Abstract
Background
The mechanisms responsible for the effects of Ginkgo biloba extract (GbE) are not fully understood. Krüppel-like factor 2 (KLF2), a zinc transcription factor, has vasculoprotective effects if activated. The present study attempted to explore whether GbE may activate KLF2 and its consequences.
Methods
To determine the effects of GbE on endothelial cells, human umbilical vein endothelial cells (HUVECs) were incubated with various concentrations of GbE. KLF2 expression levels were determined by quantitative reverse transcription polymerase chain reaction. Cytoskeleton staining and cell migration assays were performed to determine the effects of KLF2 activation. Moreover, endothelial NO synthase (eNOS) expression levels were detected by PCR and Western blot testing. Nitric oxide (NO) production was also measured with 4,5-diaminofluorescein. A knockdown of KLF2 was performed to identify the role of KLF2 in GbE-induced eNOS expression and NO production.
Results
HUVECs that were incubated with GbE increased KLF2 expression. These cells demonstrated an altered cell morphology, cytoskeleton rearrangement, and inhibited migration activity. Moreover, eNOS expression and NO production increased in a dose-dependent manner when cells were treated with GbE. Correspondingly, silencing of KLF2 in HUVECs decreased eNOS expression and NO production in GbE-treated cells.
Conclusions
GbE significantly activated KLF2 expression and KLF2-related endothelial function, including cytoskeleton rearrangement, inhibition of migration, eNOS activation, and NO production. These findings suggest that GbE may induce a vasculoprotective effect in endothelial cells.
Keywords: Endothelial cells, eNOS, Ginkgo biloba extract, KLF2, NO
INTRODUCTION
Ginkgo biloba extract (GbE), a traditional Chinese medicine, has been used to treat cerebrovascular disease and dementia.1,2 Building evidence suggests that GbE not only acts as a neuroprotective agent, but also provides a vasculoprotective effect. However, clinical observations have been conflicting. The vasculoprotective effect of GbE may be related to its modulation of lipid metabolism, reduction of blood pressure, and amelioration of atherosclerosis.3,4 Additionally, GbE has been shown to have antiplatelet and anticoagulant activity. These vasculoprotective effects of GbE may be related to activation of endothelial nitric oxide (NO) synthase (eNOS) and increased NO production in endothelial cells.5
Endothelial cells line the inner lumen of blood vessels and serve as the interface between blood and vessels. Endothelial dysfunction plays a critical role in the development of atherosclerosis. Krüppel-like factor 2 (KLF2), also referred to as lung Krüppel-like factor (LKLF), belongs to the zinc finger family of transcription factors, and plays a critical role in cellular differentiation and tissue development.6-8 A growing number of studies have suggested that KLF2 is involved in endothelial cell biology. Activation of KLF2 by shear stress may have significant vasculoprotective effects, such as a reduction in adhesion molecular expression, increased anti-thrombotic factor secretion, and enhanced NO production via an upregulation of eNOS activity.9-11 Recent studies have shown that several small molecular compounds, such as statin and resveratrol, can increase KLF2 expression, and subsequently increase eNOS activity and enhance NO production.12,13
In the present study, we investigated whether GbE can increase the eNOS expression and induce NO production via KLF2 activation.
MATERIALS AND METHODS
Cell culture
Human umbilical vein endothelial cells (HUVECs) were obtained from Lonza (Walkersville, MD, USA). Cells were cultured in sterile endothelial growth medium (EGM-2, Lonza, Walkersville, MD, USA) and maintained at 37 °C in a 5% CO2 environment throughout the experiment. HUVECs at 80-90% confluence were used in the experiment and treated with either vehicle (DMSO) or various concentrations of GbE (i.e., 10, 50, and 100 μg/mL) for 24 hours.
Ginkgo biloba extract preparation and storage
GbE originating from Indena (Settala, Italy) was provided by Yung Shin Pharmaceutical Ind. Co. Ltd. (Taiwan, ROC). The Ginkgo biloba were prepared and stored, as earlier described.5 Briefly, Ginkgo biloba, EGb 761, was used for the preparation of the GbE solution. One gram of GbE was dissolved into 10 mL of sterile phosphate-buffered saline (PBS) to make a stock solution with the concentration of 100 mg/mL. Then, the Ginkgo biloba suspension was filtered twice through a standard filter 0.45 μm, whereafter the filtrated solution was frozen in aliquots at -20 °C.
