Abstract
Background
Thrombospondin-1 (TSP-1) is known to be involved in the regulation of angiogenesis, inflammation, and vascular function. Clinical studies have demonstrated its correlation with peripheral artery disease, coronary artery disease, and pulmonary hypertension. In this study, we explored its potential roles in the background of end-stage renal disease (ESRD).
Methods
A total of 140 ESRD outpatients (ages 61.0 ± 12.4 years) were prospectively followed for 34 ± 7 months. Their TSP-1 levels were analyzed from pre-hemodialysis blood sample. Cardiovascular survey included ankle- brachial index (ABI), echocardiography and Tl-201 dipyridamole single-photon emission computed tomography (SPECT).
Results
Plasma TSP-1 levels were higher in those patients with preexisting clinical evidence of cardiovascular disease (CVD) than those without (p = 0.002). TSP-1 concentrations were also correlated with ABI, left ventricular ejection fraction, and scar burden in SPECT. Stepwise logistic regression analysis revealed that TSP-1 level was independently associated with the presence of CVD, with an odds ratio of 1.38 [95% confidence interval (CI), 1.09-1.75, p = 0.008]. In survival analyses, 31 patients (22%) died during the follow-up, 16 (52%) arising from cardiovascular causes. Cox hazards analysis revealed that the patients with TSP-1 levels in the highest tertile had a 5.32- and 6.75-fold higher risk for all-cause and cardiovascular mortality than those in the lowest tertile. This predictive value for all-cause mortality still persisted after multivariate adjustment (hazard ratio, 8.71; 95% CI, 1.36-55.68; p = 0.02).
Conclusions
This study hallmarks the association of elevated TSP-1 level with CVD and adverse outcome among hemodialysis patients.
Keywords: Thrombospondin-1, End-stage renal disease, Cardiovascular disease, Mortality
INTRODUCTION
Patients with end-stage renal disease (ESRD) are at a higher risk of cardiovascular disease (CVD), including sudden death, coronary artery disease (CAD), congestive heart failure (CHF), stroke, and peripheral artery disease (PAD) than the general population.1,2 Despite the substantial improvement in dialysis technologies in the past decades, the mortality rate associated with ESRD remains high. Renal failure is associated with numerous metabolic changes that affect almost all organ systems.3 Results from randomized placebo-controlled trials of various other treatment strategies such as lipid-lowering, increased dialysis duration, and normalization of hemoglobin were unable to provide evidence of a survival benefit. Traditional risk factor profiles are considered to differ in the prediction of morbidity and mortality between ESRD patients and the general population.4,5
Thrombospondin-1 (TSP-1), an extracellular matrix glycoprotein first discovered in activated platelets, is currently known to be involved in angiogenesis, inflammation, and fibrosis.6-8 It binds to protein components of the extracellular matrix, such as fibronectin, and is a major activator of transforming growth factor (TGF-β1). Data from TSP-1 knockout mice demonstrated that TSP-1 inhibits angiogenesis, promotes excision wound healing, and limits inflammatory responses and fibrotic remodeling after myocardial infarction.9-11 Recent in vivo and animal studies found that TSP-1 regulates cardiovascular function via CD47.12 Clinical investigations also confirmed its roles in CVD, including CAD, PAD, and pulmonary hypertension.13-15
The goal of the present study was to evaluate the association of plasma TSP-1 level with CVD in maintenance hemodialysis patients. Moreover, we examined the clinical importance of TSP-1 with regard to the outcome prediction in hemodialysis patients.
