Abstract
Background
The renal resistive index (RI) is calculated as (peak systolic velocity - minimum diastolic velocity)/peak systolic velocity, and has been significantly associated with renal function. Pulse pressure index (PPI) is derived from a formula similar to renal RI, i.e. (systolic blood pressure - diastolic blood pressure)/systolic blood pressure. The purpose of this study was to investigate whether brachial PPI had a significant correlation with renal RI and could be used in identifying patients with impaired renal function.
Methods
We consecutively enrolled 255 patients referred for echocardiographic examination. The renal RI was measured from Doppler ultrasonography and blood pressure was measured from an ABI-form device.
Results
Patients with brachial PPI ≥ 0.428 (mean value of brachial PPI) had a lower estimated glomerular filtration rate (eGFR) than those with brachial PPI < 0.428 (p < 0.001). After the multivariate analysis was completed, brachial PPI had a significant correlation with renal RI (unstandardized coefficient β = 0.53, p < 0.001). The areas under the curve for brachial PPI and renal RI in prediction of eGFR < 45 mL/min/1.73 m2 were 0.682 and 0.893 (both p < 0.001), respectively.
Conclusions
Brachial PPI was significantly correlated with renal RI. Patients with higher brachial PPI had a more reduced renal function. Hence, brachial PPI may be able to quickly reflect the intrarenal vascular hemodynamics, and may serve as an important tool for screening and follow-up for patients with abnormal renovascular resistance.
Keywords: Chronic kidney disease, Pulse pressure index, Resistive index
INTRODUCTION
Renal ultrasonography has been shown to have an important role in noninvasive assessment of renal anatomic and vascular information.1 As a supplement to renal anatomy examined by conventional gray-scale ultrasonography, Doppler ultrasonography can help to evaluate intrarenal hemodynamics. Renal resistive index (RI) which is measured by use of the Doppler spectrum of intrarenal arteries reflects renal vascular resistance.2 The formula of renal RI was derived from fluid-flow analogue of Ohm’s law (P = QR, where P is the pressure gradient, Q is the volume flow rate, and R is the resistance) and calculated as (peak systolic velocity - minimum diastolic velocity)/peak systolic velocity.1 The progressive loss of glomerular microvasculature correlates with progressive renal disease, severity of glomerular sclerosis, and interstitial fibrosis.3 Ikee et al. demonstrated that a direct relationship exists between renal RI and arteriolosclerosis in 33 patients who underwent renal biopsy.4 Furthermore, Bige´ et al. demonstrated that high renal RI was associated with severe interstitial fibrosis, severe arteriosclerosis, and the decline of renal function in 58 patients with chronic kidney disease (CKD) during 18 months of follow-up.5 Therefore, compared with traditional estimated glomerular filtration rate (eGFR), renal RI additionally provided renal vascular hemodynamics, arteriosclerotic change, and a predictor of renal function decline.
Blood pressure (BP) is a well-established and powerful risk factor for adverse cardiovascular events.6 Pulse pressure defined as systolic BP minus diastolic BP is also an independent predictor of ischemic heart disease, cerebrovascular disease, arterial stiffness, arteriosclerosis, and progression of CKD.6-10 Recently, in the similar concept of fluid-flow analog of Ohm’s law, Yang and Li derived the pulse pressure index (PPI) calculated as (systolic BP - diastolic BP)/systolic BP. The PPI, which ranges from 0 to 1, correlates negatively with vascular compliance.11 The PPI may not only reflect vascular compliance, but also predict cardiovascular outcomes. Some studies demonstrated there was a significant association of PPI with atherosclerosis, cardiovascular events, and CKD in Chinese patients.12-14 Zhao et al. showed that higher PPI was associated with lower eGFR in Chinese patients with hypertension.14 Recently, we demonstrated that increased PPI significantly correlates with elevated left ventricular filling pressure and left ventricular diastolic dysfunction in patients with CKD.15
Because the formulas to calculate the PPI and RI were similar, and both parameters were correlated with atherosclerosis and reduced renal function, PPI should have a significant correlation with RI. Tublin et al. showed renal RI was significantly correlated with renal PPI (R2 = 0.89) in isolated rabbit perfusion kidney.16 However, there has been no clinical study which investigated the correlation between brachial PPI and renal RI. Therefore, the aim of this study was to assess whether the easily-obtained parameter, brachial PPI, was significantly associated with renal RI. Furthermore, we also evaluated whether brachial PPI or renal RI was a useful parameter in identification of patients with decreased renal function.