Reverse transcription polymerase Chain reaction (RT-PCR) and quantitative real-time polymerase Chain reaction (Q-PCR/qRT-PCR)
Total RNA was isolated from HUVECs in 3.5-cm culture dishes treated with GbE in the presence of TRIzol reagent (Invitrogen Corp., Carlsbad, CA, USA), as per the manufacturer’s instructions. RNA concentrations were spectrophotometrically established at 260 nm. First-strand cDNA synthesis was performed with 2 μg of total RNA using random hexamers as primers in a final volume of 20 μ L (100 ng/μ L random hexamers, 1 mM dNTPs, 1 U/μL RNasin®, and 5 U/μL Moloney Murine Leukemia Virus Reverse Transcriptase). The reaction was carried out at 42 °C for 90 min. cDNAs encoding glyceraldehyde 3-phosphate dehydrogenase (GAPDH), KLF2, and eNOS were amplified from 2 μL of the cDNA reaction mixture using specific gene primers. The sense and antisense primers for GAPDH, KLF2, and eNOS were as follows: GAPDH: CCTCCCGCTTCGCTCTCTG and GCGCCCAATACGACCAAA-TC; KLF2: CTACACCAAGAGTTCGCATCTG and CCGTGTGCTTTCGGTAGTG; and eNOS: TGATGGCGAAGCGAGTGAAG and ACTCATCCATACACAG GACCC.
To compare the expression levels of KLF2 and eNOS mRNA among various cell lines, real-time PCR analysis was performed using the ABI Prism 7500 Sequence Detection System with the EvaGreen Master Mix (Biogenesis, Taipei, Taiwan). PCR amplification consisted of an initial denaturation step (95 °C for 3 min) and 40 cycles of denaturation (95 °C for 15 s), annealing, and extension (60 °C for 1 min). The forward and reverse primers for the amplification of the cDNA were the same as those used in RT-PCR.
Western blot analysis
Cells were washed in PBS and isolated by scraping. The scraping was performed on ice in RIPA buffer and protease-inhibiting cocktail (Roche Molecular Biochemicals, Almere, Netherlands). Western blot analysis was performed on 20 μg of total protein. Samples were run on 9% SDS-PAGE, electroblotted onto membranes, and incubated with primary antibodies against human eNOS (1:500 dilution; AP11828a; Abgent, San Diego, California, USA), p-eNOS (S1177; 1:1000 dilution; 612392; BD Biosciences, San Jose, California, USA), KLF2 (1:500 dilution; AP14973a; Abgent, San Diego, California, USA), and α-actin (1:3000 dilution; A2228; Chemicon, Temecula, CA). Proteins were detected by enhanced chemiluminescence using peroxidase-labeled luminol as the detection fluid (ECL, Amersham Life Sciences, UK).
Detection of NO by flow cytometry
HUVECs (2 × 106 cells) were seeded into 3.5 cm dishes and incubated with varying doses of GbE for 48 hours. Cells were stained with 1 μg/mL 4,5-diaminofluorescein (DAF-2, Cayman Chemicals, Ann Arbor, MI, USA) for 30 min. Cells were harvested and washed twice with PBS, re-suspended in PBS, and analyzed by flow cytometry.
Wound-healing assay
HUVECs were cultured in a 6-well plate with cell-culture insert (ibidi) and grown in Endothelial Cell Growth-2 medium to a nearly confluent cell monolayer. After overnight seeding, culture inserts were removed and a linear “wound” was introduced in the cell monolayer of each well. The monolayer was then washed twice with PBS to remove debris or detached cells. Cells were then incubated with varying doses of GbE. Cells were photographed under a light microscope (magnification, X40 and X100) at the indicated times. All experiments were performed in triplicate.