MATERIALS AND METHODS
Study design
This prospective, observational, single-center cohort study was conducted at the Taoyuan General Hospital. The local ethics committee approved the protocol, and written informed consent was obtained from each participant. A total of 140 outpatients with ESRD who had undergone maintenance hemodialysis at the hospital between April 2009 and September 2009 were enrolled in the study. Patients younger than 30 years, and those with active infection, acute heart failure, or malignancy under treatment were excluded. All the patients underwent echocardiography and ankle-brachial index (ABI) examination after inclusion. The blood pressure from the hemodialysis access side arm was not measured, and the lower ankle pressure values were used for the calculation. For clinical suspicion of myocardial ischemia or post-myocardial infarction follow-up, Tl-201 dipyridamole single-photon emission computed tomography (SPECT) was performed on 83 subjects within 6 month before enrollment. Regional myocardial uptake was normalized and assessed using the 17-segment model and 5-point semiquantitative scoring system for defect severity recommended by the American Society of Nuclear Cardiology.16 Summed scores were derived from these segmental scores, including a summed rest score (SRS; the sum of the 17 segmental rest scores) and summed stress score (SSS; the sum of the 17 segmental stress scores). A summed difference score (SDS; the difference between SSS and SRS) was also calculated. Blood samples were drawn at the start of each dialysis. After centrifugation, the plasma was stored at -80 °C before analysis. High sensitivity C-reactive protein (HsCRP) and TSP-1 levels were measured by enzyme-linked immu-noassay, according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN, USA). Patients with preexisting clinical evidence of CVD were defined as: 1) angiographically documented coronary artery stenosis (≥ 50% luminal stenosis); 2) significant myocardial ischemia and/or scar in SPECT (SRS > 4 or SDS > 4); ultrasonographically or angiographically documented significant extracranial arterial stenosis (≥ 50%); 3) history of myocardial infarction; 4) ischemic stroke or transient ischemic attack; or 4) documented peripheral arterial disease. The primary endpoint of our study was all-cause mortality. All the patients were prospectively followed-up for up to 3 years from the day of blood sampling.
Statistical analysis
Continuous variables were reported as mean ± standard deviation values, whereas categorical variables were reported as numbers and percentages. Group comparisons were performed using a 2-sample t test or Mann-Whitney U test for continuous and categorical variables, respectively. Univariable relationships between TSP-1 level and clinical variables, including ABI and left ventricular ejection fraction (LVEF), were assessed with the Pearson correlation coefficient (r). Independent predictors of underlying CVD were determined by multivariate logistic regression analysis. For outcomes analysis, circulating TSP-1 levels were divided into 3 tertiles (TSP-1 level ≤ 3 μg/mL, n = 48; 3 μg/mL < TSP-1 level ≤ 4.6 μg/mL, n = 45; TSP-1 level > 4.6 μg/mL, n = 47) and analyzed as categorical variables. Survival rates were estimated using Kaplan-Meier curves and compared using the log-rank test. Cox proportional hazards models were applied to calculate hazard ratio (HR) with or without adjustments. The results were presented as HR and 95% confidence interval (CI). Analyses were performed using the Stata statistical software (release 10.0, StataCorp LP, College Station, TX, USA). All the statistical tests were 2-sided, with p < 0.05 considered statistically significant.
RESULTS
Association between TSP-1 level and cardiovascular disease
Among the 140 maintenance hemodialysis patients (mean hemodialysis duration, 6.7 years), 61 (44%) had a diagnosis of CVD before enrollment (CAD in 40, cerebrovascular disease in 24, PAD in 21; Table 1). Overall, the mean age of the participants was 61 years, and 38% of them were male. Compared with those without CVD, the patients with CVD were older, had a higher prevalence of hypertension, diabetes, hyperlipidemia, higher percentages of antiplatelet agent use, and had lower ABI and LVEF values. In the biomarkers analyses, the circulating TSP-1 concentrations were significantly higher in hemodialysis patients with CVD than in those without CVD (5.41 ± 3.41 vs. 3.35 ± 1.61 ng/mL, p < 0.001). The difference persisted after adjusting age, gender, hyperlipidemia, hypertension, fasting glucose, duration of dialysis, and antiplatelet agent use (p = 0.002). The hsCRP levels were also higher in CVD group patients (p = 0.04). In the univariate correlation analyses, TSP-1 level was negatively correlated with ABI (r = -0.40, p < 0.001) and LVEF (r = -0.23, p = 0.01), but was not associated with hsCRP. SRS, the indicator of myocardial scar burden derived from SPECT, was positively correlated with TSP-1 (r = 0.33, p = 0.02). To evaluate the relationship between TSP-1 and CVD in the hemodialysis subjects, stepwise logistic regression analysis was performed using the presence of CVD as a dependent variable. TSP-1 level was independently associated with the presence of CVD, with an odds ratio of 1.38 (95% CI, 1.09-1.75, p = 0.008; Table 2).
