Abstract
Background
Radiofrequency catheter ablation (RFCA) for atrial tachyarrhythmias in postoperative congenital heart disease (CHD) patients has a low success rate and a high recurrence rate. This study explores the reasons for these constraints.
Methods
A total of 49 consecutive postoperative CHD patients who received RFCA for atrial tachyarrhythmias between 1993 and 2010 were enrolled.
Results
Overall, there were 86 RFCA procedures performed, 32 with the conventional method and 54 using CARTO-guided mapping. The interval between the operation and the first ablation was 13 years. Isthmus-dependent atrial flutter (AFL) was the most common type of tachycardia (37, 76%), followed by intra-atrial re-entry tachycardia (IART; 37%), and ectopic atrial tachycardia (EAT; 31%). By applying CARTO-guided mapping, the success rate was elevated compared to that of conventional ablation (84% vs. 56%, p = 0.006), but there was no improvement in the recurrence rate (22% vs. 28%, p = 0.75). Multiple atrial tachyarrhythmias occurred in 26 (53%) patients, and 17 presented during the initial electrophysiological study. The presence of multiple arrhythmias during the initial study predicted ablation failure or multiple ablations (11/17 vs. 3/32, p < 0.001). Among the 15 patients with new tachyarrhythmias, EAT and IART predominated. However, applying antiarrhythmia agents immediately following ablation may decrease arrhythmia recurrence (1/10 vs. 14/25, p = 0.02).
Conclusions
Although electroanatomical mapping improves the results of RFCA in atrial tachyarrhythmias, the recurrence rate remains high because of multiple and new atrial tachyarrhythmias. Therefore, short-term pharmacological treatment following RFCA for positive remodeling should be considered.
Keywords: Ablation, Antiarrhythmia agents, Atrial tachyarrhythmia, Congenital heart disease, Multiple arrhythmias
INTRODUCTION
With the advancement of surgical techniques and perioperative care, most congenital heart disease (CHD) patients can survive to adulthood.1 However, adult CHD patients are still at risk for several long-term cardiovascular complications, including atrial tachyarrhythmia and sudden death.2 The presence of atrial tachyarrhythmia can increase the risk of morbidity and mortality, making aggressive treatment for these tachyarrhythmias mandatory.3-5 The mechanisms of postoperative atrial tachyarrhythmias in adult CHD patients often involve macro-reentrant atrial tachycardia, including isthmus-dependent atrial flutter (isthmus-dependent AFL), intra-atrial reentry tachycardia (IART), and ectopic atrial tachycardia (EAT).2,6,7 Medical treatments to control these arrhythmias are unsatisfactory and frequently causes adverse effects, including proarrhythmia and aggravation of sinus node dysfunction.2,8 Radiofrequency catheter ablation (RFCA) is recommended for these patients. However, the success rate is low and the recurrence rate is high.9-12 To elucidate the reasons for these results, we investigated the mechanisms and outcomes of RFCA in an adult-repaired CHD cohort with postoperative atrial tachyarrhythmia.
MATERIALS AND METHODS
Patients
From January 1993 to October 2010, 49 consecutive patients (male/female ratio of 26/23) who received RFCA for atrial tachyarrhythmias following cardiac surgery for CHD in our hospital were enrolled in this study. The data collection was in accordance with the regulations of the institutional review board policy of National Taiwan University Hospital. All patients had symptomatic atrial tachyarrhythmia as documented by either a 12-lead electrocardiogram (EKG) or a 24-hour Holter examination before electrophysiologic study. The indications of catheter ablation included recurrent arrhythmia despite medication, refractory atrial tachyarrhythmia, and arrhythmia-associated heart failure. Complex cardiac surgery was defined as complex cardiac operation procedures, which include a Fontan operation, atrial switch operation, tricuspid valve surgery for Ebstein’s anomaly, or repetitive or concomitant multiple surgery procedures.
