Abstract
Background
In this study, we aimed to evaluate the effects of niacin on high sensitivity C reactive protein (hs-CRP) and cholesterol levels in non-ST elevated acute coronary syndrome (NSTE-ACS) patients.
Methods
In this prospective, open label study, 48 NSTE-ACS were randomized to niacin or control group. Patients continued their optimal medical therapy in the control group. In the niacin group patients were assigned to receive extended-release niacin 500 mg/day. Patients were contacted 1 month later to assess compliance and side effects. Blood samples for hs-CRP were obtained upon admittance to the coronary care unit, in the third day and in the first month of the treatment. Fasting blood samples for cholesterol levels were obtained before and 30 days after the treatment. The primary end point of the study was to evaluate changes in hs-CRP, cholesterol levels, short-term cardiovascular events, and the safety of niacin in NSTE-ACS.
Results
Baseline demographic, clinical and laboratory characteristics were similar between the two groups. Logarithmic transformation of baseline and 3rd day hs-CRP levels were similar between the groups; but 1 month later, logarithmic transformation of hs-CRP level was significantly lower in the niacin group (0.43 ± 0.39 to 0.83 ± 0.91, p = 0.04). HDL-C level was significantly increased in the niacin group during follow-up. Drug related side effects were seen in 7 patients in the niacin group but no patients discontinued niacin.
Conclusions
Our findings demonstrate that lower dose extended release niacin can be used safely and decreases hs-CRP and lipid parameters successfully in NSTE-ACS patients.
Keywords: Acute coronary syndrome, hs-CRP, Inflammation, Niacin
INTRODUCTION
Niacin, a potent agent for raising high density lipoprotein cholesterol levels, is able to prevent rupture by stabilizing plaque and reducing the rate of atherosclerosis progression.1 Inflammation plays a crucial role in atherogenesis2 and the inflammatory biomarker high-sensitivity C-reactive protein (hs-CRP) independently predicts vascular events.3 The magnitude of benefit associated with medical therapy relates in part to achieving levels of hs-CRP in acute coronary syndrome as well as stable patients.4
Niacin is a potent agent for raising high-density lipoprotein-cholesterol (HDL-C) levels. Recent data suggests beneficial effects of niacin on inflammation and endothelial function when added to existing statin therapy in patients with and without coronary artery disease.5,6 Niacin also has beneficial effects on plaque progression.7 Extended-release niacin (1000 mg/ day) is associated with significant decrease in carotid intima media thickness (cIMT) progression.8 However, although the effects of niacin in clinically stable patients are well-established, there is not any prospective randomized trial evaluating its effects in non-ST elevated acute coronary syndrome (NSTE-ACS) patients.
In the present study, we aimed to evaluate the effects of extended-release niacin on inflammation, short-term clinical events and cholesterol levels in NSTE-ACS patients.
MATERIALS AND METHODS
Study design and patients
Forty-eight patients who were admitted to our hospital between September 2008 and January 2010 with NSTE-ACS and accepted to participate to study were randomized to control or niacin groups. A random-generated numbers table was used for randomization. Patient information including concomitant risk factors for coronary artery disease, diagnoses, blood analyses results, recurrent angina during hospitalization, arrhythmia and cardiac arrest, possible side effects of niacin and treatments were recorded.
Inclusion criteria were: > 18 years of age, having known dyslipidemia or newly diagnosed dyslipidemia, and not having any exclusion criteria. Exclusion criteria were: anti-inflammatory drug use, liver failure, creatinin value > 1.5 mg/dL, presence of active peptic ulcer, presence of arterial hemorrhage, niacin usage and/or niacin allergy, presence of systemic inflammatory disease, or presence of active infection.
Fibrates were allowed to be used in patients as necessary, but fibrates were not needed in any of our patients. Patients were randomized to the control group (receiving optimal treatment including statin therapy) or the niacin group (that were given additional 500 mg extended-release niacin therapy to optimal treatment). We preferred to start with a lower dose because there is insufficient evidence supporting the safety of niacin use in acute coronary syndromes, and also it is recommended to start 500 mg/daily in its drug prospectus.
Blood samples for hs-CRP (Abbott, Sentinel Diagnostics, Italy) were taken three times from all patients: 1) upon admittance to coronary care unit, 2) on the third day, and 3) in the first month of the treatment. Fasting blood samples for cholesterol levels (Roche Diagnostics GmbH, Germany) were taken twice from all patients: at the time of admission to the coronary care unit and at the end of first month of the treatment. All patients were observed closely for possible side effects of niacin and clinical events during hospitalization.