Actin staining
To investigate whether GbE-induced KLF2 activation alters cytoskeleton distribution, HUVECs were incubated with DMSO or 100 μg/mL of GbE for 24 hours. Then, staining for F-actin, a cytoskeleton fiber involved in the formation of stress fibers, was performed by using fluorescent conjugates of phalloidin. Briefly, cells were washed twice with PBS and fixed in a 3.7% paraformaldehyde-PBS solution for 10 min at room temperature. After two additional washes with PBS, cells were permeabilized with a solution of 0.1% Triton X-100 in PBS for 3 to 5 min and washed again with PBS. Texas Red-X phalloidin (2 unit/mL) and Alexa Fluor 488 DNase I conjugate (9 μg/ mL) were used to localize filamentous actin (F-actin) and G-actin. Fluorescent dyes were diluted on blocking solution (1% bovine serum albumin and 0.025% saponin in PBS) and added to coverslips for 90 min at room temperature. After three washes with PBS, coverslips were mounted on a microscopy slide with Prolong Gold antifade reagent (Life Technologies). F-actin cytoskeleton imaging was performed with a confocal laser scanning microscope (ZEISS LSM510) at a magnification of X630.
Genetic knockdown using the lentivirus shRNA system
Transfection vectors of short hairpin RNA (shRNA) for KLF2 and Mock were obtained from the National RNAi Core Facility located at the Institute of Molecular Biology/Genomic Research Center, Academia Sinica. Lentiviral particles were generated in 293FT cells grown in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% FBS in an incubator at 37 °C and 5% CO2. Cells were plated to ~2.4 × 106 cells in a 10-cm dish before the day of transfection. 293FT cells were transfected with 6.0 μ g shMock [shLuc (TRCN0000072243) and shKLF2 (TRCN0000020725)], pCMVDR8.91 (5.4 μg), and pMD.G (0.6 μg) in virus LT1 transfection reagent (Mirus Bio, Madison, WI, USA), according to the manufacturer’s instructions. Plasmids were also obtained from the National RNAi Core Facility. Briefly, stock recombinant lentivirus vectors were prepared as stocks and HUVECs were infected with a multiplicity of infection (MOI) of 1 to 2. Transformants were selected with puromycin (2 μg/mL), and the selected cells were used for further experimentation.
Statistical analysis
Statistical analysis was performed with SPSS Version 17 statistics software. ANOVA and the two-tailed Student’s t-test were used for comparisons. A p < 0.05 was considered statistically significant.
RESULTS
GbE upregulated KLF2 expression and induced changes in cell morphology of endothelial cells
GbE was found to upregulate KLF2 expression in a dose-dependent manner (Figure 1A and B). It is well known that shear stress increases KLF2 expression, which alters the morphology of endothelial cells into spindle-shaped cells that are aligned in the direction of blood flow.23 Interestingly, we found that the morphology of GbE-incubated cells also changed from their typical cobblestone-shaped cells to spindle-shaped cells (Figure 2A).
Figure 1.
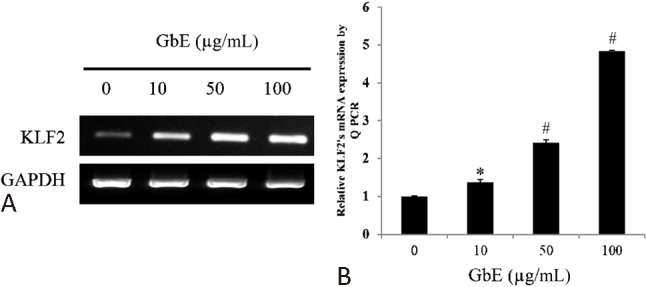
Ginkgo biloba extraction (GbE) upregulated the expression of Krüppel-like factor 2 (KLF2). Human umbilical endothelial cells (HUVECs) incubated with dimethyl sulfoxide (DSMO) or 10, 50, and 100 mM of GbE for 24 h. mRNA expression in endothelial cells was assessed by (A) polymerase chain reaction (PCR) and (B) quantitative real time PCR. GbE significantly increased KLF2 expression in a dose-dependent manner. Data expressed as mean ± SEM. * p < 0.01 and # p < 0.001 vs. control.
Figure 2.
