Abstract
Background
Pulmonary arterial hypertension (PAH) is a serious and progressive disorder that can result in right ventricular (RV) dysfunction and mortality. Consequently, it is important to monitor RV function during management of PAH. The aim of this study was to investigate the change in RV function by echocardiography before and after disease-specific therapy.
Methods
We recruited 31 PAH patients with functional class (FC) III or IV. All the patients received a comprehensive assessment before disease-specific therapy was administered, including observation of clinical symptoms, 6-min walk distance (6MWD), serum brain natriuretic peptide (BNP) level, and transthoracic echocardiography. The assessment was repeated 12 weeks after therapy.
Results
Twenty-eight patients with a mean age of 40 years completed the study, of whom 82% were women. We found that the etiologies were mainly connective tissue disease-associated and idiopathic PAH. Of the patients in our study, 36% received endothelin receptor antagonist and 64% received phosphodiesterase-5 inhibitor. There was a significant improvement in FC after disease-specific therapy (p < 0.001). The 6MWD increased from 326 to 403 m (p < 0.001), and the serum BNP level decreased from 242 to 130 pg/mL (p = 0.008) after treatment. Echocardiography showed significant reduction in the right atrial and RV areas, pulmonary artery pressure, RV free wall thickness, and inferior vena cava diameter. The myocardial performance index and left ventricular eccentricity index were significantly reduced after therapy.
Conclusions
For PAH patients in our study, disease-specific therapy for 12 weeks resulted in an improvement in FC, 6MWD, serum BNP levels, and RV function.
Keywords: Disease-specific therapy, Pulmonary arterial hypertension, Right ventricular function
INTRODUCTION
Since the Second World Symposium on Pulmonary Hypertension in 1998, pulmonary hypertension (PH) has been classified into 5 clinical groups as follows: group 1, pulmonary arterial hypertension (PAH); group 2, PH caused by left heart disease; group 3, PH caused by chronic lung disease and/or hypoxemia; group 4, chronic thromboembolic PH; and group 5, PH with unclear and/or multifactorial mechanisms.1 PAH is a serious and often progressive disorder that results in right ventricular (RV) dysfunction and impairment in activity tolerance, and may lead to right heart failure and mortality.2,3 Dramatic advancements in disease-specific therapy for PAH have been made over the past two decades. Currently, the following 3 classes of drugs have proven beneficial in PAH treatment: endothelin receptor antagonist (ERA), prostanoids, and phosphodiesterase-5 inhibitors (PDE5i).4-6 PAH is an incurable but manageable disorder at present.
Several parameters are commonly used to evaluate the clinical response to treatment and prognosis in PAH, such as the World Health Organization (WHO) functional class (FC), 6-min walk distance (6MWD), serum brain natriuretic peptide (BNP) level, tricuspid annular plane systolic excursion (TAPSE) and pericardial effusion by echocardiography, and right atrial (RA) pressure and cardiac index (CI) by right heart catheterization (RHC).5,6 RV dysfunction is an indicator of disease progression, so it is important to evaluate and monitor RV function during management of PAH.2,3 Echocardiography is an effective modality for diagnostic evaluation and follow-up examination after treatment of PAH.7,8 The aim of this study was to investigate the change in RV function using echocardiography before and after disease-specific therapy.
MATERIALS AND METHODS
Subjects
We recruited patients with PAH who visited the cardiovascular clinic of Chang Gung Memorial Hospital between August 2004 and March 2012. The inclusion criteria were PAH patients with WHO FC III or IV, who agreed to receive disease-specific therapy. The etiologies of the PAH patients included idiopathic PAH (IPAH), connective tissue disease (CTD)-associated PAH, congenital heart disease (CHD)-associated PAH, and portopulmonary hypertension. The disease-specific therapies included ERA (e.g., bosentan and ambrisentan), prostanoids (e.g., inhaled iloprost, intravenous epoprostenol or ilomedin, or subcutaneous remodulin), or PDE-5i (e.g., sildenafil). The exclusion criteria were as follows: PH other than group 1 PAH, not receiving disease-specific therapy, age < 18 years, or inability to undergo echocardiographic follow-up examination or complete 12 weeks of disease-specific therapy.
