Abstract
The ability to systematically disrupt genes serves as a powerful tool for understanding their function. The programmable Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-associated protein 9 (CRISPR/Cas9) system enables efficient targeting of large numbers of genes through the use of single-guide RNA (sgRNA) libraries. In cultured mammalian cells, collections of knockout mutants can be readily generated via transduction of Cas9/sgRNA lentiviral pools, screened for a phenotype of interest, and tracked using high-throughput DNA sequencing. This technique represents the first general method for undertaking systematic loss-of-function genetic screens in mammalian cells. In this chapter, we outline the steps for conducting CRISPR-based screens from the initial library design to final data analysis and provide guidelines for developing an appropriate screening strategy.
Introduction
The molecular underpinnings of many fundamental cellular pathways have been deciphered through unbiased genetic screens in microorganisms. However, similar studies in human cells have been hampered by a lack of suitable tools for manipulating their large, diploid genomes, limiting our understanding of the genes and biological processes unique to mammals. Recently, the bacterial CRISPR/Cas9 adaptive immune system has been co-opted to enable efficient, sequence-specific DNA cleavage in cultured cells and whole organisms, greatly expanding the toolbox for mammalian geneticists (Cong et al. 2013; Mali et al. 2013; Wang et al. 2013). In contrast to previous genome editing techniques, targeting reagents for the CRISPR/Cas9 system can be rapidly generated as the target specificity is dictated by a short 20-bp sequence at the 5’-end of the sgRNA. This ease of construction allows the generation of large scale libraries targeting all (or a desired subset) of the protein-coding genes encoded in a mammalian genome using microarray-based oligonucleotide synthesis. Using this approach, we and others have developed a general method for performing systematic loss-of-function genetic screens in mammalian cells (Shalem et al. 2014; Wang et al. 2014).