This study has been approved by the Baskent University Clinical Research Ethics Committee with the decision number of 08/158 at 06 Aug 2008. Written informed consents were taken from all patients.
Statistical analyses
The statistical package program SPSS 11.5 (Statistical Package for the Social Sciences, version 11.5, SPSS Inc, Chicago, IL, USA) was used for statistical analyses. The results of statistical analysis were expressed with n (%) for categorical variables and average ± standard deviation and the median for continuous variables. A level of p < 0.05 was accepted as statistically significant for all tests. For continuous variables, the Kolmogorov-Smirnov distribution test was used to measure whether they have normal distribution or not. To compare the normal distributed quantitative data, initial values, first control values and second control values, the paired sample t-test was used. For the parameters that do not have normal distribution, the Mann-Whitney U Test” was utilized. Logarithmic transformation (log) was performed for parameters not showing normal distribution. To measure change depends on time span at hs-CRP, HDL, low-density lipoprotein (LDL), and triglyceride (TG) for their repeated measurements, repeated measure ANOVA model was used.
We determined the sample sizes for the statistical tests by power/sample size formulas. The power analyses of the tests were conducted to determine the number of participants needed to detect the critical value with an adequate level of statistical power. Alpha levels for the analyses were set at 0.05. To achieve power of 0.80 sample size of 25 in per groups were adequate. We expected 15% decrease in hs-CRP levels under niacin therapy and calculated sample size according to this alpha value.
RESULTS
Patient characteristics
A total of 48 patients (30 men and 18 women) with a mean age of 63 were included in the study. All patients were dyslipidemic and already on statin therapy. There were no significant differences between groups with regard to demographics and baseline characteristics (Table 1). Distributions of risk factors were also similar between groups. The medications that patients received during hospitalization were similar between the groups as seen in Table 1. Two patients refused coronary intervention in the niacin group and 1 patient in control group was in a low-risk group and treated medically. All other patients underwent coronary intervention.
Table 1. Clinical and laboratory characteristics of patients .
| Niacin group (n = 25) | Control group (n = 23) | p value | |
| Age, years | 63 ± 12 | 64 ± 13 | 0.64 |
| Gender, Female, n (%) | 9 (36) | 9 (39) | 0.82 |
| Male, n (%) | 16 (64) | 14 (61) | 0.76 |
| Diagnose, n (%) | 0.28 | ||
| Unstable angina pectoris | 16 (64) | 18 (78) | |
| NSTE-MI | 9 (36) | 5 (21) | |
| Treatment strategy, n (%) | 0.50 | ||
| Invasive | 24 | 21 | |
| Conservative | 1 | 2 | |
| Dislipidemia, n (%) | 25 (100) | 23 (100) | 1.0 |
| Diabetes mellitus, n (%) | 11 (44) | 8 (34) | 0.51 |
| Hypertension, n (%) | 14 (56) | 17 (74) | 0.20 |
| Smoking, n (%) | 16 (64) | 9 (39) | 0.09 |
| Heart failure, n (%) | 6 (24) | 10 (43) | 0.15 |
| Medications | |||
| ASA, n (%) | 23 (92) | 21 (91) | 0.93 |
| β-blocker, n (%) | 19 (76) | 17 (73) | 0.92 |
| ACEİ, n (%) | 21(84) | 19 (82) | 0.90 |
| Nitrate, n (%) | 18 (72) | 19 (82) | 0.38 |
| Heparin (UFH/LMWH), n (%) | 24 (96) | 23 (100) | 0.33 |
| Statin, n (%) | |||
| Atorvastatin 10 mg | 9 (36) | 7 (30) | 0.73 |
| Atorvastatin 20 mg | 7 (28) | 7 (30) | 0.87 |
| Atorvastatin 40 mg | 7 (28) | 6 (26) | 0.62 |
| Rosuvastatin 10 mg | 2 (8) | 3 (13) | 0.24 |
| CCB, n (%) | 9 (36) | 5 (21) | 0.28 |
| Clopidogrel, n (%) | 14 (56) | 12 (52) | 0.79 |
| Laboratory | |||
| Troponin-I (ng/mL) | 4.6 ± 13.7 | 6.7 ± 16.2 | 0.63 |
| Creatin (mg/dL) | 0.9 ± 0.2 | 0.9 ± 0.1 | 0.64 |
| Hemoglobin (g/dL) | 13.9 ± 1.5 | 13.8 ± 1.7 | 0.80 |
ACEi, angiotensin converting enzyme inhibitors; ASA, acetyl salicylic aside; CCB, calcium channel blockers; dL, deciliter, LMWH, low molecule weight heparin; mg, milligram; NSTE-MI, non ST elevated myocardial infarction; UFH, unfractioned heparin.