MATERIALS AND METHODS
Study subjects and design
This study consecutively included patients referred for echocardiographic examinations in a regional hospital in southern Taiwan due to suspected coronary artery disease, heart failure, hypertension, abnormal results on cardiac physical examination, dyspnea survey, or a pre-operative cardiac function survey. Patients with atrial fibrillation, significant aortic or mitral valve diseases, or inadequate image visualization were excluded. Additionally, patients with a history of unilateral or bilateral renal artery stenosis, unilateral or bilateral nephrectomy, end stage renal disease receiving renal replacement or renal transplantation therapy, acute kidney injury, and acute unilateral or bilateral hydronephrosis were also excluded. Finally, 255 patients were enrolled in this study from July 2012 to December 2012.
Ethics statement
The protocol for this study was approved by the institutional review board of the Kaohsiung Medical University Hospital (KMUHIRB-20120162). Informed consents have been obtained in writing from patients, and all clinical investigation was conducted according to the principles expressed in the Declaration of Helsinki. The patients gave consent for the publication of the clinical details.
Renal Doppler ultrasonography study
Ultrasonographic examinations were performed using duplex Doppler ultrasonography with a CX50 (Philips Compact Xtreme System, USA) ultrasound machine with a 2.5-MHz pulsed Doppler frequency and a 3.5-MHz convex array transducer. The image of the kidneys was determined by B-mode and renal blood flow was visualized with color-Doppler sonography superimposed on B-mode image while the patient was in the supine position. Then, intrarenal Doppler signals were obtained from the arcuate arteries at the corticomedullary junction. The renal RI was calculated as (peak systolic velocity - minimum diastolic velocity)/peak systolic velocity.1,17,18 The renal RI was determined at least three times for each kidney and then the values from bilateral kidneys were averaged to obtain the mean value for later analysis. All measurements were performed by one experienced physician who was blinded to the other data of the subjects.
BP measurement
After ultrasonographic examinations and ten minutes of rest, BPs were measured by using an ABI-form device (VP1000; Colin Co. Ltd., Komaki, Japan), which automatically and simultaneously measures BPs in both arms and ankles using an oscillometric method.19-22 After obtaining bilateral values of brachial BPs, their mean values were used for later analysis. The pulse pressure was calculated as the difference between brachial systolic BP and diastolic BP. The mean BP was calculated as (brachial systolic BP + 2 × brachial diastolic BP)/3. The PPI was calculated as (brachial systolic BP - brachial diastolic BP)/brachial systolic BP.
Collection of demographic, medical, and laboratory data
Demographic and medical data, including age, gender, smoking history, and comorbid conditions, were obtained from medical records or interviews with patients. Body mass index was calculated as the ratio of weight in kilograms divided by the square of height in meters. Laboratory data were measured from fasting blood samples using an autoanalyzer (Roche Diagnostics GmbH, Mannheim, Germany and COBAS Integra 400, Roche Diagnostics GmbH). Serum creatinine was measured by the compensated Jaffe´ (kinetic alkaline picrate) method with a Roche/Integra 400 Analyzer (Roche Diagnostics, GmbH), using a calibrator traceable to isotope-dilution mass spectrometry.23 The value of eGFR was calculated using the four-variable equation in the Modification of Diet in Renal Disease study.24 The definition of CKD was according to the National Kidney Foundation-Kidney Disease Outcomes Quality Initiative guidelines.25 Our patients with evidence of kidney damage lasting for over 3 months into CKD stages was based on having an eGFR level (ml/min/1.73 m2) less than 60. Blood samples were obtained within 1 month of enrollment. In addition, information regarding patient medications, including angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, β-blockers, calcium channel blockers, and diuretics during the study period, was obtained from medical records.
Reproducibility
Thirty patients were randomly selected for evaluation of the interobserver variability of renal RI measurement by two independent observers. To obtain intraobserver variability, the same measurement was repeated after 1 week. Mean percentage error was calculated as the absolute difference divided by the average of the two observations. The intraobserver and interobserver mean percentage errors for renal RI measurement were 3.29 ± 2.18% and 6.58 ± 4.36%, respectively.