Table 1. Baseline characteristics of study population with and without preexisting clinical evidence of cardiovascular disease .
| Parameter | All patients N = 140 | CVD n = 61 | Non-CVD n = 79 | p value |
| Age (yr) | 61.0 ± 12.4 | 64.0 ± 10.4 | 58.7 ± 13.4 | 0.01 |
| Male gender (%) | 53 (38%) | 27 (44%) | 26 (33%) | 0.17 |
| Duration of dialysis (yr) | 6.7 ± 3.5 | 7.6 ± 3.8 | 6.1 ± 3.2 | 0.01 |
| Hypertension (%) | 78 (56%) | 43 (70%) | 35 (44%) | 0.002 |
| Diabetes mellitus (%) | 69 (49%) | 40 (66%) | 29 (37%) | < 0.001 |
| Hyperlipidemia (%) | 53 (38%) | 26 (49%) | 23 (31%) | 0.04 |
| Smoker (%) | 24 (17%) | 15 (25%) | 9 (12%) | 0.04 |
| Medications (%) | ||||
| Statins | 17 (12%) | 8 (13%) | 9 (11%) | 0.76 |
| Beta-blockers | 28 (20%) | 15 (25%) | 13 (16%) | 0.23 |
| ACEi/ARBs | 30 (21%) | 17 (28%) | 13 (16%) | 0.10 |
| Aspirin | 47 (34%) | 30 (49%) | 17 (22%) | < 0.001 |
| Cilostazol | 20 (14%) | 13 (21%) | 7 (9%) | 0.04 |
| BUN (mg/dl) | 64.3 ± 19.8 | 66.4 ± 17.3 | 62.7 ± 21.5 | 0.27 |
| Creatinine (mg/dl) | 10.7 ± 2.6 | 10.4 ± 2.1 | 11.0 ± 2.8 | 0.14 |
| Albumin (g/dl) | 3.87 ± 0.35 | 3.83 ± 0.28 | 3.91 ± 0.39 | 0.19 |
| Hemoglobin (g/dl) | 9.9 ± 1.2 | 9.8 ± 1.2 | 9.9 ± 1.1 | 0.67 |
| Fasting glucose (mg/dl) | 148 ± 70 | 168 ± 81 | 133 ± 56 | 0.003 |
| Hemoglobin A1c (%) | 7.3 ± 1.5 | 7.9 ± 1.7 | 6.8 ± 1.4 | 0.01 |
| Total cholesterol (mg/dl) | 187 ± 40 | 192 ± 41 | 183 ± 40 | 0.20 |
| LDL-C (mg/dl) | 120 ± 30 | 123 ± 34 | 118 ± 27 | 0.38 |
| Intact PTH (pg/ml) | 301 ± 298 | 247 ± 292 | 332 ± 342 | 0.07 |
| LVEF | 0.60 ± 0.14 | 0.55 ± 0.14 | 0.64 ± 0.11 | 0.01 |
| Ankle-brachial index | 0.93 ± 0.22 | 0.84 ± 0.26 | 0.99 ± 0.16 | < 0.001 |
| hsCRP (mg/dl) | 2.34 ± 1.96 | 2.77 ± 2.02 | 2.05 ± 1.87 | 0.04 |
| Thrombospondin-1 (ug/ml) | 4.25 ± 2.74 | 5.41 ± 3.41 | 3.35 ± 1.61 | < 0.001 |
ACEi/ARBs, angiotensin-converting enzyme inhibitor/angiotensin receptor blockers; BUN, blood urea nitrogen; CVD, cardiovascular disease; hsCRP, high sensitivity C-reactive protein; LDL-C, low-density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; PTH, parathyroid hormone.