Lipid parameters
Baseline and 1 month of fasting HDL-C, LDL-C, triglyceride, creatinine, and haemoglobin levels were similar between the niacin and control groups (Table 1, Table 2, Table 3). In the follow-up, treatment with niacin was associated with a significant increase in HDL-C (35 ± 7 mg/dL at baseline; 40 ± 10 mg/dL 1 month later, p = 0.049). Whereas in the control group, HDL-C was unchanged after 1 month (39 ± 12 mg/dL at baseline; 37 ± 11 mg/dL 1 month later). LDL-C levels decreased significantly in the niacin group (105 ± 35 mg/dl at baseline to 88 ± 28 mg/dl 1 month later, p = 0.004) and were unchanged in the control group (92 ± 28 mg/dl at baseline and 76 ± 25 mg/dl 1 month later, p = 0.054). TG levels also decreased significantly in the niacin group (148 ± 65 mg/dl at baseline and 137 ± 58 mg/dl 1 month later, p = 0.04) whereas no significant difference was detected in the control group (156 ± 55 mg/dl at baseline and 148 ± 80 mg/dl 1 month later, p = 0.61). However, although group changes of lipid parameters were significant in the Niacin group, changes between groups were not significant (Table 3).
Table 2. Baseline and follow up log [hs-CRP] levels .
| Niacin group (n = 25) | Control group (n = 23) | p value | |
| log [Baseline hs-CRP (mg/L)] | 0.61 ± 0.42 | 0.49 ± 0.71 | 0.49 |
| log [3rd day hs-CRP (mg/L)] | 0.72 ± 0.39 | 0.84 ± 0.77 | 0.58 |
| log [1 month later hs-CRP (mg/L)] | 0.43 ± 0.39 | 0.83 ± 0.91 | 0.04 |
p value of hs-CRP changes between groups = 0.783.
hs-CRP, high sensitive C-reactive protein; log, logarithmic transformation; mg/L, milligram/liter.
Table 3. Baseline and follow up HDL-C, LDL-C and TG levels .
| Niacin | Control | p valuea | p valueb | p valuec | |||
| Baseline | 1 month later | Baseline | 1 month later | ||||
| HDL-C (mg/dL) | 35 ± 7 | 40 ± 10 | 39 ± 12 | 37 ± 11 | 0.049 | 0.86 | 0.97 |
| LDL-C (mg/dL) | 105 ± 35 | 88 ± 28 | 92 ± 28 | 76 ± 25 | 0.004 | 0.054 | 0.24 |
| TG (mg/dL) | 148 ± 65 | 137 ± 58 | 156 ± 55 | 148 ± 80 | 0.04 | 0.61 | 0.48 |
a p value of HDL-C, LDL-C, TG level changes within niacin group; b p value HDL-C, LDL-C, TG level changes within control group; c p value of HDL-C, LDL-C, TG level changes between groups.
dL, deciliter; HDL-C, high density lipoprotein; LDL-C, low density lipoprotein; mg, milligram; TG, triglyceride.
Changes in hs-CRP
Logarithmic transformation was performed for hs-CRP levels because hs-CRP levels did not show normal distribution. Log [Baseline hs-CRP level]s were similar between the two groups (0.61 ± 0.42 to 0.49 ± 0.71, p = 0.49). Level of hs-CRP decreased significantly in patients treated with niacin for 1 month (0.61 ± 0.42 to 0.43 ± 0.39, p < 0.001), whereas hs-CRP was statistically unchanged after 1 month in the control group. hs-CRP levels 1 month later were significantly higher in the control group than in the niacin group (p = 0.04; Table 2).
On the 3rd day, hs-CRP levels increased in both groups. There was a further increase in the control group compared to baseline, but this increase was not significant between the groups (0.72 ± 0.39 to 0.84 ± 0.77, p = 0.58) (Table 2).
Alteration of hs-CRP levels are seen in Figure 1. Alteration of hs-CRP during follow-up was significant in the niacin group when compared to baseline levels (p < 0.001) but this alteration is not statistically significant when compared between the groups (p = 0.78).
Figure 1.