Electrophysiological study and radiofrequency ablation
Clinical evaluations, operation history reviews, EKGs, and echocardiographic studies were performed on all patients prior to the commencement of our electrophysiological study. Informed consent was obtained from the patients or their parents if they were younger than 18 years. The patients received the procedure under intravenous propofol anesthesia. EAT was defined as atrial activation beginning from a small focus and spreading centrifugally during arrhythmia. If the atrial activation waveform revolved around a large central obstacle with reentrant nature, it was defined as macroreentrant atrial tachycardia. This group was further divided into isthmus-dependent AFL and IART. Isthmus-dependent AFL used the tricuspid ring as the central obstacle. If the central obstacle was a surgical scar or other anatomical barrier other than the tricuspid ring, it was defined as IART.13
Conventional versus CARTO-3D electroanatomical mapping
At the beginning of the study period, most of the patients received conventional mapping. During the conventional mapping, we inserted one quadripolar catheter into the right atrial free wall, one octapolar catheter into the coronary sinus, and one multipolar Halo catheter (Irvine Biomedical Inc, Irvine, CA, USA) into the right atrium. The activation sequence of the tachycardia was then recorded. In addition, we introduced a 4-mm tip mapping/ablation catheter into the right atrium. Entrainment pacing was performed to differentiate the EAT from reentry atrial tachyarrhythmia. When the difference of the post-pacing interval and tachycardia cycle length was less than 30 ms, it was defined as concealed entrainment, and the mapping electrode was considered to be located in the reentry circuit.5
Since the introduction of CARTO-3D electroanatomical mapping (CARTO, Biosense Webster, Diamond Bar, CA, USA) in 2002, the number of CARTO-guided ablations has gradually increased. Since 2008, most patients have received CARTO-guided ablations. During the CARTO-guided ablation, we inserted one octapolar deflectable catheter into the coronary sinus as a reference catheter for the local activation time, although sometimes one quadripolar catheter in the right atrial appendage was used if a coronary sinus catheter insertion was unfeasible. We next inserted a 4-mm tip mapping/ablation catheter (Navistar, Biosense Webster, Diamond Bar, CA, USA) for roving intracardiac mapping. The entire right atrium was plotted by dragging the mapping catheter over the endocardium. The surgical incision lines were identified as double potentials or long-fragmented potentials, and the surgical scar was identified as either silent or as having a low-voltage potential (< 0.05 mV).5,13,14 Anatomical barriers were tagged, such as tricuspid rings, coronary sinus, and superior and inferior vena cava. The mechanism was postulated to be reentrant atrial tachycardia when the continuous atrial activation sequence around one anatomical barrier using mapped activation duration encompassed more than 90% of the tachycardia’s cycle length. EAT was suggested if the activation sequence spread centrifugally from a single early point with a mapped activation duration less than 90% of the tachycardia’s cycle length.13 We used entrainment pacing to delineate the mechanisms of arrhythmia in some of the participants if it could not be defined clearly using CARTO mapping.
Ablation
The ablation target for EAT was the earliest activation site. For the reentry atrial tachyarrhythmia, we targeted areas with slow conduction zones, such as the cavotricuspid isthmus in the isthmus-dependent AFL. We dragged an ablation line joining two anatomical barriers. We delivered radiofrequency energy using a temperature control mode with an upper limit temperature set at 60-65 °C, and a power limit of 50 Watts for 1 minute at each site. To create the linear ablation line for the reentry atrial tachyarrhythmia, we used the point-by-point method. Targeted local electrograms were ablated until either complete elimination or they were less than 25% of their initial amplitude. If the local electrogram did not change following energy delivery, repeated energy delivery was applied. If the arrhythmia persisted despite repeat ablation at a satisfactory site, a saline irrigated-tip catheter with temperature set at 45 °C or an 8-mm tipped electrode with a maximum temperature set at 55 °C and an energy limit of 80 Watts was used for the arrhythmic foci.
Following arrhythmia termination by ablation, we rechecked the arrhythmia inducibility using programmed atrial stimulation under isoproterenol infusion. For the isthmus-dependent AFL, we performed atrial pacing to document the presence of bidirectional blocks across the ablation line. The procedure was deemed successful if no atrial tachyarrhythmia was induced under isoproterenol infusion or bidirectional block following the ablation. Recurrence was defined if an arrhythmia recurred within the same foci as the previous ablated one. If the recurrence was caused by an arrhythmia focus that differed from the previously ablated one, it was defined as a new arrhythmia. The presence of multiple arrhythmias was defined as at least 2 types of arrhythmia mechanisms or one type of arrhythmia mechanism with different foci or reentry circuits.
Statistics
The statistical methods used in our study included a Chi-square study and Fisher’s exact test for the comparison of the categorical data between groups. A Wilcoxon rank-sum test was applied for the comparison of the numerical data. Statistical significance was defined as a p value less than .05.
RESULTS
General characteristics
In total, 86 RFCA procedures were performed on the 49 patients: 32 procedures used a conventional mapping and ablation method, and 54 were guided by CARTO 3D electroanatomical mapping. The operation age for CHD patients ranged from 3 months to 70 years, with a median age of 16 (mean 20 ± 18) years. The median age of the first RFCA was 36 (mean 34 ± 18) years, and the median duration between the operation and RFCA was 13 (mean 14 ± 9.2) years. The underlying cardiac anomalies are shown in Table 1A. Atrial septal defects and ventricular septal defects accounted for half of the patients. Four patients were post-Fontan operation patients, and 2 had transposition of the great arteries after atrial switch operation.