We enrolled 31 consecutive patients with PAH. Three patients withdrew from the study: 1 because of death from rapid progression of right heart failure within 2 weeks after start of treatment; 1 because of schizophrenia; and 1 because of Down syndrome. All the patients received a comprehensive assessment before disease-specific therapy was administered, including clinical symptoms, FC, functional capacity by 6MWD, cardiac biomarker BNP level, and transthoracic echocardiography. According to the National Health Bureau (NHB) in Taiwan, all 3 classes of drugs can be used in IPAH treatment.9 The choice of medication in this study was based on the following two factors: first, the route of drug administration (oral form followed by inhalation and subcutaneous or intravenous medication as the choice for patients’ convenience); second, improved clinical outcomes such as symptoms, functional capacity, hemodynamics, and survival during clinical trials.10 Most drugs have to be approved by the NHB before use in IPAH patients. For CTD-associated PAH, only PDE5i sildenafil is approved for use. For all the other types of PAH, such as CHD-associated PAH or portopulmonary hypertension, no disease-specific drug has been approved by the NHB, and patients have to pay for the drugs (Revatio was approved to be used in CHD with Eisenmenger syndrome since August 1, 2013). We selected the PDE5i sildenafil because of economic concerns. The comprehensive assessment of clinical responses was repeated 12 weeks after therapy. This study was approved by the Chang Gung Memorial Hospital Institutional Review Board, and informed consent was obtained from each patient.
Clinical symptoms, FC, functional capacity, and cardiac biomarker evaluation
All of the patients were followed up at cardiovascular clinics. FC and clinical symptoms of dyspnea, fatigue, chest pain, near syncope, or syncope were evaluated during monthly follow-up examinations. The functional capacity of each patient was assessed by 6MWD. This was performed by technicians in the chest department who encouraged the patients during the test. Serum BNP level was measured using a commercial kit (Triage BNP Test, Biosite Incorporated, San Diego, CA, USA).
Echocardiographic and Doppler measurements
Transthoracic 2-dimensional (2D) echocardiography was performed using a GE ultrasound system (Vivid E9, GE Vingmed Ultrasound, Horten, Norway). The echocardiography was performed by the same physician (W-JH) who was blinded to the patients’ clinical details. The echocardiographic parameters of left ventricular (LV) and RV functions were recorded. For each variable, 3 representative beats were measured, and the mean results were obtained. The echocardiographic parameters of the right heart were analyzed as follows. RA area was measured by tracing the endocardium from the lateral aspect of the tricuspid annulus to the septal aspect at end-systole in the apical 4-chamber view. RV basal diameter was measured at end-diastole using 2D echocardiography in the apical 4-chamber view. RV end-diastolic area (EDA) and end-systolic area (ESA) were measured by tracing the RV endocardium from the medial annulus along the septum, apex, free wall, and then back to the lateral annulus in the apical 4-chamber view. The RV fractional area change (RVFAC) defined as RVFAC = (EDA - ESA)/ EDA × 100%. The peak tricuspid regurgitation jet velocity (TRV) was obtained using continuous-wave Doppler from the apical view. The tricuspid regurgitation pressure gradient (TRPG) was calculated using a simplified Bernoulli equation as TRPG = 4 × TRV2. The systolic pulmonary artery pressure (SPAP) was obtained by the summation of TRPG and RA pressure.11 The RA pressure was estimated by the diameter and inspiratory collapse of the inferior vena cava (IVC) from the subcostal view.8 The mean pulmonary artery pressure (MPAP) was calculated as MPAP = SPAP × 0.61 + 2 mm Hg.12,13 RV ejection time, velocity, and velocity-time integral (VTI) were measured at the RV outflow tract (RVOT) using pulsed-wave Doppler in the parasternal short-axis view. RV free wall thickness was measured at end-diastole by 2D or M-mode echocardiography in the subcostal view. The accuracy of measurements was maximized by excluding trabeculations, papillary muscle, and epicardial fat. Minimal IVC diameter was measured with the long-axis view of the IVC proximal to the junction of the hepatic veins and approximately 0.5-3.0 cm to the ostium of RA during respiration and a sniff in the subcostal view.8 TAPSE, the distance of systolic excursion at the lateral aspect of the tricuspid annulus along its longitudinal plane, was measured using M-mode echocardiography in the apical 4-chamber view.14 The maximal systolic velocity at the lateral aspect of the tricuspid annulus (S’) was measured using pulsed tissue Doppler in the apical 4-chamber view.15 The RV myocardial performance index (MPI) was measured using pulsed tissue Doppler at the lateral tricuspid annulus or pulsed-wave Doppler of the tricuspid inflow and RVOT. MPI was calculated as (tricuspid valve closing to opening time - RV ejection time)/RV ejection time. This was also expressed as (isovolumic contraction time + isovolumic relaxation time)/ejection time.8,16 The ratio of TRV and VTI at RVOT was calculated. This ratio was positively correlated with the pulmonary vascular resistance (PVR).17,18