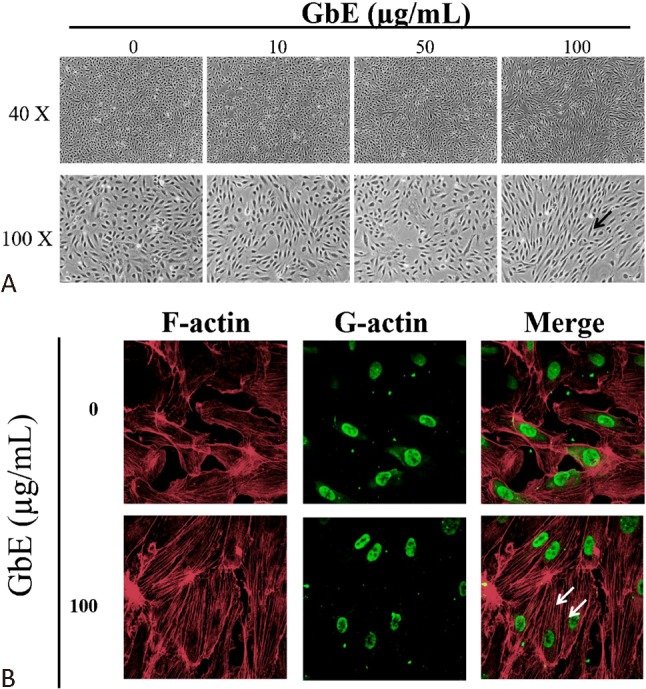
Ginkgo biloba extraction (GbE) altered cell morphology and cytoskeleton arrangement. Human umbilical endothelial cells (HUVECs) were treated with different concentrations of GbE or DMSO as control for 24 h. (A) Cell morphology gradually shifted from a typical cobble stone shape to a spindle shape (black arrow) when cells were incubated with increasing GbE concentrations. (B) Immunofluorescence Texas Red-X phalloidin staining for F-actin stress fibers revealed cytoskeletal rearrangement and increased stress fiber formation (white arrow) after treatment with 100 mg/mL of GbE. Alexa Fluor 488 DNase I conjugate was used for G actin staining to localize the nucleus. Images were acquired at 40X and 100X magnification. A confocal laser scanning microscope was used for the 400X magnification (n = 3).
GbE altered endothelial cell cytoskeleton distribution and inhibited cell migration
KLF2 activation has been shown to involve cytoskeleton distribution and contributes to stress fiber formation when endothelial cells are exposed to shear stress. GbE-treated cells exhibited a reorganization of stress fibers and stress fiber formation (Figure 2B). Moreover, recent studies demonstrated that an overexpression of KLF2 may inhibit endothelial cell migration. To evaluate whether GbE induction of KLF2 affects migration, a wound-healing assay were performed. It was found that endothelial cells incubated with GbE had inhibited cell migration (Figure 3).
Figure 3.
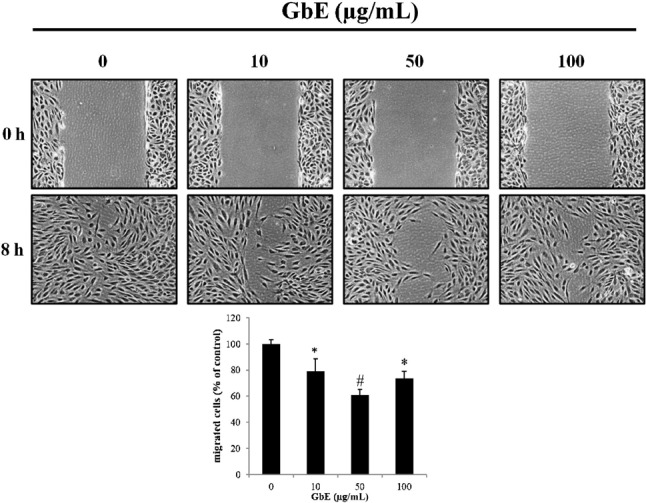
Effects of GbE on endothelial cell migration. Representative examples of the wound-healing assay 8 h after HUVECs were treated with either DMSO or different concentrations of GbE (i.e., 10, 50, and 100 μg/mL). Cells incubated with GbE demonstrated a decrease in migration compared with control. A microscope was used to obtain a magnification of 100X. Data are representative of a sample size of 3. * p < 0.05 and # p < 0.01 vs. control.
GbE increased eNOS expression and NO production
In endothelial cells, KLF2 activation plays a critical role in the stimulation of eNOS activity. We found that GbE increased eNOS expression in a dose-dependent manner both at the RNA and protein level (Figure 4A and B). Furthermore, NO production was also increased in a dose-dependent manner in GbE-treated cells (Figure 4C), which is consistent with the upregulated eNOS expression.
Figure 4.