Statistical analysis
Statistical analysis was performed using SPSS 18.0 for windows (SPSS Inc. Chicago, IL, USA). Data are expressed as percentages and the mean ± standard deviation. The differences between groups were evaluated using a Chi-square test for categorical variables or by an independent t-test for continuous variables. The relationship between two continuous variables was assessed using a bivariate correlation method (Pearson’s correlation). Significant variables in the univariable analysis were further analyzed by multivariable linear regression analysis to identify the determinants of renal RI. Receiver operating characteristic (ROC) curve was constructed for prediction of eGFR < 45 mL/min/1.73 m2. A difference was considered significant if the p value was less than 0.05.
RESULTS
There were 255 patients (age 63.1 ± 12.9 years, male 58.4%, and PPI 0.428 ± 0.059) included in this study. Table 1 shows the comparison of baseline characteristics between two groups (brachial PPI ≥ 0.428 versus PPI < 0.428) divided by mean PPI. There was a significant difference in age, gender, diabetes, systolic BP, pulse pressure, mean BP, heart rate, hematocrit, eGFR, eGFR < 45 mL/min/1.73 m2, renal RI, and brachial PPI between groups. Figure 1 shows the scatter plot between renal RI and brachial PPI. Table 2 displays the determinants of renal RI according to univariate and multivariate analyses in the study subjects. Old age, diabetes, hypertension, cerebrovascular disease, increased pulse pressure, decreased heart rate, use of angiotensin II receptor blockers, β blockers, diuretics, and α blockers, low hematocrit, and increased brachial PPI were associated with renal RI in the univariate analysis. Old age (unstandardized coefficient β = 0.002, p < 0.001), diabetes (unstandardized coefficient β = 0.03, p = 0.001), hematocrit (unstandardized coefficient β = -0.003, p < 0.001), and brachial PPI (unstandardized coefficient β = 0.53, p < 0.001) were significantly associated with renal RI in the multivariate analysis. The areas under the ROC curve (AUCs) for renal RI and brachial PPI in prediction of < 45 mL/min/1.73 m2 were shown in Figure 2. The AUCs for brachial PPI and renal RI in the prediction of eGRF < 45 mL/min/1.73 m2 were 0.682 (p < 0.001) and 0.893 (p < 0.001), respectively. Table 3 shows the statistical values of brachial PPI and renal RI in prediction of eGFR < 45 mL/min/1.73 m2.
Table 1. Comparison of baseline characteristics according to mean value of brachial PPI (0.428 ± 0.059) .