The echocardiographic parameters of the left heart were analyzed as follows. Left atrial (LA) area and LV EDA and ESA were obtained by a method similar to that for the RA and RV measurements of area in the apical 4-chamber view. LA and LV end-diastolic and end-systolic diameters were obtained using M-mode echocardiography from the parasternal long-axis view. Mitral E- and A-waves, and VTI were measured using pulsed-wave Doppler of mitral inflow signals in the apical 4-chamber view. The diameter of the LV outflow tract (LVOT) was measured at the annulus from the inner edge to edge, utilizing zoom images in the parasternal long-axis view. The velocity and VTI at the LVOT were measured using pulsed-wave Doppler in the apical 5-chamber view. The left ventricular eccentricity index (LVeI) is a ratio of the minor-axis diameter parallel to the septum and the minor-axis diameter perpendicular to the septum in the parasternal short-axis views of the LV at the level of the chordae tendineae at end-systole and end-diastole.19 Stroke volume (SV) was calculated by multiplying the cross-sectional area and VTI of the LVOT. Cardiac output (CO) was obtained by multiplying SV and heart rate. CI was derived by dividing CO by body surface area. The pericardial effusion size was evaluated by parasternal long- and short-axis views and graded as follows: absent (score = 0), trace (score = 1; separation of posterior pericardial layers in both systole and diastole), small (score = 2; diastolic separation < 1 cm), moderate (score = 3; diastolic separation 1-2 cm), or large (score = 4; diastolic separation > 2 cm).20
Hemodynamics of cardiac catheterization
Both left and right heart catheterizations were performed in IPAH. Hemodynamic indices including mean RA pressure, SPAP, MPAP, pulmonary arterial wedge pressure (PAWP), PVR, systemic vascular resistance, CO, and CI were obtained. CO was measured by the thermodilution method, except for use of the Fick method in CHD. The acute vasoreactivity test with nitric oxide inhalation was performed during cardiac catheterization to evaluate the vasodilator response. For etiologies of PAH other than IPAH, cardiac catheterization was performed only in patients with an uncertain diagnosis after a series of noninvasive examinations or absence of tricuspid regurgitation (TR) on echocardiography and after estimation of PAP could not be obtained.
Statistical analyses
Data were expressed as mean ± SD for continuous variables and number of subjects (%) for categorical variables. Variables before and after disease-specific therapy were compared using the nonparametric Wil-coxon signed-rank test. The number of patients with absence or presence of pericardial effusion after therapy was analyzed using McNemar’s test. A p < 0.05 was considered statistically significant.
RESULTS
The demographic characteristics of the patients are shown in Table 1. There were 28 patients with PAH who completed the study, of whom 82% were women. The mean age of the patients was 40 years (range, 25-70 years). The etiologies of patient PAH were mainly CTD and IPAH. Among the patients, 96% had FC III or IV. The mean 6MWD and serum BNP levels were 326 m and 242 pg/mL, respectively. Twenty-seven patients with mild or moderate TR had a mean estimated SPAP of 93 mm Hg and MPAP of 58 mm Hg. In 1 patient, SPAP was difficult to obtain because there was no TR. Overall, 15 patients completed RHC. The mean SPAP and MPAP were 95 and 61 mm Hg, respectively, and the PVR was 15 WU. Comorbidities were found in 7 patients, of whom 3 had hypertension, 2 had bronchial asthma, and 2 had pulmonary fibrosis. Thirty-six percent of the patients received ERA, and 64% received PDE5i as disease-specific therapy.
Table 1. Demographic characteristics of the patients with pulmonary arterial hypertension .
| Variable | n (%) or mean ± SD |
| Patients, n | 28 |
| Sex, female | 23 (82) |
| Age, years | 40 ± 12 |
| Etiology of PAH | |
| Idiopathic | 8 (29) |
| Connective tissue disease | 14 (50) |
| SLE | 10 (36) |
| Scleroderma | 2 (7) |
| Other | 2 (7) |
| Congenital heart disease | 4 (14) |
| ASD | 2 (7) |
| VSD | 2 (7) |
| Portopulmonary | 2 (7) |
| WHO functional class | |
| II | 1 (4) |
| III | 22 (75) |
| IV | 6 (21) |
| 6MWD, m | 326 ± 90 |
| BNP, pg/mL | 242 ± 265 |
| O2 saturation*, % | 90 ± 8 |
| SBP, mm Hg | 118 ± 17 |
| SPAP#, mm Hg | 93 ± 31 |
| MPAP#, mm Hg | 58 ± 19 |
| Hemodynamics by RHC† | |
| RAP, mm Hg | 10 ± 7 |
| SPAP, mm Hg | 95 ± 29 |
| MPAP, mm Hg | 61 ± 17 |
| PAWP, mm Hg | 10 ± 3 |
| CO, L/min | 3.59 ± 0.95 |
| CI, L·min-1·m-2 | 2.33 ± 0.66 |
| PVR, WU | 15.02 ± 4.83 |
| Comorbidities | |
| Hypertension | 3 (11) |
| Asthma | 2 (7) |
| Pulmonary fibrosis | 2 (7) |
| Disease-specific therapy | |
| Endothelin receptor antagonists | 10 (36) |
| Bosentan | 9 (32) |
| Ambrisentan | 1 (4) |
| Phosphodiesterase-5 inhibitor | |
| Sildenafil | 18 (64) |
Data are expressed as mean ± SD for continuous variables and as a number with percentage for categorical variables.