Table 2. Forward stepwise logistic regression analysis showing odds ratios for the prevalence of cardiovascular disease in the hemodialysis patients .
| Parameters | OR | 95% CI | p value |
| Thrombopondin-1 | 1.38 | 1.09-1.75 | 0.008 |
| Age | 1.04 | 1.00-1.09 | 0.03 |
| Smoking | 3.18 | 1.07-9.48 | 0.04 |
| Diabetes | 2.92 | 1.28-6.69 | 0.01 |
| Hyperlipidemia | 3.01 | 1.26-7.19 | 0.01 |
Variables included in the original model are thrombospondin-1, age, sex, duration of dialysis, hypertension, diabetes, hyperlipidemia, smoking, creatinine, fasting glucose, HbA1c, total cholesterol, LDL-C, and hsCRP.
CI, confidence internal; OR, odds ratio.
Association between TSP-1 level and outcomes
During the follow-up period (34 ± 7 months), 31 patients (22%) died, 16 (51.6%) of them caused by CVD. None of the patient were lost follow-up. Non-cardiovascular causes of death consisted of sepsis in 8 patients, hyperkalemia in 2, chronic obstructive pulmonary disease in 2, gastrointestinal bleeding in 1, and other causes in 2. The characteristic differences of sur-vivors and nonsurvivors were demonstrated in Table 3. Nonsurvivor patients were older, with poor sugar control, impaired LVEF, lower albumin level, and higher TSP-1 concentrations (5.66 ± 3.93 vs. 3.84 ± 2.16 μg/mL, p < 0.001).
Table 3. Baseline characteristics of study population according to survival or not .
| Parameter | Alive N = 109 | Expired N = 31 | p value |
| Age (year) | 59.8 ± 12.1 | 65.4 ± 12.7 | 0.03 |
| Male gender (%) | 40 (37%) | 13 (42%) | 0.60 |
| Hypertension (%) | 55 (50%) | 23 (74%) | 0.02 |
| Diabetes mellitus (%) | 49 (45%) | 20 (65%) | 0.06 |
| Hyperlipidemia (%) | 42 (39%) | 11 (35%) | 0.79 |
| Smoker (%) | 16 (15%) | 8 (26%) | 0.15 |
| Duration of dialysis (yr) | 6.5 ± 3.4 | 7.5 ± 3.7 | 0.16 |
| Cilostazol treatment (%) | 10 (9%) | 10 (32%) | 0.001 |
| Albumin (g/dl) | 3.91 ± 0.32 | 3.75 ± 0.40 | 0.03 |
| Hemoglobin (g/dl) | 9.8 ± 1.2 | 9.9 ± 1.2 | 0.84 |
| Fasting glucose (mg/dl) | 141 ± 64 | 173 ± 85 | 0.03 |
| Total cholesterol (mg/dl) | 187 ± 39 | 186 ± 44 | 0.91 |
| LVEF | 0.62 ± 0.13 | 0.55 ± 0.15 | 0.04 |
| Ankle-brachial index | 0.94 ± 0.21 | 0.87 ± 0.26 | 0.11 |
| hsCRP (mg/dl) | 2.32 ± 1.95 | 2.42 ± 2.01 | 0.82 |
| Thrombospondin-1 (ug/ml) | 3.84 ± 2.16 | 5.66 ± 3.93 | < 0.001 |
hsCRP, high sensitivity C-reactive protein; LVEF, left ventricular ejection fraction.
The outcomes analysis included all-cause mortality and cardiovascular mortality. Figure 1 shows the Kaplan-Meier survival curves for all-cause and cardiovascular mortality according to TSP-1 levels. The patients with TSP-1 levels in the highest tertile (TSP-1 level > 4.6 μg/mL, mortality rate 34%) and middle tertile (3 μg/mL < TSP-1 level ≤ 4.6 μg/mL, mortality rate 25%) had a significantly higher mortality rate than those with TSP-1 levels in the lowest tertile (TSP-1 level ≤ 3 μg/mL, mortality rate 8%). The patients in the highest tertile of TSP-1 levels also had higher cardiovascular mortality than those in the lowest tertile. After adjusting for age and gender, the patients with TSP-1 levels in the highest tertile had a 5.32- and 6.75-fold higher risk for all-cause and cardiovascular mortality than those in the lowest tertile (model 1, Table 4). Because the baseline CVD diagnosis was believed to be an important confounder, the Cox proportional hazard model analysis, including preexisting CVD and parameters with p value < 0.1 in Table 3, was performed. As shown in model 2, higher TSP-1 level still predicted a worse outcome (highest tertile vs. lowest tertile: HR, 8.71; 95% CI, 1.36-55.68; p = 0.02), although this impact was attenuated in cardiovascular mortality analysis.