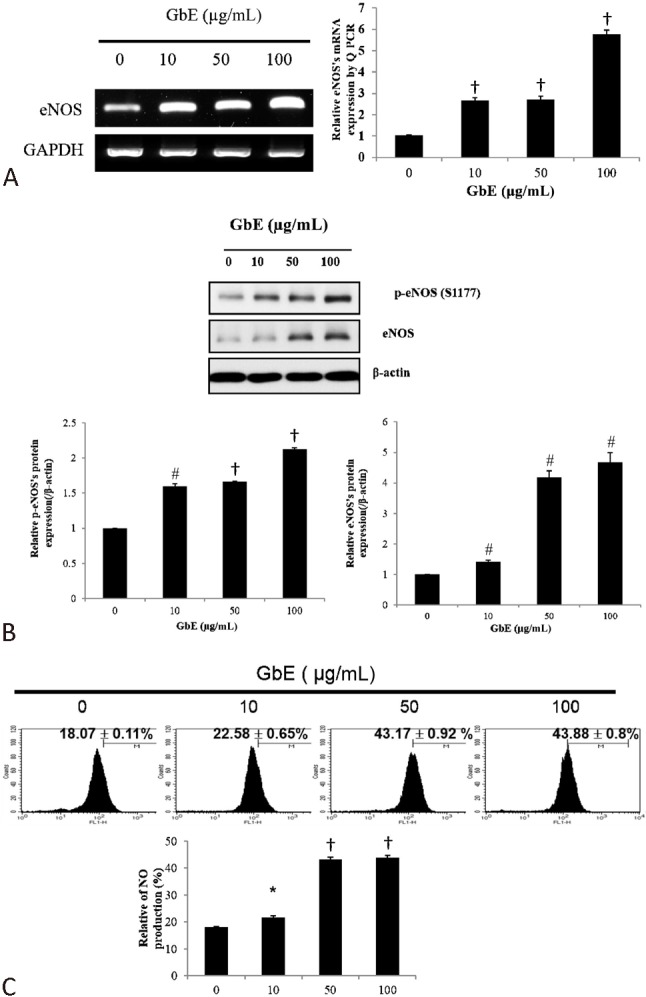
GbE increased endothelial NO synthase (eNOS) expression at the RNA and protein level, which subsequently increased nitric oxide (NO) production. HUVECs were treated with DMSO (control) or GbE (10, 50, or 100 μg/mL) for 24 h. Cells were collected and eNOS mRNA and protein expression levels were determined with PCR and Western blot, respectively. eNOS expression levels increased in a dose-dependent manner at the (A) mRNA and (B) protein level, which coincided with increases in (C) NO production, as detected by flow cytometry with 4,5-diaminofluorescein. Data are presented as mean ± SD of experiments performed in triplicate. * p < 0.05 and # p < 0.001 vs. control.
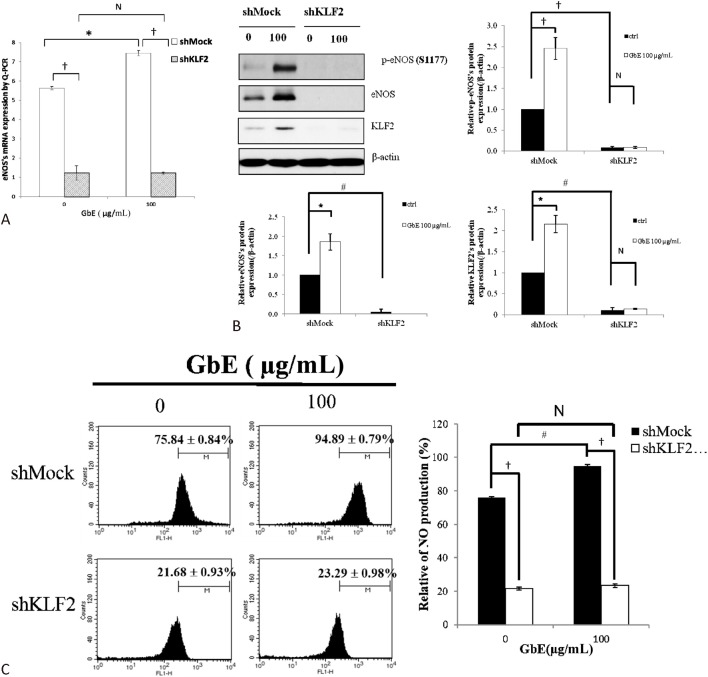
KLF2 expression was critical for eNOS induction and NO production in GbE-treated HUVECs
KLF2 has been previously shown to induce eNOS expression and subsequently NO production when HUVECs are applied to shear stress or statin. HUVECs transfected with shMock had significantly greater in total or phosphorylation form eNOS expression levels and NO production when treated with GbE. However, KLF2 silenced cells had lower eNOS expression level and NO production, even when incubated with GbE (Figure 5). Thus, KLF2 appears to be critical for GbE-induced eNOS activation and NO production.