* Data obtained by pulse oximeter; # n = 27, data obtained by echocardiography; † n = 15, data obtained by right heart catheterization.
ASD, atrial septal defect; BNP, brain natriuretic peptide; CI, cardiac index; CO, cardiac output; MPAP, mean pulmonary artery pressure; PAH, pulmonary arterial hypertension; PAWP, pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure; RHC, right heart catheterization; SBP, systolic blood pressure; SLE, systemic lupus erythematosis; SPAP, systolic pulmonary artery pressure; VSD, ventricular septal defect; WHO, World Health Organization; 6MWD, 6-min walk distance.
FC, 6MWD, and serum BNP levels during follow-up examination
There was a significant improvement in FC after disease-specific therapy (Figure 1). Twenty-two of 28 patients had improvement in FC after therapy (p < 0.001). Of the 22 patients, 19 improved by 1 class and 3 improved by 2 classes after 12 weeks of treatment. Six patients remained in the same FC without improvement or deterioration. There was a significant increase in 6MWD from 326 to 403 m after therapy (p < 0.001; Figure 2). In addition, the serum BNP level decreased from 242 to 130 pg/mL (p = 0.008; Figure 3).
Figure 1.
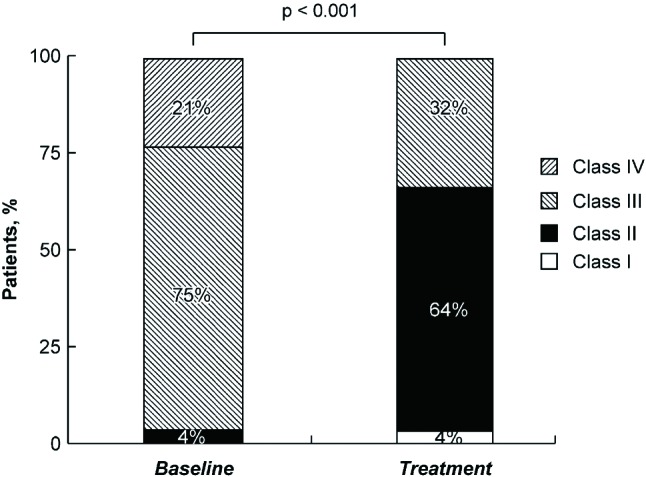
Improvement in functional class of patients with pulmonary arterial hypertension after disease-specific therapy.
Figure 2.
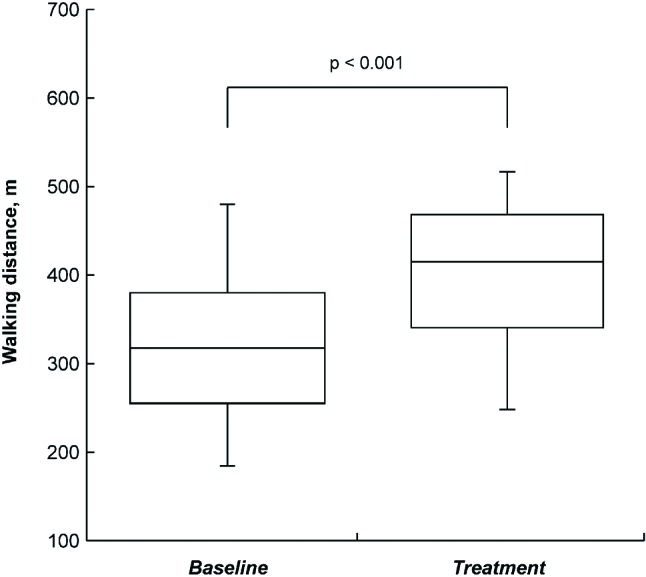
Increase in 6-min walk distance in the patients with pulmonary arterial hypertension after disease-specific therapy (median distance, 327 m vs. 423 m, p < 0.001).
Figure 3.

Decrease in serum brain natriuretic peptide levels in the patients with pulmonary arterial hypertension after disease-specific therapy (mean level, 242 pg/mL vs. 130 pg/mL, p = 0.008).