In this chapter, we outline the steps required to carry out a CRISPR-based screen. Additional considerations relating to the design of screens and validation of hits will not be discussed at length in this chapter. For these topics, we refer the reader to (Moffat and Sabatini 2006), (Boutros and Ahringer 2008), and (Kaelin 2012).
Screen principle
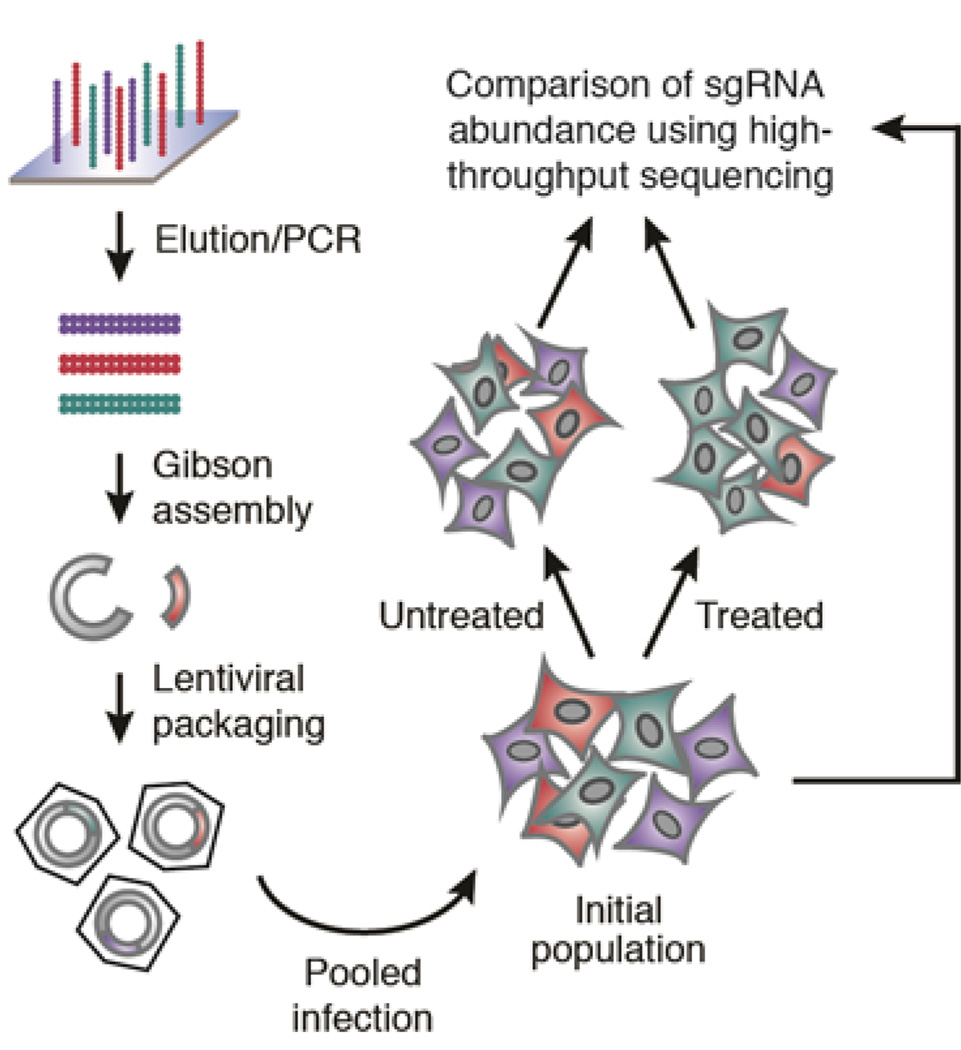
The bacterial CRISPR/Cas9 system has been co-opted for mammalian genome editing, allowing for the rapid generation of isogenic cell lines and mice with modified alleles. By using pooled libraries expressing tens of thousands of sgRNAs, the scale of this technology can be greatly expanded, enabling loss-of-function genetic screening of all protein-coding genes in mammalian cells. In this method, sgRNA expression constructs are generated by array-based oligonucleotide library synthesis and packaged into lentiviral particles (Fig. 1). Target cells of interest can then be transduced with the lentiviral sgRNA pools to generate a collection of knockout mutants via Cas9-mediated genomic cleavage. Finally, through high-throughput sequencing of the integrated expression cassettes, the number of cells bearing each sgRNA in the mutant collection can be monitored over time to pinpoint the mutants of interest.
Figure 1. Schematic of sgRNA library construction and genetic screening strategy.
Positive selection screens
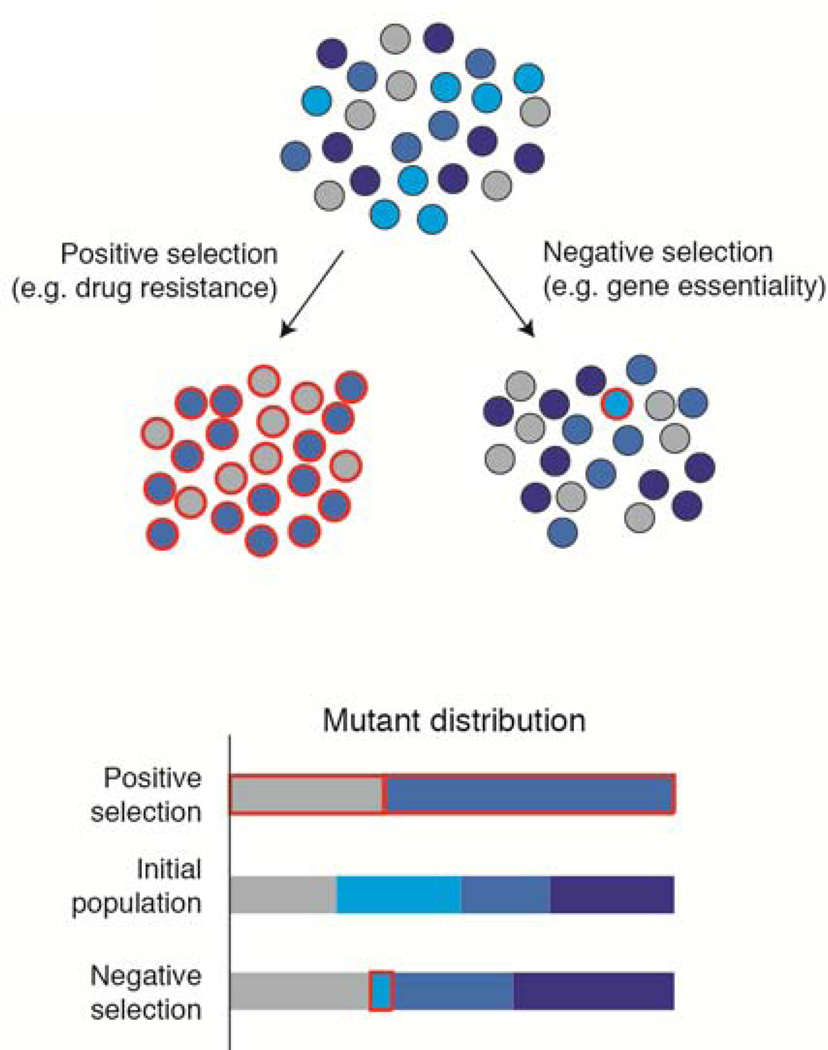
Pooled screens can be divided into two classes, positive selection and negative selection, which can often reveal complementary biological information (Fig. 2). In positive selection screens, disruption of the genes of interest confers a selective advantage on cells, allowing them to rise to high frequency. As a result, gene candidates can be readily identified.