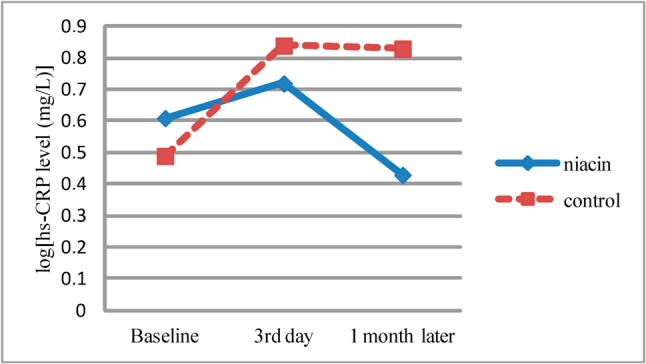
Alteration of log[hs-CRP levels]. p value of hs-CRP changes in niacin group < 0.001; p value of hs-CRP changes between groups = 0.783. hs-CRP, high sensitive C-reactive protein, log, logarithmic transformation, mg/L, milligram/liter.
Clinical events and side effects
Groups were similar in terms of early clinical events like recurrent angina, arrhythmia and cardiac arrest. During one month follow-up, 2 deaths were noted in the control group. There were no deaths in the niacin group but this finding was not statistically significant. (p = 0.21) (Table 4).
Table 4. Distribution of early clinical events and possible side effects of niacin in the 1 month follow up .
| Niacin group (n = 25) | Control group (n = 23) | p value | |
| Recurrent angina, n (%) | 3 (12) | 6 (26) | 0.27 |
| Arrhythmia, n (%) | 1 (4) | 3 (13) | 0.61 |
| Death, n (%) | 0 (0) | 2 (8) | 0.21 |
| Flushing, n (%) | 4 (16) | 0 (0) | 0.97 |
| Myalgia, n (%) | 2 (8) | 1 (4) | 0.88 |
| Swelling, n (%) | 1 (4) | 2 (8) | 0.88 |
Possible side effects of niacin were seen in 7 patients and the most common side effect was flushing (4 patients) (Table 4). The remaining patients tolerated treatment without subjective complaints and drug discontinuation was not needed in any patients.
DISCUSSION
In the present study, we demonstrate that additional lower dose extended release niacin therapy has beneficial effects on lipoproteins and inflammatory markers, but no beneficial effects on short term clinical events in NSTE-ACS patients. We also demonstrate that lower dose extended release niacin therapy can be used safely in NSTE-ACS patients.
Previously, the effects of niacin on inflammation and lipid parameters in patients with NSTE-ACS had not been sufficiently evaluated. In our study, compared with the control group, one month niacin therapy significantly increased HDL-C levels and decreased TG levels. These findings are compatible with previous studies.8-10 Additionally, LDL-C levels decreased significantly in the niacin group. This impact of niacin may be associated with the inhibition of free fatty acid (FAA) release from adipose tissue and reduction of TG-rich very low density lipoprotein (VLDL) production in the liver.11 Niacin activates Gi-protein coupled receptors in adipose tissue, which inhibits adenylate cyclase activity and lowers intracellular cyclic adenosine monophosphate (cAMP). Reduction in cAMP reduces the formation and release of FAA into plasma.12 In animal models, reduced FFA release attenuated the activity of peroxisome and assembly of TG-rich VLDL in the liver.13 Since VLDL is converted into IDL and then LDL by lipoprotein lipase, reductions in VLDL lead to lower LDL-C levels.11
A one month interval was chosen to measure cholesterol and hs-CRP levels because both inflammatory process and cholesterol levels become within normal ranges one month after NSTE-ACS.
In addition to having a favorable effect on lipoproteins, we also established that niacin therapy can lead to a significant decrease in hs-CRP levels. This finding provides evidence that niacin therapy may improve the vascular environment, and is compatible with previous studies evaluating the use of extended release niacin in patients with coronary disease.5 This finding may also be interpreted as evidence showing that the anti-inflammatory effect of niacin therapy starts in the acute phase and continues in the sub-acute phase of vascular disease, similar to statins.
Previously, no significant hs-CRP reduction was detected with niacin therapy in the ARBITER-2 trial.8 This difference may be associated with the drug compound because we used the commercially available form of extended release niacin in our country (Niascor ER), and not the same compound studied in the ARBITER-2 trial (Niaspan).
In our study, baseline hs-CRP levels were above 3 mg/L in both groups (4.4 ± 3.4 mg/L to 4.3 ± 5.6 mg/L); these elevated hs-CRP levels support the fact that the patients enrolled to the study were indeed high risk patients. The distributions of risk factors were similar between the groups, thus manifesting similar effects on hs-CRP in the two groups.