Table 1. (A) The underlying congenital heart diseases in the 49 patients with atrial tachyarrhythmia. (B) The atrial arrhythmia mechanisms in these patients.
| (A) | Underlying disease | Number (%) |
| Atrial septal defect | 15 (31%) | |
| Ventricular septal defect | 8 (16%) | |
| Tetralogy of Fallot | 7 (14%) | |
| Ebstein anomaly | 4 (8%) | |
| Post Fontan operation | 4 (8%) | |
| Post atrial switch operation | 2 (4%) | |
| Endocardial cushion defect | 3 (6%) | |
| Others | 6 (12%) | |
| (B) | Arrhythmia mechanism | Number (%) |
| Atrial flutter | 17(35%) | |
| IART | 7 (14%) | |
| EAT | 3 (6%) | |
| Atrial flutter and EAT | 9 (18%) | |
| Atrial flutter and IART | 6 (12%) | |
| EAT and IART | 1 (2%) | |
| Atrial flutter, EAT and IART | 2 (4%) | |
| Atrial flutter and atrial fibrillation | 3 (6%) | |
| IART and atrial fibrillation | 1 (2%) |
EAT, ectopic atrial tachycardia; IART, intra-atrial reentry tachycardia.
Arrhythmia mechanisms
The mechanisms of atrial tachyarrhythmia were isthmus-dependent AFL in 37 (76%) patients, IART in 18 (37%) patients, and EAT in 15 (31%) patients (Table 1B). The median cycle length of the isthmus-dependent AFL, IART, and EAT were 278, 331, and 335 milliseconds (mean 285 ± 67 vs. 311 ± 61 vs. 364 ± 87 ms), respectively. The IART and isthmus-dependent AFL had shorter cycles lengths than that of the EAT (p = 0.004). The substrates of the IART and EAT in these patients are shown in Table 2. We found that the lateral atriotomy scar was the most common substrate in IART, followed by the superior right atrial scar (or upper crista terminalis) and inferior right atrium. There were minor differences regarding arrhythmia substrates between the initial attack and new IART recurrences. For EAT, the possible foci varied, but mostly involved right atrium sites, including the superior, inferior, and anterior right atriums.
Table 2. Arrhythmia substrate of initial and new intra-atrial reentry tachycardia and ectopic atrial tachycardia in the 49 postoperative congenital heart disease patients.
| Initial arrhythmia substrate | New arrhythmia substrate | ||
| IART | |||
| Lateral atriotomy | 6 | Lateral atriotomy | 5 |
| Inferior RA (or around IVC) | 2 | Inferior RA (or around IVC) | 1 |
| Anterior RA* | 1 | Superior RA scar (or CT) | 3 |
| LA | 2 | LA | 1 |
| Middle posterior RA† | 1 | Coronary sinus ostium | 2 |
| EAT | |||
| Superior lateral RA | 3 | Superior lateral RA | 1 |
| Inferior RA | 2 | Inferior RA | 3 |
| Anterolateral RA | 2 | Anterolateral RA | 1 |
| Inferior RA | 2 | Posterior RA | 2 |
| RA septal | 2 | Inferior anterior RA | 2 |
| LA | 1 |
CT, crista terminalis; EAT, ectopic atrial tachycardia; IART, intra-atrial reentry tachycardia; IVC, inferior vena cava; LA, left atrium; RA, right atrium.
* The patient of Bjork operation with IART reentrant around anterior suture line (right atrial appendage to right ventricle anastomoses); † The patient of atrial septal defect with IART reentrant around middle posterior area which may related to atrial septal defect patch.
Ablation outcomes
Among the 49 patients, the tachycardia substrates in 35 (71%) patients were successfully ablated during the 1st or 2nd RFCA (Figure 1). The other 14 patients either received multiple ablations (i.e., more than 3 times) or failed the RFCA. The presence of multiple arrhythmias at the time of initial electrophysiological study predicted failure or multiple ablations (11/17 vs. 3/32, p < 0.001). Among the 35 patients with successful RFCA within the first two ablations, 15 had arrhythmia recurrences that were caused by the same arrhythmia (8) or a new arrhythmia (7). Complex cardiac surgery and IART as the initial arrhythmia mechanisms predicted arrhythmia recurrences (p = 0.001 and 0.03, respectively). Immediate post-catheterization antiarrhythmic medication (either beta-blockers or Class IA or III antiarrhythmic agents) decreased arrhythmia recurrence (1/10 vs. 14/25, p = 0.02).
Figure 1.