| Brachial PPI < 0.428, n = 126 | Brachial PPI ≥ 0.428, n = 129 | p value | Total patients, n = 255 | |
| Age (years) | 58.2 ± 12.3 | 67.9 ± 11.6 | < 0.001 | 63.1 ± 12.9 |
| Male gender (%) | 73.8 | 43.4 | < 0.001 | 58.4 |
| Smoking history (%) | 26.2 | 17.1 | 0.09 | 21.6 |
| Diabetes mellitus (%) | 22.2 | 34.9 | 0.03 | 28.6 |
| Hypertension (%) | 66.7 | 76.7 | 0.10 | 71.8 |
| Coronary artery disease (%) | 15.9 | 13.2 | 0.60 | 14.5 |
| Cerebrovascular disease (%) | 7.1 | 11.6 | 0.28 | 9.4 |
| Systolic blood pressure (mmHg) | 128.4 ± 17.8 | 139.2 ± 19.4 | < 0.001 | 133.9 ± 19.3 |
| Diastolic blood pressure (mmHg) | 79.3 ± 11.4 | 73.1 ± 10.0 | < 0.001 | 76.2 ± 11.1 |
| Pulse pressure (mmHg) | 49.1 ± 8.4 | 66.1 ± 12.5 | < 0.001 | 57.7 ± 13.7 |
| Mean blood pressure (mmHg) | 95.7 ± 13.3 | 95.1 ± 12.5 | 0.72 | 95.4 ± 12.9 |
| Heart rate (beats/min) | 72.4 ± 12.6 | 66.1 ± 9.2 | < 0.001 | 69.2 ± 11.5 |
| Body mass index (kg/m2) | 26.7 ± 3.9 | 26.5 ± 3.6 | 0.64 | 26.6 ± 3.7 |
| Antihypertensive medication | ||||
| ACEIs | 20.6 | 14.7 | 0.25 | 17.6 |
| ARBs | 35.7 | 47.3 | 0.08 | 41.6 |
| β-blockers | 38.9 | 48.8 | 0.13 | 43.9 |
| Calcium channel blockers | 39.7 | 47.3 | 0.26 | 43.5 |
| Diuretics | 32.5 | 32.6 | 1.00 | 32.5 |
| α-blockers | 3.2 | 4.7 | 0.75 | 3.9 |
| Laboratory parameters | ||||
| Fasting glucose (mg/dL) | 121.8 ± 49.6 | 116.7 ± 28.9 | 0.34 | 119.3 ± 40.6 |
| Triglyceride (mg/dL) | 144.7 ± 91.5 | 137.5 ± 83.0 | 0.54 | 141.1 ± 87.2 |
| Total cholesterol (mg/dL) | 188.9 ± 34.1 | 190.2 ± 45.7 | 0.81 | 189.6 ± 40.3 |
| Hematocrit (%) | 41.6 ± 5.1 | 38.4 ± 5.1 | < 0.001 | 39.9 ± 5.3 |
| Baseline eGFR (mL/min/1.73 m2) | 66.5 ± 19.1 | 58.3 ± 22.9 | 0.002 | 62.4 ± 21.5 |
| eGFR < 45 (mL/min/1.73 m2) | 8.7 | 24.0 | 0.001 | 16.5 |
| Renal resistive index | 0.64 ± 0.07 | 0.73 ± 0.07 | < 0.001 | 0.68 ± 0.08 |
ACEI, angiotensin-converting-enzyme inhibitor; ARB, angiotensin II receptor blocker; eGFR, estimated glomerular filtration rate; PPI, pulse pressure index.
Figure 1.
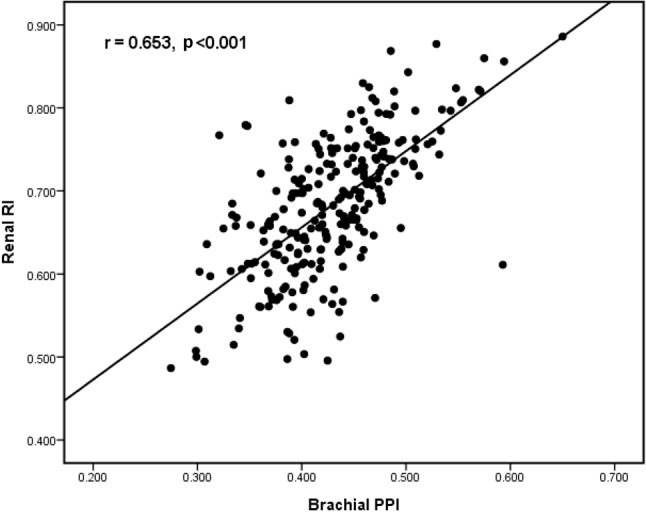
The scatter plot between brachial pulse pressure index (PPI) and renal resistive index (RI) in all study patients.
Table 2. Determinants of renal resistive index by univariate and multivariate analyses in all patients .
| Univariate | Multivariate | |||||||
| Unstandardized coefficient β | SD | 95% CI | p value | Unstandardized coefficient β | SD | 95% CI | p value | |
| Age (year) | 0.004 | < 0.001 | (0.003, 0.004) | < 0.001 | 0.002 | < 0.001 | (0.001, 0.003) | < 0.001 |
| Male gender (%) | -0.02 | 0.01 | (-0.04, 0.002) | 0.08 | - | |||