Echocardiographic and Doppler measurements during follow-up examination
The changes in the echocardiographic parameters of the right heart at baseline and after 12 weeks of therapy are shown in Table 2. There were significant reductions in the RA and RV areas. The TRV, TRPG, and estimated SPAP also decreased significantly. There was no significant change in RVFAC after treatment. The reduction in the RV free wall thickness and IVC diameter were significant. An increasing trend in TAPSE and decreasing trend in tissue Doppler MPI were additionally noted. However, the change in tissue Doppler maximal systolic velocity at the tricuspid annulus was insignificant. The MPI by pulsed-Doppler (Tei index) was significantly reduced after therapy. The significant reduction in the ratio of TRV/VTIRVOT implied a decrease in PVR after treatment.
Table 2. Echocardiographic parameters of the right heart in pulmonary arterial hypertension at baseline and after treatment .
| Variable | Baseline | After treatment | p value |
| RA area, cm2 | 24 ± 8 | 21 ± 8 | 0.010 |
| RV basal diameter, mm | 43 ± 8 | 41 ± 9 | 0.207 |
| RVEDA, cm2 | 22 ± 7 | 20 ± 7 | 0.004 |
| RVESA, cm2 | 17 ± 6 | 15 ± 6 | 0.005 |
| RVFAC, % | 25 ± 9 | 27 ± 12 | 0.202 |
| TRV, m/s | 4.53 ± 0.74 | 4.24 ± 0.71 | 0.003 |
| TRPG, mm Hg | 84 ± 30 | 73 ± 27 | 0.003 |
| SPAP, mm Hg | 93 ± 31 | 80 ± 28 | 0.001 |
| RVOT velocity, m/s | 0.68 ± 0.24 | 0.75 ± 0.30 | 0.064 |
| RVOT VTI, cm | 11 ± 6 | 13 ± 9 | 0.029 |
| RVOT diameter, mm | 27 ± 6 | 25 ± 5 | 0.079 |
| RV thickness, mm | 8 ± 2 | 7 ± 2 | 0.048 |
| Minimal IVC diameter, mm | 12 ± 5 | 10 ± 5 | 0.046 |
| TAPSE, cm | 1.73 ± 0.59 | 1.89 ± 0.51 | 0.069 |
| S′, cm/s | 10 ± 2 | 11 ± 3 | 0.121 |
| MPI by tissue Doppler | 0.59 ± 0.29 | 0.46 ± 0.10 | 0.070 |
| Tei index | 0.81 ± 0.41 | 0.62 ± 0.35 | 0.004 |
| TRV/VTIRVOT ratio | 0.47 ± 0.18 | 0.39 ± 0.15 | 0.002 |
Values are expressed as mean ± SD.
IVC, inferior vena cava; MPI, myocardial performance index; RA, right atrium; RV, right ventricle; RVEDA, right ventricular end-diastolic area; RVESA, right ventricular end-systolic area; RVFAC, right ventricular fractional area change; RVOT, right ventricular outflow tract; S´, tissue Doppler maximal systolic velocity at the tricuspid annulus; SPAP, systolic pulmonary artery pressure; TRPG, tricuspid regurgitation pressure gradient; TAPSE; tricuspid annular plane systolic excursion; TRV, tricuspid regurgitation velocity; VTI, velocity-time integral.
The changes in echocardiographic parameters of the left heart at baseline and after 12 weeks of therapy are shown in Table 3. The increment in the LV end-diastolic diameter was significant, but the increment in the LV end-diastolic area was only borderline significant. There was a trend toward an increase in LA diameter after treatment. However, the changes in LA area and LV ejection fraction were insignificant. The LVeI was significantly reduced at diastole but insignificantly reduced at systole. The changes in SV, CO, and CI were insignificant. In addition, pericardial effusion was found in 14 patients (50%) at baseline and in 9 patients (32%) after therapy. There was a significant reduction in the pericardial effusion score in the PAH patients after therapy (p = 0.033), but the reduction in the number of patients with pericardial effusion after treatment was insignificant.
Table 3. Echocardiographic parameters of the left heart in pulmonary arterial hypertension at baseline and after treatment .