Figure 1.
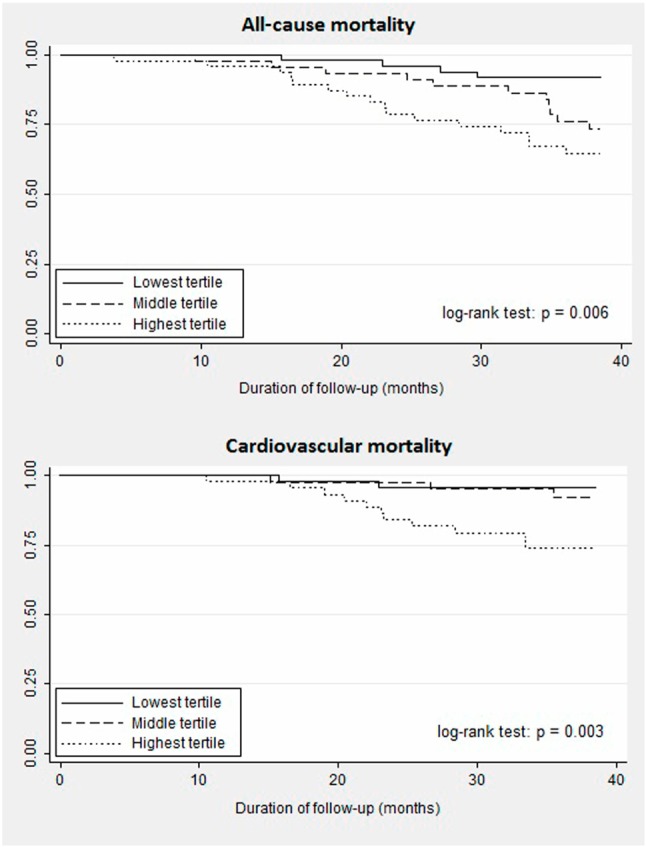
Kaplan-Meier survival curves for all-cause mortality and cardiovascular mortality according to cut-off values of thrombospondin-1 in hemodialysis patients. (Highest tertile: TSP-1 level > 4.6 μg/mL, middle tertile: 3 μg/mL < TSP-1 level ≤ 4.6 μg/mL, lowest tertile: TSP-1 level ≤ 3 μg/mL). TSP-1, Thrombospondin-1.
Table 4. Multivariate Cox proportional hazards analyses of thrombospondin-1 level for all-cause and cardiovascular mortality during 3-year follow-up .
| n | Model 1 | Model 2 | |||||
| HR | 95% CI | p | HR | 95% CI | p | ||
| All-cause mortality | |||||||
| Lowest tertile | 48 | 1.0 | 1.0 | ||||
| Middle tertile | 45 | 3.15 | 1.00-9.91 | 0.05 | 2.05 | 0.28-14.89 | 0.48 |
| Highest tertile | 47 | 5.32 | 1.78-15.94 | 0.003 | 8.71 | 1.36-55.68 | 0.02 |
| p for trend | 0.002 | 0.009 | |||||
| Cardiovascular mortality | |||||||
| Lowest tertile | 48 | 1.0 | 1.0 | ||||
| Middle tertile | 45 | 1.76 | 0.29-10.55 | 0.57 | 1.34 | 0.02-88.03 | 0.89 |
| Highest tertile | 47 | 6.75 | 1.49-30.60 | 0.01 | 25.19 | 0.76-834.65 | 0.07 |
| p for trend | 0.005 | 0.04 |
Model 1 adjusted for age and gender; Model 2 adjusted for age, gender, hypertension, diabetes, fasting glucose, albumin, left ventricular ejection fraction, cilostazol treatment, and pre-existing CVD. CI, confidence internal; HR, hazard ratio.