| Smoking history (%) | -0.01 | 0.01 | (-0.04, 0.01) | 0.27 | - | |||
| Diabetes mellitus (%) | 0.06 | 0.01 | (0.03, 0.08) | < 0.001 | 0.03 | 0.009 | (0.01, 0.05) | 0.001 |
| Hypertension (%) | 0.04 | 0.01 | (0.01, 0.06) | 0.002 | 0.003 | 0.009 | (-0.02, 0.02) | 0.76 |
| Coronary artery disease (%) | 0.03 | 0.02 | (-0.003, 0.06) | 0.08 | - | |||
| Cerebrovascular disease (%) | 0.04 | 0.02 | (0.006, 0.08) | 0.02 | 0.01 | 0.01 | (-0.01, 0.03) | 0.42 |
| Pulse pressure (mmHg) | 0.004 | < 0.001 | (0.003, 0.004) | < 0.001 | < 0.001 | < 0.001 | (-0.001, 0.001) | 0.59 |
| Heart rate (beats/min) | -0.002 | < 0.001 | (-0.002, -0.001) | < 0.001 | < 0.001 | < 0.001 | (-0.001, < 0.001) | 0.23 |
| Body mass index (kg/m2) | 0.001 | 0.001 | (-0.002, 0.004) | 0.54 | - | |||
| Antihypertensive medication | ||||||||
| ACEI | -0.02 | 0.01 | (-0.05, 0.008) | 0.17 | - | |||
| ARB | 0.03 | 0.01 | (0.01, 0.05) | 0.002 | -0.01 | 0.008 | (-0.03, 0.004) | 0.14 |
| Beta blocker | 0.03 | 0.01 | (0.009, 0.05) | 0.005 | 0.02 | 0.008 | (< 0.001, 0.03) | 0.05 |
| Calcium channel blocker | 0.02 | 0.01 | (-0.006, 0.04) | 0.15 | - | |||
| Diuretics | 0.02 | 0.01 | (0.002, 0.05) | 0.04 | 0.01 | 0.008 | (-0.003, 0.03) | 0.10 |
| Alfa blocker | 0.06 | 0.03 | (0.01, 0.11) | 0.02 | 0.03 | 0.02 | (-0.008, 0.08) | 0.12 |
| Laboratory parameters | ||||||||
| Fasting glucose (mg/dL) | < 0.001 | < 0.001 | (< 0.001, < 0.001) | 0.24 | - | |||
| Triglyceride (mg/dL) | -3.62 | < 0.001 | (< 0.001, < 0.001) | 0.58 | - | |||
| Total cholesterol (mg/dL) | < 0.001 | < 0.001 | (< 0.001, < 0.001) | 0.10 | - | |||
| Hematocrit (%) | -0.007 | 0.001 | (-0.009, -0.005) | < 0.001 | -0.003 | 0.001 | (-0.004, -0.002) | < 0.001 |
| Brachial pulse pressure index | 0.92 | 0.07 | (0.79, 1.05) | < 0.001 | 0.53 | 0.11 | (0.32, 0.74) | < 0.001 |
ACEI, angiotensin-converting-enzyme inhibitor; ARB, angiotensin II receptor blocker; CI, confidence interval; SD, standard deviation.
Figure 2.
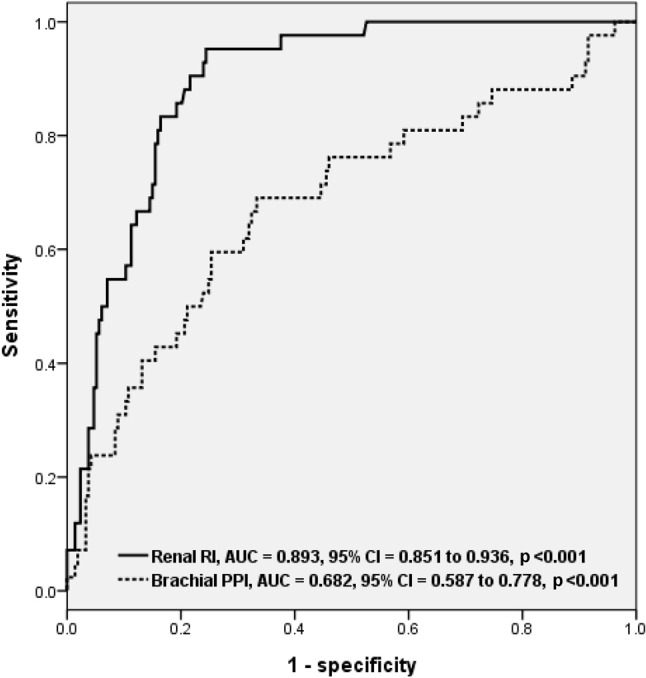
The areas under the receiver operating characteristic curves (AUCs) for brachial pulse pressure index (PPI) and renal resistive index (RI) in the prediction of estimated glomerular filtration rate (eGFR) < 45 mL/min/1.73 m2.
Table 3. Statistical values of brachial PPI and renal RI for predicting eGFR < 45 mL/min/1.73 m2 .
| Sensitivity (%) | Specificity (%) | Accuracy (%) | |
| Brachial PPI > 0.449 | 61.9 | 69.0 | 65.5 |
| Renal RI > 0.718 | 95.2 | 75.6 | 85.4 |
eGFR, estimated glomerular filtration rate; PPI, pulse pressure index; RI, resistive index.
