference range. Clin Chim Acta. 2002;322:163–168. doi: 10.1016/s0009-8981(02)00172-9. [DOI] [PubMed] [Google Scholar]
- 28.Steering Committee of the Western Pacific Region of the World Health Organization, the International Obesity Task Force. The Asia-Pacific Perspective: Redefining Obesity and its Treatment. Available at http://www.who.int/nutrition/publications/obesity/09577082_1_1/en/ [Google Scholar]
- 29.Bonora E, Kiechl S, Willeit J, et al. Prevalence of insulin resistance in metabolic disorders: the Bruneck Study. Diabetes. 1998;47:1643–1649. doi: 10.2337/diabetes.47.10.1643. [DOI] [PubMed] [Google Scholar]
- 30.Faggioni R, Feingold KR, Grunfeld C. Leptin regulation of the immune response and the immunodeficiency of malnutrition. FASEB J. 2001;15:2565–2571. doi: 10.1096/fj.01-0431rev. [DOI] [PubMed] [Google Scholar]
- 31.Wolf AM, Wolf D, Rumpold H, et al. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun. 2004;323:630–635. doi: 10.1016/j.bbrc.2004.08.145. [DOI] [PubMed] [Google Scholar]
- 32.Tartaglia LA, Dembski M, Weng X, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 33.Ouchi N, Kihara S, Arita Y, et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 34.Baskin DG, Blevins JE, Schwartz MW. How the brain regulates food intake and body weight: the role of leptin. J Pediatr Endocrinol Metab. 2001;14:1417–1429. [PubMed] [Google Scholar]
- 35.Ceddia RB. Direct metabolic regulation in skeletal muscle and fat tissue by leptin: implications for glucose and fatty acids homeostasis. Int J Obes. 2005;29:1175–1183. doi: 10.1038/sj.ijo.0803025. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Scarpace PJ. The role of leptin in leptin resistance and obesity. Physiol Behav. 2006;88:249–256. doi: 10.1016/j.physbeh.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 37.Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 38.Huang KC, Lin RC, Kormas N, et al. Plasma leptin is associated with insulin resistance independent of age, body mass index, fat mass, lipids, and pubertal development in nondiabetic adolescents. Int J Obes Relat Metab Disord. 2004;28:470–475. doi: 10.1038/sj.ijo.0802531. [DOI] [PubMed] [Google Scholar]
- 39.Satoh N, Naruse M, Usui T, et al. Leptin-to-adiponectin ratio as a potential atherogenic index in obese type 2 diabetic patients. Diabetes Care. 2004;27:2488–2490. doi: 10.2337/diacare.27.10.2488. [DOI] [PubMed] [Google Scholar]
- 40.Norata GD, Raselli S, Grigore L, et al. Leptin:adiponectin ratio is an independent predictor of intima media thickness of the common carotid artery. Stroke. 2007;38:2844–2846. doi: 10.1161/STROKEAHA.107.485540. [DOI] [PubMed] [Google Scholar]
- 41.Matsuda M, Shimomura I, Sata M, et al. Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. J Biol Chem. 2002;277:37487–37491. doi: 10.1074/jbc.M206083200. [DOI] [PubMed] [Google Scholar]
- 42.Ding PYA, Hsu PF, Lu TM. Statin therapy on insulin resistance and plasma level of adiponectin in non-diabetic, hypercholesterolemic patients. Acta Cardiol Sin. 2009;25:183–189. [Google Scholar]