In both groups, although not statistically significant in the niacin group, the hs-CRP levels measured at the 3rd day were higher than the baseline level. This increase, which is expected in the natural history of acute coronary syndromes, may be interpreted as niacin treatment having no acute effects on inflammation. This circumstance may be explained by lower doses of niacin, duration of therapy and the limited number of patients. Nearly all of the previous studies that evaluated effects of niacin and niacin therapy used a dosage of 1000 mg/day.1 We chose 500 mg/day because there was not enough data about the safety of niacin in NSTE-ACS patients. The existing data concerning use of niacin in NTE-ACS patients is derived from 2 case reports of worsening angina after application of crystallized niacin.14 Although the peripheral vasodilatation effect of niacin is well-known, its effects on coronary circulation are unclear. A possible mechanism of worsening angina may be associated with coronary hypoperfusion that occurred as a result of the peripheral vasodilatation effect of niacin.15 Another mechanism of worsening angina may be associated with coronary steal phenomenon caused by selective coronary dilatation in collateral coronary arteries.14 In our study, there were no significant differences between the two groups at the end points of angina, arrhythmia and death. This may be explained by usage of a more tolerable form of the drug (extended release form) instead of crystallized niacin, and successful percutaneous reperfusion treatments that prevent coronary steal. Our entire study population was already on statin therapy. This situation may be interpreted as confirmation of statins’ positive effects on hs-CRP levels, and that such positive effects were similar between the two groups.
Although niacin is used in complicated dyslipidemia, its use is often neglected in daily practice. A 3-month follow-up study that investigated the effects of niacin on lipoproteins and CRP levels in stable coronary artery disease showed a significant increase in HDL levels (7.5%, p < 0.005), a 15% decrease in TG levels (p < 0.005) and a 15% decrease in CRP levels (p < 0.005) with a 3-month niacin treatment.5 Our results are consistent with these findings. We found a statistically significant decrease in hs-CRP levels with just a one-month lower-dose niacin treatment, compared to baseline levels. We also observed a significant increase in the one month HDL-C level in the niacin group, whereas the significance of the TG decrease stayed in the border. However, although variation of the HDL-C level was significant in the niacin group, variation of HDL-C levels between groups was not significant. The use of a lower dose of niacin may explain why this decrease was not significant. Additionally, observation of the control group at just a 1 month interval may be too early to evaluate the effects of niacin on cholesterol levels.
Previously, the HDL-Atherosclerosis Treatment Study (HATS) showed a significant decrease (0.4%) in coronary stenosis with simvastatin and niacin combination therapy (p < 0.001).16 A significant slowdown in coronary lesion progression and a 73% decrease in clinical events with lovastatin and niacin combination therapy were found in the Familial Atherosclerosis Treatment Study (FATS).17
However, we did not find a significant decrease in short-term clinical events with niacin therapy. Repeated angina, arrhythmia, and death ratio were similar between the two groups. Two deaths occurred in the control group, and there was no death in niacin group; but in our opinion, this was an incidental finding and the difference was not statistically significant.
The most common side effect of niacin is flushing. In addition, myalgia, bulge, vertigo, palpitations, shortness of breath, sweat, chills, and oedema can be seen as other side effects arising from niacin use. Taking the drug after acetyl salicylic acid treatment and using the extended - release form helps to prevent side effects such as flushing attacks. A possible side effect that may be related to niacin was seen in 7 patients in niacin group, and there was no significant difference detected compared to the control group. Therefore, drug discontinuation was not required in any patients.
Study limitations
There were several limitations of this study. First, our population was small. Types of statin that patients were already using, doses and time length were not taken into consideration and this situation may result in different effects on hs-CRP. Statin therapies after hospitalization were not standardized and statin types and doses were adjusted by physicians. We used a lower dose of extended-release niacin (500 mg/day) and have no data regarding a dose effect of niacin. The follow-up period was only 1 month, limiting our ability to monitor long-term cardiovascular events. Placebo was not used in the study, thus making it difficult to evaluate the side effects of niacin objectively. The extent of myocardial injury which obviously would influence the increase of hs-CRP and would affect clinical events was not controlled.
Glucose tolerance abnormalities are a well-known side effect of niacin, but sufficient data is not available to evaluate this side effect.