DISCUSSION
In this study, we evaluated the relationship between brachial PPI and renal RI measured from renal Doppler ultrasonography. We found that patients with higher brachial PPI had more impaired renal function. Brachial PPI also had a significant correlation with renal RI and was higher in patients with decreased eGFR. Although renal ultrasonography is a useful tool in the assessment of renal parenchymal and vascular disease, it is still operator-dependent and relatively time-consuming. In contrast, brachial PPI is an easily-obtained and operator-independent parameter and can be rapidly acquired in daily clinical practice. Hence, brachial PPI may be able to quickly reflect the intrarenal vascular resistance.
Renal RI is an important parameter used to evaluate renal function and intrarenal vascular hemodynamics. The renal RI can be used to assess renal vascular resistance, predict the decline of renal function and cardiovascular events, and reflect the pathological change in CKD and target organ damage in patients with essential hypertension.1,4,5,17,26-29 There have been limited studies that investigated the association between renal RI and brachial PPI. In an isolated perfused rabbit kidney model, Tublin et al. had demonstrated that renal RI was affected by age, heart rate, vascular stiffness, pulse pressure, and renal blood flow.16,30,31 Furthermore, in this animal model, RI was significantly correlated with renal PPI.16 Jensen et al. showed there was a significant correlation between BP index, calculated as (systolic BP - diastolic BP)/mean BP, and renal blood flow in hypertensive patients.32 Dimsdale et al. showed that a change in main renal artery RI was associated with a transient increase in BP (r = 0.25, p < 0.05) in subjects without structural renal disease.33 The possible reasons why the change in local renal vascular resistance and blood flow correlated with the change in systemic BP are the result of hemodynamic and neurohormonal influence. In the present study, we consistently found brachial PPI had a moderate correlation with renal RI (r = 0.65, p < 0.001).
PPI based on elastic chamber theory is the recently emerged parameter which is theoretically illustrated by internal and dynamic vascular compliance.11 Studies of Chinese populations have shown that PPI is significantly associated with atherosclerosis and cardiovascular events.13 Our recent study found that CKD patients with a ratio of transmitral E wave velocity (E) to early diastole mitral annulus velocity (Ea) ≥ 15 had a higher systolic BP, higher pulse pressure, and higher PPI compared to those patients with E/Ea < 15. In multivariate analysis, PPI was an independent determinant of E/Ea and left ventricular diastolic dysfunction in study subjects with CKD.15 In addition, there were few studies that had researched the relationship between PPI and CKD. Zhao et al. investigated the relationship among pulse pressure, PPI, and eGFR in 742 Chinese patients with essential hypertension.14 The eGFR was significantly lower in the group with PP > 55 mmHg and PPI > 0.45 than in the group with PP ≤ 55 mmHg and PPI ≤ 0.45. In our study, we similarly found brachial PPI > 0.449 was useful in the prediction of eGFR < 45 mL/min/1.73 m2. Hence, brachial PPI was not only positively correlated with renal RI, but also higher in patients with impaired renal function.
There were several limitations to this study. The majority of our patients were treated chronically with antihypertensive medications. Previous studies have shown that antihypertensive agents can affect RI.4,34,35 For ethical reasons, we did not withdraw these medications. Therefore, we could not exclude the influence of antihypertensive agents on our findings. Besides, because the measurements of renal RI during atrial fibrillation were difficult because of beat-to-beat variation, we excluded patients with atrial fibrillation. Finally, patients with a history of renal artery stenosis, nephrectomy, end stage renal disease receiving renal replacement or renal transplantation therapy, acute kidney injury, and hydronephrosis were also excluded. Therefore, our results could not be applied to these patients.
CONCLUSIONS
In conclusion, the present study demonstrated that brachial PPI was significantly correlated with renal RI obtained from Doppler ultrasonography. The patients with higher brachial PPI had an increased impaired renal function. Hence, brachial PPI may be able to quickly and roughly reflect the intrarenal vascular hemodynamics and may serve as an important tool for screening and following up patients with abnormal renovascular resistance. However, the clinical application of brachial PPI needs to be investigated in future large-scale studies.
Acknowledgments
The research presented in this article is supported by a grant from Kaohsiung Municipal Hsiao-Kang Hospital (kmhk-101-019), Kaohsiung Medical University, Kaohsiung, Taiwan and the statistical work by the department of Research Education and Training at Kaohsiung Municipal Hsiao-Kang Hospital.