Figure 5.
Knockdown of KLF2 decreases eNOS expression and NO production in GbE-treated endothelial cells. HUVECs were transfected with either short hairpin Klf2 (shKLF2) or a mock vector (control). Transfected cells were treated with DMSO or 100 μg/mL of GbE. Real time PCR and Western blot were used to determine eNOS RNA and protein expression, respectively. Furthermore, NO production was measured by 4,5-diaminofluorescein. shMock-infected HUVECs treated with GbE had increased eNOS expression at the (A) RNA and (B) protein level, which was blocked by KLF2 silencing. Additionally, (C) NO production also increased when incubated with GbE and abolished when KLF2 was knocked down. * p < 0.05; # p < 0.01, and † p < 0.001 vs. control. Data are presented as mean ± SEM.
DISCUSSION
In the past decade, GbE has gained popularity for the treatment of dementia and hypertension in the clinical setting.14-16 From a clinical perspective, its use is controversial for the treatment of cardiovascular disease. Additionally, despite its growing use, the molecular mechanisms responsible for the beneficial effects of GbE are not yet fully understood. Recently, KLF2 activation was found to have a vasculoprotective effect when endothelial cells are exposed to shear stress. The activation of KLF2 can significantly increase eNOS expression, and subsequently NO production. NO, an important mediator of endothelial cell function, can inhibit the adhesion and migration of leukocytes, prevent platelet aggregation, and decrease vessel tone through smooth muscle cells relaxation. Most of NO production in endothelial cells is related to the activity of eNOS.17 It is well-known that eNOS can be activated by several factors, such as shear stress. Recently, an increasing number of studies have also demonstrated that shear stress increases eNOS expression by enhancing KLF2 activity.10,18 The mechanisms behind the effects of KLF2 on endothelial cells had been widely explored, and the role of KLF2 on the functioning of endothelial cells had been clarified. Additionally, subsequent studies were performed to determine what small molecules can stimulate KLF2 activity, and in turn, enhance endothelial cell function and inhibit the progression of atherosclerosis. Several well-known medicinal compounds, such as resveratrol and statin, have been shown to possess vasculoprotective effects in clinical practice, as well as suggested to be involved in the activation of KLF2 expression.13 Gracia-Sancho et al. demonstrated that resveratrol increased KLF2 expression through sirtuin 1 activation in human endothelial cells, where treated cells had a vasculoprotective phenotype.12 Cui and colleagues reported that grape seed proanthocyanidin extracts potentially increase eNOS activity, which is mediated through KLF2 activation.19 In the present study, we found that GbE can increase the expression of KLF2, which is then followed by enhanced eNOS expression and NO production. These findings point to a novel mechanism by which GbE may exert its vasculoprotective effects.
In a previous study by Parmar and colleagues, it was demonstrated that shear stress activates KLF2 through a mitogen-activated protein kinase 5/extracellular signal-regulated kinase 5/myocyte enhancer factor 2 [MEK5/ERK5/myocyte enhancer factor 2(MEF2)] signalling pathway.20 Additionally, shear stress appears to also enhance the eNOS-mediated phosphatidylinositol 3′-kinase (PI3K)/AKT pathway.21,22 Recently, Sako et al. showed that angiopoietin 1 (Ang1)/Tie2 enhances MEF2 activity via a PI3K/AKT pathway, and in turn, induces KLF2 expression in endothelial cells.23 These studies suggested that the PI3K/AKT pathway may be involved in the regulation of KLF2. Additionally, Koltermann et al. demonstrated that GbE increases eNOS via the PI3K/AKT pathway.5 Thus, we hypothesize that GbE activates KLF2, and in turn, increases eNOS expression. The present study demonstrated for the first time that KLF2 acts as a positive regulator of eNOS expression, when endothelial cells are exposed to GbE. HUVECs treated with GbE can induce KLF2 expression and modulate KLF2-related endothelial cell function, cytoskeleton rearrangement, cell migration, and NO production. Furthermore, we demonstrated that GbE increases eNOS expression at the RNA and protein level via an upregulation of KLF2 expression. Furthermore, after silencing KLF2 expression in HUVECs, there was a decrease in eNOS expression and NO production, even when cells were treated with GbE. Our findings suggest that KLF2 plays a critical role in GbE-induced eNOS activation and NO production.