| Variable | Baseline | After treatment | p value |
| LA diameter, mm | 30 ± 6 | 31 ± 5 | 0.070 |
| LA area, cm2 | 13 ± 6 | 12 ± 5 | 0.568 |
| Mitral inflow E, m/s | 0.52 ± 0.20 | 0.55 ± 0.18 | 0.404 |
| Mitral inflow A, m/s | 0.63 ± 0.16 | 0.60 ± 0.14 | 0.294 |
| Mitral inflow VTI, cm | 12 ± 4 | 14 ± 4 | 0.064 |
| LVOT diameter, mm | 19 ± 2 | 18 ± 2 | 0.568 |
| LVOT velocity, m/s | 0.82 ± 0.25 | 0.84 ± 0.16 | 0.477 |
| LVOT VTI, cm | 14 ± 6 | 15 ± 4 | 0.072 |
| LVEDD, mm | 36 ± 6 | 39 ± 6 | 0.035 |
| LVESD, mm | 20 ± 5 | 21 ± 6 | 0.110 |
| LVEFm, % | 76 ± 9 | 76 ± 9 | 0.964 |
| LVEDA, cm2 | 15 ± 5 | 16 ± 4 | 0.052 |
| LVESA, cm2 | 6 ± 2 | 7 ± 3 | 0.116 |
| LVEFs, % | 75 ± 8 | 73 ± 12 | 0.400 |
| Diastolic LVeI | 1.62 ± 0.41 | 1.42 ± 0.45 | 0.003 |
| Systolic LVeI | 1.70 ± 0.48 | 1.58 ± 0.50 | 0.121 |
| Stroke volume, mL | 38 ± 19 | 41 ± 15 | 0.349 |
| Cardiac output, L/min | 3.22 ± 1.39 | 3.20 ± 1.13 | 0.943 |
| Cardiac index, L·min-1·m-2 | 2.11 ± 0.84 | 2.10 ± 0.72 | 0.962 |
Values are expressed as mean ± SD.
LA, left atrium; LVeI, left ventricular eccentricity index; LVEDA, left ventricular end-diastolic area; LVEDD, left ventricular end-diastolic diameter; LVEFm, left ventricular ejection fraction by M-mode; LVEFs, left ventricular ejection fraction by modified Simpson; LVESA, left ventricular end-systolic area; LVESD, left ventricular end-systolic diameter; LVOT, left ventricular outflow tract; VTI, velocity-time integral.
DISCUSSION
In this study, there were improvements in FC, functional capacity, and RV function after the 12 weeks of disease-specific therapy. Since the introduction of disease-specific therapy in PAH, there have been improvements in symptoms, functional capacity, and survival of patients.10,21,22 This is in line with our study in which there was an improvement in FC and 6MWD after treatment. The drug of choice in our patients depended on the ACC/ESC guidelines and reimbursement by the NHB.5,6,9 Therefore, ERA was mainly used to treat IPAH. Only 2 patients with CTD-associated PAH were treated with ERA, while the remainder received PDE5i. A meta-analysis by Galiè et al. showed no difference in the outcome of all-cause mortality among the 3 classes of drugs.10
RV dysfunction and failure is the most common cause of mortality in PAH.2,3 Therefore, improvement of RV function after therapy can be seen as a favorable determinant. RV function can be studied noninvasively by echocardiography. Parameters such as RVFAC, TAPSE, tissue Doppler maximal systolic velocity at the tricuspid annulus, MPI by pulsed-wave (Tei index) or tissue Doppler, and LVeI are frequently used in the evaluation of RV function.7,22 Not all the parameters were significantly improved in this study. This may be due to small population and short follow-up period. A significant decrease in the Tei index was found in this study. In addition, a trend toward a reduction in MPI by tissue Doppler and increment in TAPSE were also seen in the study. The significant reduction in LVeI at diastole shows not only a decline in RV pressure overload but also an improvement in interventricular interaction.19 However, the change in RVFAC and tissue Doppler maximal systolic velocity at the tricuspid annulus were insignificant after therapy. PVR is important in the progression of PAH.2,3,23 Therefore, a reduction in PVR after treatment also implies a simultaneous reduction in disease progression. The ratio of TRV/VTIRVOT determined by echocardiography is closely related to the PVR obtained by RHC.18 A ratio > 0.38 indicated a PVR > 8 WU with sensitivity of 75% and specificity of 100%.24 There was a decrease in the TRV/(TVI)RVOT ratio after disease-specific therapy in our study. This is in accordance with a decrease in PVR after therapy in many clinical trials.10 A decrease in the size of the RA, RV, and IVC, as well as a reduction in the RV free wall thickness was found in the present study. Together, these findings may be caused by an improvement in RV function and PVR. Although right heart catheterization is the gold standard for hemodynamics and RV function evaluation, it is an invasive procedure. The noninvasive study of RV function by echocardiography is more acceptable to patients, with easy access and repeatability by physicians. More importantly we can actively and aggressively participate in treatment of PAH patients,. However, once there is no additional improvement in RV function or other targets are reached, further treatment such as combination drug therapy, atrial septostomy, or lung transplantation should be considered.25
Pericardial effusion is seen as an unfavorable outcome factor in PAH.23,26 In our short-term study, there was a significant decrease in pericardial effusion scores, but the reduction in patients with pericardial effusion after treatment was insignificant. Five of 14 patients with pericardial effusion had resolution after therapy, and none of the patients developed pericardial effusion during treatment. More time may be necessary to observe a significant number of patients with resolution of pericardial effusion.