REFERENCES
- 1.Bude RO, Rubin JM. Relationship between the resistive index and vascular compliance and resistance. Radiology. 1999;211:411–417. doi: 10.1148/radiology.211.2.r99ma48411. [DOI] [PubMed] [Google Scholar]
- 2.Lubas A, Kade G, Niemczyk S. Renal resistive index as a marker of vascular damage in cardiovascular diseases. Int Urol Nephrol. 2014;46:395–402. doi: 10.1007/s11255-013-0528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang DH, Kanellis J, Hugo C, et al. Role of the microvascular endothelium in progressive renal disease. J Am Soc Nephrol. 2002;13:806–816. doi: 10.1681/ASN.V133806. [DOI] [PubMed] [Google Scholar]
- 4.Ikee R, Kobayashi S, Hemmi N, et al. Correlation between the resistive index by doppler ultrasound and kidney function and histology. Am J Kidney Dis. 2005;46:603–609. doi: 10.1053/j.ajkd.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Bige N, Levy PP, Callard P, et al. Renal arterial resistive index is associated with severe histological changes and poor renal outcome during chronic kidney disease. BMC Nephrol. 2012;13:139. doi: 10.1186/1471-2369-13-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Safar ME, Levy BI, Struijker-Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation. 2003;107:2864–2869. doi: 10.1161/01.CIR.0000069826.36125.B4. [DOI] [PubMed] [Google Scholar]
- 7.Miura K, Dyer AR, Greenland P, et al. Pulse pressure compared with other blood pressure indexes in the prediction of 25-year cardiovascular and all-cause mortality rates:the Chicago heart association detection project in industry study. Hypertension. 2001;38:232–237. doi: 10.1161/01.hyp.38.2.232. [DOI] [PubMed] [Google Scholar]
- 8.Moody WE, Edwards NC, Chue CD, et al. Arterial disease in chronic kidney disease. Heart. 2013;99:365–372. doi: 10.1136/heartjnl-2012-302818. [DOI] [PubMed] [Google Scholar]
- 9.Weitzman D, Goldbourt U. The significance of various blood pressure indices for long-term stroke, coronary heart disease, and all-cause mortality in men: the Israeli Ischemic Heart Disease study. Stroke. 2006;37:358–363. doi: 10.1161/01.STR.0000198869.84540.80. [DOI] [PubMed] [Google Scholar]
- 10.Arulkumaran N, Diwakar R, Tahir Z, et al. Pulse pressure and progression of chronic kidney disease. J Nephrol. 2010;23:189–193. [PubMed] [Google Scholar]
- 11.Yang PL, Li YC. Pulse pressure index (pulse pressure/systolic pressure) may be better than pulse pressure for assessment of cardiovascular outcomes. Medical Hypotheses. 2009;72:729–731. doi: 10.1016/j.mehy.2008.12.041. [DOI] [PubMed] [Google Scholar]
- 12.Chen P, Yang PL, Wang KH, et al. The relationship between pulse pressure index and carotid intima-media thickness in elderly patients. Chin J Arterioscler. 2003;11:250–253 (Article in Chinese). [Google Scholar]
- 13.Zhang XG, Sun ZQ, Zheng LQ, et al. Relationship between pulse pressure,pulse pressure index and prevalence of stroke among rural population in China. Zhonghua Yi Xue Za Zhi. 2007;87:468–470 (Article in Chinese). [PubMed] [Google Scholar]
- 14.Zhao YJ, Shen LH, Wang W, et al. Clinical value of pulse pressure,pulse pressure index and estimated glomerular filtration rate in patients with essential hypertension. Journal of Shanghai Jiaotong University (Medical Science) 2007;27:1258–1260 (Article in Chinese). [Google Scholar]
- 15.Lee WH, Hsu PC, Chu CY, et al. Associations of pulse pressure index with left ventricular filling pressure and diastolic dysfunction in patients with chronic kidney disease. Am J Hypertens. 2013;27:454–459. doi: 10.1093/ajh/hpt228. [DOI] [PubMed] [Google Scholar]
- 16.Tublin ME, Tessler FN, Murphy ME. Correlation between renal vascular resistance, pulse pressure, and the resistive index in isolated perfused rabbit kidneys. Radiology. 1999;213:258–264. doi: 10.1148/radiology.213.1.r99oc19258. [DOI] [PubMed] [Google Scholar]
- 17.Doi Y, Iwashima Y, Yoshihara F, et al. Association of renal resistive index with target organ damage in essential hypertension. Am J Hypertens. 2012;12:1292–1298. doi: 10.1038/ajh.2012.113. [DOI] [PubMed] [Google Scholar]