Lastly, a large number of studies demonstrated that linear shear stress can alter cell morphology and inhibit cell migration via KLF2 activation. Dekker and colleagues demonstrated that prolonged KLF2 overexpression can lead to morphological changes in HUVECs and inhibit their migration activity which is unrelated to shear stress exposure.24 Interestingly, we also found that HUVECs incubated with GbE for 24 hours had alterations in cellular morphology, specifically cells went from a typical cobblestone shape to a spindle form. F-actin, an indicator of cytoskeleton distribution, also increased unidirectional stress fiber formation. Moreover, we found that the migratory activity of treated HUVECs was inhibited. These findings suggest that, in addition to eNOS activation, GbE-induced KLF2 activation results in other changes in endothelial cells. Nonetheless, the mechanisms by which GbE alters the migration of endothelial cells were not explored in the present study, and therefore, further investigation is warranted.
CONCLUSIONS
In conclusion, we revealed that GbE may potentially activate KLF2 transcription, resulting in increased eNOS expression and NO production. Activation of KLF2 also results in cytoskeleton rearrangement and inhibits HUVEC migration. These observations point to a possible molecular mechanism by which GbE induces vasculoprotective effects in endothelial cells.
Acknowledgments
This work was supported, in part, by a grant from the Tri-Service General Hospital (TSGH-C95-5-S03).
DISCLOSURE
The authors have no competing financial interests to declare.
REFERENCES
- 1.Ahlemeyer B, Krieglstein J. Pharmacological studies supporting the therapeutic use of Ginkgo biloba extract for Alzheimer’s disease. Pharmacopsychiatry. 2003;36:S8–S14. doi: 10.1055/s-2003-40454. [DOI] [PubMed] [Google Scholar]
- 2.Pietri S, Maurelli E, Drieu K, Culcasi M. Cardioprotective and anti-oxidant effects of the terpenoid constituents of ginkgo biloba extract (EGb 761) J Mol Cell Cardiol. 1997;29:733–742. doi: 10.1006/jmcc.1996.0316. [DOI] [PubMed] [Google Scholar]
- 3.Jezova D, Duncko R, Lassanova M, et al. Reduction of rise in blood pressure and cortisol release during stress by ginkgo biloba extract (EGb 761) in healthy volunteers. J Physiol Pharmacol. 2002;53:337–348. [PubMed] [Google Scholar]
- 4.Liebgott T, Miollan M, Berchadsky Y, et al. Complementary cardioprotective effects of flavonoid metabolites and terpenoid constituents of ginkgo biloba extract (EGb 761) during ischemia and reperfusion. Basic Res Cardiol. 2000;95:368–377. doi: 10.1007/s003950070035. [DOI] [PubMed] [Google Scholar]
- 5.Koltermann A, Hartkorn A, Koch E, et al. Ginkgo biloba extract EGb 761 increases endothelial nitric oxide production in vitro and in vivo. Cell Mol Life Sci. 2007;64:1715–1722. doi: 10.1007/s00018-007-7085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson KP, Kern CB, Crable SC, Lingrel JB. Isolation of a gene encoding a functional zinc finger protein homologous to erythroid Kruppel-like factor:identification of a new multigene family. Mol Cell Biol. 1995;15:5957–5965. doi: 10.1128/mcb.15.11.5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wani MA, Wert SE, Lingrel JB. Lung Kruppel-like factor, a zinc finger transcription factor, is essential for normal lung development. J Biol Chem. 1999;274:21180–21185. doi: 10.1074/jbc.274.30.21180. [DOI] [PubMed] [Google Scholar]
- 8.Chiplunkar AR, Lung TK, Alhashem Y, et al. Kruppel-like factor 2 is required for normal mouse cardiac development. PLoS One. 2013;8:e54891. doi: 10.1371/journal.pone.0054891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin Z, Kumar A, SenBanerjee S, et al. Kruppel-like factor 2 (KLF2) regulates endothelial thrombotic function. Circ Res. 2005;96:e48–e57. doi: 10.1161/01.RES.0000159707.05637.a1. [DOI] [PubMed] [Google Scholar]