CONCLUSIONS
In this study, we showed that extended-release niacin can be used safely and effectively in NSTE-ACS patients. Early application of niacin provides better cholesterol levels in NSTE-ACS patients. In addition to niacin’s beneficial effects on inflammation, it may also confer atheroprotection. However, larger clinical trials are needed to evaluate niacin’s effects on cardiovascular events. Further investigation is needed to suggest routine use in acute coronary syndromes.
Acknowledgments
None.
REFERENCES
- 1.Thoenes M, Oguchi A, Nagamia S, et al. The effects of extended-release niacin on carotid intimal media thickness, endothelial function and inflammatory markers in patients with the metabolic syndrome. Int J Clin Pract. 2007;61:1942–1948. doi: 10.1111/j.1742-1241.2007.01597.x. [DOI] [PubMed] [Google Scholar]
- 2.Hansson GK, Libby P. The immune response in atherosclerosis:a double-edged sword. Nat Rev Immunology. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 3.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 4.Morrow DA, de Lemos JA, Sabatine MS, et al. Clinical relevance of c-reactive protein during follow-up of patients with acute coronary syndromes in the Aggrastat-to-Zocor Trial. Circulation. 2006;114:281–288. doi: 10.1161/CIRCULATIONAHA.106.628909. [DOI] [PubMed] [Google Scholar]
- 5.Kuvin JT, Dave DM, Sliney KA, et al. Effects of extended release niacin on lipoprotein particle size,distribution and inflammatory markers in patients with coronary artery disease. Am J Cardiol. 2006;98:743–745. doi: 10.1016/j.amjcard.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Benjó AM, Maranhão RC, Coimbra SR, et al. Accumulation of chylomicron remnants and impaired vascular reactivity occur in subjects with isolated low HDL cholesterol:effects of niacin treatment. Atherosclerosis. 2006;187:116–122. doi: 10.1016/j.atherosclerosis.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 7.Hodis HN, Mack WJ, LaBree L, et al. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998;128:262–269. doi: 10.7326/0003-4819-128-4-199802150-00002. [DOI] [PubMed] [Google Scholar]
- 8.Taylor AJ, Sullenberger LE, Lee HJ, et al. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind,placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation. 2004;110:3512–3517. doi: 10.1161/01.CIR.0000148955.19792.8D. [DOI] [PubMed] [Google Scholar]
- 9.Morgan JM, Capuzzi DM, Baksh RI, et al. Effects of extended release niacin on lipoprotein subclass profile. Am J Cardiol. 2003;91:1432–1436. doi: 10.1016/s0002-9149(03)00394-1. [DOI] [PubMed] [Google Scholar]
- 10.Kuvin JT, Ramet ME, Patel AR, et al. A novel mechanism for the beneficial vascular effects of high density lipoprotein cholesterol:enhanced vasorelaxation and increased endothelial nitric oxide synthase expression. Am Heart J. 2002;144:165–172. doi: 10.1067/mhj.2002.123145. [DOI] [PubMed] [Google Scholar]
- 11.Tavintharan S, Kashyap ML. The benefits of niacin in atherosclerosis. Curr Atheroscler Rep. 2001;3:74–82. doi: 10.1007/s11883-001-0014-y. [DOI] [PubMed] [Google Scholar]
- 12.Gille A, Bodor ET, Ahmed K, Offermanns S. Nicotinic acid:pharmacological effects and mechanisms of action. Annu Rev Pharmacol Toxicol. 2008;48:79–106. doi: 10.1146/annurev.pharmtox.48.113006.094746. [DOI] [PubMed] [Google Scholar]
- 13.Hernandez C, Molusky M, Li Y, et al. Regulation of hepatic ApC3 expression by PGC-1b mediates hypolipidemic effect of notinic acid. Cell Metab. 2010;12:411–419. doi: 10.1016/j.cmet.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pasternak RC, Kolmas BS. Unstable myocardial ischemia after the initiation of niacin therapy. Am J Cardiol. 1991;67:904–906. doi: 10.1016/0002-9149(91)90631-t. [DOI] [PubMed] [Google Scholar]
- 15.Weiner M, van Eys J, New York. Nicotinic acid effects on vascular tone. In: Nicotinic Acid: Nutrient Cofactor-Drug. New York: NY: Marcel Dekker; 1983. pp. 273–280. [Google Scholar]
- 16.Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin,antioxidant vitamins,or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583–1592. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 17.Brown G, Albers JJ, Fisher LD, et al. Regression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B. N Engl J Med. 1990;323:1289–1298. doi: 10.1056/NEJM199011083231901. [DOI] [PubMed] [Google Scholar]