- 18.Sugiura T, Wada A. Resistive index predicts renal prognosis in chronic kidney disease. Nephrol Dial Transplant. 2009;24:2780–2785. doi: 10.1093/ndt/gfp121. [DOI] [PubMed] [Google Scholar]
- 19.Yamashina A, Tomiyama H, Takeda K, et al. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res. 2002;25:359–364. doi: 10.1291/hypres.25.359. [DOI] [PubMed] [Google Scholar]
- 20.Su HM, Lin TH, Hsu PC, et al. A comparison between brachial and echocardiographic systolic time intervals. PLoS One. 2013;8:e55840. doi: 10.1371/journal.pone.0055840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen SC, Chang JM, Tsai YC, et al. Brachial-ankle pulse wave velocity and brachial pre-ejection period to ejection time ratio with renal outcomes in chronic kidney disease. Hypertens Res. 2012;12:159–163. doi: 10.1038/hr.2012.114. [DOI] [PubMed] [Google Scholar]
- 22.Su HM, Lin TH, Hsu PC, et al. Brachial-ankle pulse wave velocity and systolic time intervals in risk stratification for progression of renal function decline. Am J Hypertens. 2012;25:1002–1010. doi: 10.1038/ajh.2012.77. [DOI] [PubMed] [Google Scholar]
- 23.Vickery S, Stevens PE, Dalton RN, et al. Does the id-ms traceable mdrd equation work and is it suitable for use with compensated jaffe and enzymatic creatinine assays? Nephrol Dial Transplant. 2006;21:2439–2445. doi: 10.1093/ndt/gfl249. [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 25.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease:evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 26.Doi Y, Iwashima Y, Yoshihara F, et al. Renal resistive index and cardiovascular and renal outcomes in essential hypertension. Hypertension. 2012;60:770–777. doi: 10.1161/HYPERTENSIONAHA.112.196717. [DOI] [PubMed] [Google Scholar]
- 27.Ennezat PV, Marechaux S, Six-Carpentier M, et al. Renal resistance index and its prognostic significance in patients with heart failure with preserved ejection fraction. Nephrol Dial Transplant. 2011;26:3908–3913. doi: 10.1093/ndt/gfr116. [DOI] [PubMed] [Google Scholar]
- 28.Radermacher J, Ellis S, Haller H. Renal resistance index and progression of renal disease. Hypertension. 2002;39:699–703. doi: 10.1161/hy0202.103782. [DOI] [PubMed] [Google Scholar]
- 29.Pearce JD, Craven TE, Edwards MS, et al. Associations between renal duplex parameters and adverse cardiovascular events in the elderly:a prospective cohort study. Am J Kidney Dis. 2010;55:281–290. doi: 10.1053/j.ajkd.2009.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy ME, Tublin ME. Understanding the doppler RI: impact of renal arterial distensibility on the RI in a hydronephrotic ex vivo rabbit kidney model. J Ultrasound Med. 2000;19:303–314. [PubMed] [Google Scholar]
- 31.Tublin ME, Bude RO, Platt JF. Review. The resistive index in renal doppler sonography: where do we stand? AJR Am J Roentgenol. 2003;180:885–892. doi: 10.2214/ajr.180.4.1800885. [DOI] [PubMed] [Google Scholar]
- 32.Jensen G, Bardelli M, Volkmann R, et al. Renovascular resistance in primary hypertension: experimental variations detected by means of doppler ultrasound. J Hypertens. 1994;12:959–964. [PubMed] [Google Scholar]
- 33.Dimsdale JE, Berry C, O'Boyle M, et al. Renal ultrasonography as a tool for detecting dynamic changes in blood pressure. J Ultrasound Med. 1995;14:715–718. doi: 10.7863/jum.1995.14.10.715. [DOI] [PubMed] [Google Scholar]
- 34.Leoncini G, Martinoli C, Viazzi F, et al. Changes in renal resistive index and urinary albumin excretion in hypertensive patients under long-term treatment with lisinopril or nifedipine gits. Nephron. 2002;90:169–173. doi: 10.1159/000049038. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe S, Okura T, Kurata M, et al. Valsartan reduces serum cystatin c and the renal vascular resistance in patients with essential hypertension. Clin Exp Hypertens. 2006;28:451–461. doi: 10.1080/10641960600798671. [DOI] [PubMed] [Google Scholar]