Figure 2. Positive and negative selection screens.
The mutants of interest (outlined in red) rise to high frequency in positive selection screens but are underrepresented in negative selection screens, requiring more precise methods for their detection.
Some biological processes, such as drug resistance or anchorage-independent growth, are ideally suited for positive selection screening as they are intrinsically linked to cellular proliferation and survival. For studying other processes, additional selection strategies can be devised. For example, cells can be engineered to express a selectable marker in a pathway activity dependent manner or isolated in screens using fluorescence-activated cell sorting (FACS) (Duncan et al. 2012; Lee et al. 2013). Together, these approaches can greatly broaden the diversity of phenotypes amenable for screening.
Negative selection screens
Negative selection screens seek to identify genes whose inactivation is detrimental to cells. Such genes can be recognized by a decrease in the abundance of corresponding sgRNAs during the course of a screen. While conceptually simple, identifying such sgRNAs poses a significant technical challenge for pooled CRISPR-based screens. First, negative selection screens require potent sgRNAs, because depletion of a sgRNA can only be observed if the gene target is cleaved and inactivated in a large proportion of the cells carrying the sgRNA. Additionally, during the infection of the library, it is necessary to introduce each sgRNA into a large number (≈1000) of target cells. This high level of representation of the library in the initial cell population ensures that a ‘drop-out’ of cells carrying a deleterious sgRNA can be reliably distinguished from random changes in abundance resulting from sampling fluctuations. For genome-wide screens, particularly in primary cells or in vivo, it may be impractical or impossible to infect and culture cells at the required scale. In these cases, secondary screens using a sub-library of sgRNAs targeting candidate genes can serve as a powerful tool for validation.
Acknowledgments
This work was supported by the U.S. National Institutes of Health (CA103866) (D.M.S.), National Human Genome Research Institute (2U54HG003067-10) (E.S.L.), the Broad Institute of MIT and Harvard (E.S.L.), and an award from the U.S. National Science Foundation (T.W.). T.W, E.S.L., and D.M.S. are inventors on a patent application relating to aspects of the work described in this chapter.
References
- Boutros M, Ahringer J. The art and design of genetic screens: RNA interference. Nat Rev Genet. 2008;9:554–566. doi: 10.1038/nrg2364. [DOI] [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, et al. Multiplex Genome Engineering Using CRISPR/Cas Systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan LM, Timms RT, Zavodszky E, Cano F, Dougan G, Randow F, Lehner PJ. Fluorescence-Based Phenotypic Selection Allows Forward Genetic Screens in Haploid Human Cells. PLoS ONE. 2012;7:e39651. doi: 10.1371/journal.pone.0039651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin WG. Use and Abuse of RNAi to Study Mammalian Gene Function. Science. 2012;337:421–422. doi: 10.1126/science.1225787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Carette JE, Brummelkamp TR, Ploegh HL. A Reporter Screen in a Human Haploid Cell Line Identifies CYLD as a Constitutive Inhibitor of NF-κB. PLoS ONE. 2013;8:e70339. doi: 10.1371/journal.pone.0070339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM. RNA-Guided Human Genome Engineering via Cas9. Science. 2013;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat J, Sabatini DM. Building mammalian signalling pathways with RNAi screens. Nat Rev Mol Cell Biol. 2006;7:177–187. doi: 10.1038/nrm1860. [DOI] [PubMed] [Google Scholar]
- Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelsen TS, Heckl D, Ebert BL, Root DE, Doench JG, et al. Genome-Scale CRISPR-Cas9 Knockout Screening in Human Cells. Science. 2014;343:84–87. doi: 10.1126/science.1247005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yang H, Shivalila Chikdu S, Dawlaty Meelad M, Cheng Albert W, Zhang F, Jaenisch R. One-Step Generation of Mice Carrying Mutations in Multiple Genes by CRISPR/Cas-Mediated Genome Engineering. Cell. 2013;153:910–918. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Wei JJ, Sabatini DM, Lander ES. Genetic Screens in Human Cells Using the CRISPR-Cas9 System. Science. 2014;343:80–84. doi: 10.1126/science.1246981. [DOI] [PMC free article] [PubMed] [Google Scholar]