DISCUSSION
To the best of our knowledge, this is the first presented study to evaluate the association of circulating TSP-1 levels with CVD and mortality in patients under regular hemodialysis. We demonstrated that TSP-1 concentrations were significantly higher in the ESRD patients with CVD than those without CVD. Higher TSP-1 levels were independently associated with worse overall survival within the 3-year follow-up period.
Previous studies on TSP-1 expression mostly focused on its role in tissue repair, including hemostasis, cell adhesion, migration, proliferation, extracellular matrix expression and organization, and regulation of growth factor activity.17 Nevertheless, it is also involved in multiple steps of the inflammatory process and regulates cardiovascular function. TSP-1 is produced by all vascular cell types, and it modifies cellular response from an extracellular position. TSP-1 limits the angiogenic activity of NO in endothelial cells, its vasodilator activity in vascular smooth muscle, and its antithrombotic activity in platelets.18 Loss of either TSP-1 expression or its receptor CD47 in transgenic mice results in hyperdynamic response to NO and shows improved abilities to respond to ischemic stress.19 In an animal model of atherosclerosis, an increased TSP-1 level in the matrix surrounding arterial vascular smooth muscle cell also supported its role in vascular function regulation.20 A study conducted by Smadja et al. demonstrated that PAD patients had higher TSP-1 concentrations than the healthy controls, and the increased TSP-1 level was believed to reflect an overexpression in ischemic tissues.15 This result was supported by our finding that patients with abnormal ABI had significantly higher TSP-1 levels than those with normal ABI (5.00 ± 3.60 vs. 3.80 ± 1.96 μg/mL, p = 0.01). Moreover, the cross-sectional analysis not only confirmed the correlation between TSP-1 level and CVD in ESRD patients, the prospective survival analysis in our study further extended its role into outcome prediction.
Another notable finding in our study was the relationship between plasma TSP-1 concentration, LVEF, and scar burden in ESRD patients. Because TSP-1 level was negatively correlated with LVEF but positively correlated with scar burden, we suggest that the elevated TSP-1 concentrations in the patients with higher scar burden and impaired LVEF reflect the ongoing process of tissue fibrosis, remodeling, and angiogenesis, which might not be limited in the myocardium. In fact, the roles of TSP-1 expression in cardiac remodeling and heart failure have been investigated recently. TSP-1 is a known regulator of latent TGF-β activation in multiple cell types, including cardiac fibroblast, and plays a role in wound healing and fibrosis. Induced in healing myocardial infarcts, TSP-1 is found to suppress postinfarction inflammatory response, inhibit local angiogenesis, and limit expansion of granulation tissue into the noninfarcted area.21 In diabetic cardiomyopathy, cardiac fibrosis is associated with marked TSP-1 upregulation, which is believed to play an important role in matrix preservation and dilative remodeling prevention.22 In an animal model of age-related heart remodeling and heart failure, increased TSP-1 levels in cardiomyocytes identified the failure-prone heart.23 Circulating TSP-1 levels were also demonstrated to be higher in patients with acute heart failure than in healthy controls.24
The causal relationship between TSP-1 and CVD in ESRD is another important issue. Plasma TSP-1 concentrations may indicate the process of fibrosis, remodeling, and angiogenesis after arterial stenosis, and the upregulated TSP-1 could have direct impact on vascular function. In our study, the major causes of renal failure consisted of diabetes, hypertension, glomerulonephritis, and interstitial nephritis, which might have contributed and/or interfered with the predictive value of TSP-1 expression. A growing body of evidence suggests that high glucose stimulates TSP-1 expression in various cell types, and leptin upregulates TSP-1 expression in vascular smooth muscle cell.25,26 Furthermore, TSP-1 modulates TGF-β related renal injury in patient with diabetes and obesity.27,28 Our results also demonstrated that TSP-1 levels were higher in those with diabetes (4.80 ± 3.50 vs. 3.71 ± 1.57, p = 0.02). However, compared with 40 age- and sex-matched controls at risk for CVD with preserved renal function, our hemodialysis patients did not have significantly higher TSP-1 concentrations (p = 0.37, data not shown).