- 10.SenBanerjee S, Lin Z, Atkins GB, et al. KLF2 Is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med. 2004;199:1305–1315. doi: 10.1084/jem.20031132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atkins GB, Jain MK. Role of Kruppel-like transcription factors in endothelial biology. Circ Res. 2007;100:1686–1695. doi: 10.1161/01.RES.0000267856.00713.0a. [DOI] [PubMed] [Google Scholar]
- 12.Gracia-Sancho J, Villarreal G, Jr., Zhang Y, García-Cardeña G. Activation of SIRT1 by resveratrol induces KLF2 expression conferring an endothelial vasoprotective phenotype. Cardiovasc Res. 2010;85:514–519. doi: 10.1093/cvr/cvp337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parmar KM, Nambudiri V, Dai G, et al. Statins exert endothelial atheroprotective effects via the KLF2 transcription factor. J Biol Chem. 2005;280:26714–26719. doi: 10.1074/jbc.C500144200. [DOI] [PubMed] [Google Scholar]
- 14.Mahadevan S, Park Y. Multifaceted therapeutic benefits of ginkgo biloba L.: chemistry, efficacy, safety, and uses. J Food Sci. 2008;73:R14–R19. doi: 10.1111/j.1750-3841.2007.00597.x. [DOI] [PubMed] [Google Scholar]
- 15.Bone KM. Potential interaction of ginkgo biloba leaf with antiplatelet or anticoagulant drugs: what is the evidence? Mol Nutr Food Res. 2008;52:764–771. doi: 10.1002/mnfr.200700098. [DOI] [PubMed] [Google Scholar]
- 16.Weinmann S, Roll S, Schwarzbach C, et al. Effects of ginkgo biloba in dementia: systematic review and meta-analysis. BMC Geriatr. 2010;10:14. doi: 10.1186/1471-2318-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang KC, Sasano T, Wang YC, Huang SKS. Nitric oxide synthase 1 adaptor protein, an emerging new genetic marker for QT prolongation and sudden cardiac death. Acta Cardiol Sin. 2013;29:217. [PMC free article] [PubMed] [Google Scholar]
- 18.Dekker RJ, van Thienen JV, Rohlena J, et al. Endothelial KLF2 links local arterial shear stress levels to the expression of vascular tone-regulating genes. Am J Pathol. 2005;167:609–618. doi: 10.1016/S0002-9440(10)63002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui X, Liu X, Feng H, et al. Grape seed proanthocyanidin extracts enhance endothelial nitric oxide synthase expression through 5’-AMP activated protein kinase/Surtuin 1-Krupple like factor 2 pathway and modulate blood pressure in ouabain induced hypertensive rats. Biol Pharm Bull. 2012;35:2192–2197. doi: 10.1248/bpb.b12-00598. [DOI] [PubMed] [Google Scholar]
- 20.Parmar KM, Larman HB, Dai G, et al. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest. 2006;116:49–58. doi: 10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dimmeler S, Fleming I, Fisslthaler B, et al. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 22.Fisslthaler B, Dimmeler S, Hermann C, et al. Phosphorylation and activation of the endothelial nitric oxide synthase by fluid shear stress. Acta Physiol Scand. 2000;168:81–88. doi: 10.1046/j.1365-201x.2000.00627.x. [DOI] [PubMed] [Google Scholar]
- 23.Sako K, Fukuhara S, Minami T, et al. Angiopoietin-1 induces Kruppel-like factor 2 expression through a phosphoinositide 3-kinase/AKT-dependent activation of myocyte enhancer factor 2. J Biol Chem. 2009;284:5592–5601. doi: 10.1074/jbc.M806928200. [DOI] [PubMed] [Google Scholar]
- 24.Dekker RJ, Boon RA, Rondaij MG, et al. KLF2 provokes a gene expression pattern that establishes functional quiescent differentiation of the endothelium. Blood. 2006;107:4354–4363. doi: 10.1182/blood-2005-08-3465. [DOI] [PubMed] [Google Scholar]