FC is a powerful prognostic factor in PAH.21,27 Seventy-nine percent of the patients in this study showed an improvement in FC by 1 or 2 classes after receiving disease-specific therapy. We have seen that 6MWD is a surrogate end point frequently used in clinical trials to evaluate the response to treatment.22,28 A 6MWD ≥ 380 m indicates a better survival than that < 380 m.29 Most of the patients in this study reached the target of 380 m after 12 weeks of treatment (Figure 2). The biomarker BNP is an indicator used in left heart failure; a serum level < 100 pg/mL makes the diagnosis of dyspnea caused by congestive heart failure less likely.30 However, there is no consensus on the cutoff value for BNP in the diagnosis or prognosis of PAH. Of note, BNP is a determinant used in the evaluation of the clinical response during PAH treatment.5,6 We have reported serial BNP testing in the clinical management of PAH which showed a decline in serum BNP levels by treatment with oral bosentan.31 In addition, Nagaya et al. found that serum BNP levels decreased after 3 months of oral prostanoid beraprost therapy with better survival than that in patients with increased serum levels in primary pulmonary hypertension.32 Our patients showed a decline in serum BNP levels after treatment, except in 3 patients (Figure 3).
This study had several limitations. First, the population recruited into the study was small. Second, no controls were used in the study because of ethical considerations.22 Third, no subgroup analysis, such as for the difference between the etiologies or classes of drugs, was performed owing to the limited number of cases. Fourth, treatment bias might have occurred because of the choice of different drugs. Finally, half of the patients had CTD-associated PAH, and treating underlying disease activity might have contributed to the clinical responses determined by FC, function capacity, or even the echocardiographic results, although we enrolled patients who were stable for at least 2 weeks. PAH is uncommon in clinical practice. Although we included only a small number of cases in our study, we demonstrated the benefit of disease-specific therapy in PAH with short-term 12-week treatment.
CONCLUSIONS
Disease-specific therapy for 12 weeks in PAH patients resulted in an improvement in FC, increased exercise capacity shown by 6MWD, and reduction in levels of the heart failure biomarker BNP. The echocardiographic evaluations showed improvement in RV function, reduction in RV pressure overload, improvement in interventricular interaction between RV and LV, and a reduction in PVR.
Acknowledgments
This study was supported by grants from the Chang Gung Research Grant Foundation (grant Nos. CMRPG 391243, CMRPG3B1021 and CMRPG3B1022).
Conflict of interest statement
No conflicts of interest to declare.
REFERENCES
- 1.Fishman AP. Primary pulmonary arterial hypertension: a look back. J Am Coll Cardiol. 2004;43:2S–4S. doi: 10.1016/j.jacc.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 2.Haddad F, Doyle R, Murphy DJ, Hunt SA. Right ventricular function in cardiovascular disease, part II, pathophysiology, clinical importance, and management of right ventricular failure. Circulation. 2008;117:1717–1731. doi: 10.1161/CIRCULATIONAHA.107.653584. [DOI] [PubMed] [Google Scholar]
- 3.Galiè N, Palazzini M, Manes A. Pulmonary arterial hypertension: from the kingdom of the near-dead to multiple clinical trial analyses. Eur Heart J. 2010;31:2080–2086. doi: 10.1093/eurheartj/ehq152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Eng J Med . 2004;351:1425–1436. doi: 10.1056/NEJMra040291. [DOI] [PubMed] [Google Scholar]
- 5.Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the task force for diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur Heart J. 2009;30:2493–2537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 6.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 Expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on expert consensus documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53:1573–1619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Forfia PR, Vachièry JL. Echocardiography in pulmonary arterial hypertension. Am J Cardiol. 2012;110:16S–24S. doi: 10.1016/j.amjcard.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Hsu CH, Glassner C, Hsu HH, Maitland MG. Current therapeutics for pulmonary arterial hypertension. Acta Cardiol Sin. 2012;28:267–278. [Google Scholar]
- 10.Galiè N, Manes A, Negro L, et al. A meta-analysis of randomized controlled trials in pulmonary arterial hypertension. Eur Heart J. 2009;30:374–403. doi: 10.1093/eurheartj/ehp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yock PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation. 1984;70:657–662. doi: 10.1161/01.cir.70.4.657. [DOI] [PubMed] [Google Scholar]