Our present study had some limitations. First, the sample size and follow-up duration were inadequate to obtain conclusive data. Second, the causal relationship between TSP-1 and CVD in patients with ESRD could not be clarified in this cross-sectional study design. Finally, the differences in plasma level between before and those after hemodialysis need further investigation. However, TSP-1 is a large homotrimeric molecule (420 kDa), a feature precluding its dialysability.
CONCLUSIONS
Plasma TSP-1 concentration was higher in hemo-dialysis patients with preexisting clinical evidence of CVD than those without, and was also an independent predictor of all-cause mortality during the 3-year follow-up period. These findings provide evidence of the correlation between TSP-1 and CVD in ESRD. TSP-1 expression deserves further comprehensive pathophysiological study and should be considered as a novel parameter in outcome prediction in patients undergoing hemodialysis.
Acknowledgments
We are grateful to those colleagues from Taoyuan General Hospital for referring hemodialysis patients and also acknowledge Ms. Yu-Shuan Hung for her technical assistance.
REFERENCES
- 1.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32:S112–S119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 2.Sarnak MJ, Levey AS, Schoolwerth AC, et al. American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 3.Cibulka R, Racek J. Metabolic disorders in patients with chronic kidney failure. Physiol Res. 2007;56:697–705. doi: 10.33549/physiolres.931128. [DOI] [PubMed] [Google Scholar]
- 4.Kalantar-Zadeh K, Block G, Humphreys MH, et al. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003;63:793–808. doi: 10.1046/j.1523-1755.2003.00803.x. [DOI] [PubMed] [Google Scholar]
- 5.Stenvinkel P, Carrero JJ, Axelsson J, et al. Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: how do new pieces fit into the uremic puzzle? Clin J Am Soc Nephrol. 2008;3:505–521. doi: 10.2215/CJN.03670807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawler JW, Slayter HS, Coligan JE. Isolation and characterization of a high molecular weight glycoprotein from human blood platelets. J Biol Chem. 1978;253:8609–8616. [PubMed] [Google Scholar]
- 7.Lopez-Dee Z, Pidcock K, Gutierrez LS. Thrombospondin-1:multiple paths to inflammation. Mediators Inflamm. 2011;2011:296069. doi: 10.1155/2011/296069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawler PR, Lawler J. Molecular basis for the regulation of angiogenesis by thrombospondin-1 and -2. Cold Spring Harb Perspect Med. 2012;2:a006627. doi: 10.1101/cshperspect.a006627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ren B, Yee KO, Lawler J, et al. Regulation of tumor angiogenesis by thrombospondin-1. Biochim Biophys Acta. 2006;1765:178–188. doi: 10.1016/j.bbcan.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Agah A, Kyriakides TR, Lawler J, et al. The lack of thrombospondin-1 (TSP1) dictates the course of wound healing in double-TSP1/TSP2-null mice. Am J Pathol. 2002;161:831–839. doi: 10.1016/S0002-9440(10)64243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frangogiannis NG, Ren G, Dewald O, et al. Critical role of endogenous thrombospondin-1 in preventing expansion of healing myocardial infarcts. Circulation. 2005;111:2935–2942. doi: 10.1161/CIRCULATIONAHA.104.510354. [DOI] [PubMed] [Google Scholar]