- 12.Chemla D, Castelain V, Humbert M, et al. New formula for predicting mean pulmonary artery pressure using systolic pulmonary artery pressure. Chest. 2004;126:1313–1317. doi: 10.1378/chest.126.4.1313. [DOI] [PubMed] [Google Scholar]
- 13.Steckelberg RC, Tseng AS, Nishimura R, et al. Derivation of mean pulmonary artery pressure from noninvasive parameters. J Am Soc Echocardiogr. 2013;26:464–468. doi: 10.1016/j.echo.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Miyatake K, Yamagishi M, Tanak N, et al. New method for evaluating left ventricular wall motion by color-coded tissue Doppler imaging: in vitro and in vivo studies. J Am Coll Cardiol. 1995;25:717–724. doi: 10.1016/0735-1097(94)00421-L. [DOI] [PubMed] [Google Scholar]
- 15.Meluzin J, Spinarova L, Bakala J, et al. Pulsed Doppler tissue imaging of the velocity of tricuspid annular systolic motion: a new, rapid, and non-invasive method of evaluating right ventricular systolic function. Eur Heart J. 2001;22:340–348. doi: 10.1053/euhj.2000.2296. [DOI] [PubMed] [Google Scholar]
- 16.Tei C, Dujardin KS, Hodge DO, et al. Doppler echocardiographic index for assessment of global right ventricular function. J Am Soc Echocardiogr. 1996;9:838–847. doi: 10.1016/s0894-7317(96)90476-9. [DOI] [PubMed] [Google Scholar]
- 17.Abbas AE, Fortuin FD, Schiller NB, et al. A simple method for noninvasive estimation of pulmonary vascular resistance. J Am Coll Cardiol. 2003;41:1021–1027. doi: 10.1016/s0735-1097(02)02973-x. [DOI] [PubMed] [Google Scholar]
- 18.Opotowsky AR, Clair M, Afilalo J, et al. A simple echocardiographic method to estimate pulmonary vascular resistance. Am J Cardiol. 2013;112:873–882. doi: 10.1016/j.amjcard.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryan T, Petrovic O, Dillon JC, et al. An echocardiographic index for separation right ventricular volume and pressure overload. J Am Coll Cardiol. 1985;5:918–924. doi: 10.1016/s0735-1097(85)80433-2. [DOI] [PubMed] [Google Scholar]
- 20.Eysmann SB, Palevsky HI, Reichek N, et al. Two-dimensional and Doppler echocardiographic and cardiac catheterization correlates of survival in primary pulmonary hypertension. Circulation. 1989;80:353–360. doi: 10.1161/01.cir.80.2.353. [DOI] [PubMed] [Google Scholar]
- 21.Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation. 2010;122:156–163. doi: 10.1161/CIRCULATIONAHA.109.911818. [DOI] [PubMed] [Google Scholar]
- 22.McLaughlin VV, Badesch DB, Delcroix M, et al. End points and clinical trial design in pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S97–S107. doi: 10.1016/j.jacc.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Benza RL, Gomberg-Maitland M, Miller DP, et al. The REVEAL registry risk score calculator in patients newly diagnosed with pulmonary artery hypertension. Chest. 2012;141:354–362. doi: 10.1378/chest.11-0676. [DOI] [PubMed] [Google Scholar]
- 24.Vlahos AP, Feinstein JA, Schiller NB, Silverman NH. Extension of Doppler-derived echocardiographic measures of pulmonary vascular resistance to patients with moderate or severe pulmonary vascular disease. J Am Soc Echocardiogr. 2008;21:711–714. doi: 10.1016/j.echo.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Sitbon O, Baloira Villar AB, Bauer F, et al. Treat-to-target approach in pulmonary arterial hypertension: a consensus-based proposal. Eur Respir Rev. 2012;21:259–262. doi: 10.1183/09059180.00003612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raymond RJ, Hinderliter AL, Willis PW, et al. Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J Am Coll Cardiol. 2002;39:1214–1219. doi: 10.1016/s0735-1097(02)01744-8. [DOI] [PubMed] [Google Scholar]
- 27.Nickel N, Golpon H, Greer M, et al. The prognostic impact of follow-up assessments in patients with idiopathic pulmonary arterial hypertension. Eur Respir J. 2012;39:589–596. doi: 10.1183/09031936.00092311. [DOI] [PubMed] [Google Scholar]
- 28.Savarese G, Paolillo S, Costanzo P, et al. Do changes of 6-minute walk distance predict clinical events in patients with pulmonary hypertension? : A meta-analysis of 22 randomized trials. J Am Coll Cardiol. 2012;60:1192–1201. doi: 10.1016/j.jacc.2012.01.083. [DOI] [PubMed] [Google Scholar]
- 29.Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346:896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 30.Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Eng J Med. 2002;347:161–167. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 31.Ho WJ, Hsu TS, Tsay PK, et al. Serial plasma brain natriuretic peptide testing in clinical management of pulmonary arterial hypertension. Acta Cardiol Sin. 2009;25:147–153. [Google Scholar]
- 32.Nagaya N, Nishikimi T, Uematsu M, et al. Plasma brain natriuretic peptide as a prognostic indicator in patients with primary pulmonary hypertension. Circulation. 2000;102:865–870. doi: 10.1161/01.cir.102.8.865. [DOI] [PubMed] [Google Scholar]