- 12.Roberts DD, Miller TW, Rogers NM, et al. The matricellular protein thrombospondin-1 globally regulates cardiovascular function and responses to stress via CD47. Matrix Biol. 2012;31:162–169. doi: 10.1016/j.matbio.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi KY, Kim DB, Kim MJ, et al. Higher plasma thrombospondin-1 levels in patients with coronary artery disease and diabetes mellitus. Korean Circ J. 2012;42:100–106. doi: 10.4070/kcj.2012.42.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bauer PM, Bauer EM, Rogers NM, et al. Activated CD47 promotes pulmonary arterial hypertension through targeting caveolin-1. Cardiovasc Res. 2012;93:682–693. doi: 10.1093/cvr/cvr356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smadja DM, d'Audigier C, Bièche I, et al. Thrombospondin-1 is a plasmatic marker of peripheral arterial disease that modulates endothelial progenitor cell angiogenic properties. Arterioscler Thromb Vasc Biol. 2011;31:551–559. doi: 10.1161/ATVBAHA.110.220624. [DOI] [PubMed] [Google Scholar]
- 16.Tilkemeier PL, Wackers FJ Quality Assurance Committee of the American Society of Nuclear Cardiology. Myocardial perfusion planar imaging. J Nucl Cardiol. 2006;13:e91–e96. doi: 10.1016/j.nuclcard.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Sweetwyne MT, Murphy-Ullrich JE. Thrombospondin1 in tissue repair and fibrosis:TGF-β-dependent and independent mechanisms. Matrix Biol. 2012;31:178–186. doi: 10.1016/j.matbio.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isenberg JS, Frazier WA, Krishna MC, et al. Enhancing cardiovascular dynamics by inhibition of thrombospondin-1/CD47 signaling. Curr Drug Targets. 2008;9:833–841. doi: 10.2174/138945008785909338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isenberg JS, Hyodo F, Matsumoto K, et al. Thrombospondin-1 limits ischemic tissue survival by inhibiting nitric oxide-mediated vascular smooth muscle relaxation. Blood. 2007;109:1945–1952. doi: 10.1182/blood-2006-08-041368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riessen R, Kearney M, Lawler J, et al. Immunolocalization of thrombospondin-1 in human atherosclerotic and restenotic arteries. Am Heart J. 1998;135:357–364. doi: 10.1016/s0002-8703(98)70105-x. [DOI] [PubMed] [Google Scholar]
- 21.Frangogiannis NG, Ren G, Dewald O, et al. Critical role of endo-genous thrombospondin-1 in preventing expansion of healing myocardial infarcts. Circulation. 2005;111:2935–2942. doi: 10.1161/CIRCULATIONAHA.104.510354. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez-Quesada C, Cavalera M, Biernacka A, et al. Thrombospondin-1 induction in the diabetic myocardium stabilizes the cardiac matrix,while promoting vascular rarefaction through angiopoietin-2 upregulation. Circ Res. 2013;113:1331–1344. doi: 10.1161/CIRCRESAHA.113.302593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Almen GC, Verhesen W, van Leeuwen RE, et al. MicroRNA-18 and microRNA-19 regulate CTGF and TSP-1 expression in age-related heart failure. Aging Cell. 2011;10:769–779. doi: 10.1111/j.1474-9726.2011.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vila V, Martínez-Sales V, Almenar L, et al. Inflammation, endothelial dysfunction and angiogenesis markers in chronic heart failure patients. Int J Cardiol. 2008;130:276–277. doi: 10.1016/j.ijcard.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Chavez RJ, Haney RM, Cuadra RH, et al. Upregulation of thrombospondin-1 expression by leptin in vascular smooth muscle cells via JAK2- and MAPK-dependent pathways. Am J Physiol Cell Physiol. 2012;303:C179–C191. doi: 10.1152/ajpcell.00008.2012. [DOI] [PubMed] [Google Scholar]
- 26.Maier KG, Han X, Sadowitz B, et al. Thrombospondin-1: a proatherosclerotic protein augmented by hyperglycemia. J Vasc Surg. 2010;51:1238–1247. doi: 10.1016/j.jvs.2009.11.073. [DOI] [PubMed] [Google Scholar]
- 27.Lu A, Miao M, Schoeb TR, et al. Blockade of TSP1-dependent TGF-beta activity reduces renal injury and proteinuria in a murine model of diabetic nephropathy. Am J Pathol. 2011;178:2573–2586. doi: 10.1016/j.ajpath.2011.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cui W, Maimaitiyiming H, Qi X, et al. Thrombospondin 1 mediates renal dysfunction in a mouse model of high-fat diet-induced obesity. Am J Physiol Renal Physiol. 2013;305:F871–F880. doi: 10.1152/ajprenal.00209.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]