The flow chart of the success rate, recurrence, and failed ablation in the 49 postoperative congenital heart disease patients receiving catheter ablation. The underlying electrophysiological mechanism of atrial arrhythmia at the initial electrophysiological study was shown below the blocks. AFL, isthmus-dependent atrial flutter; IART, intra-atrial reentry tachycardia; EAT, ectopic atrial tachycardia. * means successful catheter ablation within first two electrophysiological study; # means failed ablation or repeated recurrence with multiple ablation more than three times needed.
The comparison of the ablation outcomes between the conventional ablation and CARTO-guided ablation method are summarized and presented in Table 3. The success rate improved to 84% (46/55) for those receiving CARTO-guided ablation, considerably higher than for those who received conventional ablation (18/32, 56%, p = 0.006). This difference was most prominent in patients with multiple arrhythmias, regardless whether they had various mechanisms or the same mechanism with multiple foci (0/5 vs. 11/13, p = 0.002).
Table 3. The comparisons of the success rate and recurrence rate between those using conventional ablation and CARTO-guided electroanatomical ablation methods in the 86 procedures in these postoperative congenital heart disease patients.
| Conventional ablation (32) | CARTO-guided ablation (54) | p value | |
| Success rate overall | 18/32 (56%) | 45/54 (83%) | 0.006 |
| Isthmus dependent AFL | 11/14 (79%) | 23/25 (92%) | 0.48 |
| EAT | 3/4 (75%) | 3/5 (60%) | 1 |
| IART | 4/9 (44%) | 8/11 (73%) | 0.36 |
| Multiple | 0/5 (0%) | 11/13 (85%) | 0.002 |
| Recurrence of same arrhythmia | 5/18 (28%) | 10/45 (22%) | 0.75 |
| Isthmus dependent AFL | 1/11 (9%) | 6/23 (26%) | 0.63 |
| EAT | 2/3 (67%) | 0/3 (0%) | 0.4 |
| IART | 2/4 (50%) | 1/8 (13%) | 0.24 |
| Multiple | NA | 3/11 (27%) | NA |
| Recurrence of all arrhythmia (either same or new arrhythmia) | 11/18 (61%) | 18/45 (40%) | 0.13 |
| Isthmus dependent AFL | 5/11 (46%) | 11/23 (48%) | 1 |
| EAT | 2/3 (67%) | 0/3 (0%) | 0.4 |
| IART | 4/4 (100%) | 4/8 (50.0%) | 0.21 |
| Multiple | NA | 3/11 (27.3%) | NA |
AFL, atrial flutter; EAT, ectopic atrial tachycardia; IART, intra-atrial reentry tachycardia.
After 2065 person-months of follow-up (ranging from 1 to 164 months with a mean of 33 ± 41 months), 2 patients died: One died from cerebral infarction caused by atrial thrombus and recurrent atrial tachyarrhythmia, and one died from heart failure without arrhythmia recurrence. The recurrence rates in the patients receiving conventional and CARTO-guided ablation were 28% and 22%, respectively. Although the recurrence rate tended to be higher in those receiving conventional ablation methods for the EAT and IART, the difference was not significant.
Multiple atrial tachyarrhythmias
More than half (26, 53%) of the patients had multiple atrial tachyarrhythmias: 17 (35%) were noted at the initial electrophysiology study (Figure 2) and 15 (6 had multiple atrial tachyarrhythmias at the initial exam and additional tachyarrhythmias during the electrophysiology study) had new atrial tachyarrhythmias during the follow-ups (Figure 3). Compared to those patients without multiple atrial tachyarrhythmias, the success rate of conventional ablation in those with multiple atrial tachyarrhythmias was extremely low (67% vs. 0%, p = 0.01). However, this difference did not exist when using the CARTO-guided ablation (83% vs. 85%, p = 1.00). We did not identify any risk factors for the presence of multiple atrial tachyarrhythmias, including age during cardiac operation, postoperative follow-up period, and sex. Those receiving complex cardiac surgery tended to have later multiple atrial tachyarrhythmias, with recurrence rates that were not significantly different statistically (9/13 vs. 17/36, p = 0.21). If we excluded the atrial fibrillation, we found that those patients undergoing complex cardiac surgery had a higher trend for multiple atrial tachyarrhythmias (9/13 vs. 13/36, p = 0.05).
Figure 2.
(A) The 14 years-old patient of tetralogy of Fallot received total repair 10 years ago. He suffered from palpitation and electrophysiological study with electroanatomical mapping showed the typical counterclockwise atrial flutter as (B). We then applied radiofrequency energy on the cavotricuspid isthmus. During ablation, the arrhythmia changed with cycle length from 299ms to 418ms. (C) We remapped this arrhythmia and found it was ectopic atrial tachycardia near the isthmus. After we ablated this new ectopic focus, arrhythmia ceased, and he was free of arrhythmia symptoms during 18 months of follow-up.
Figure 3.
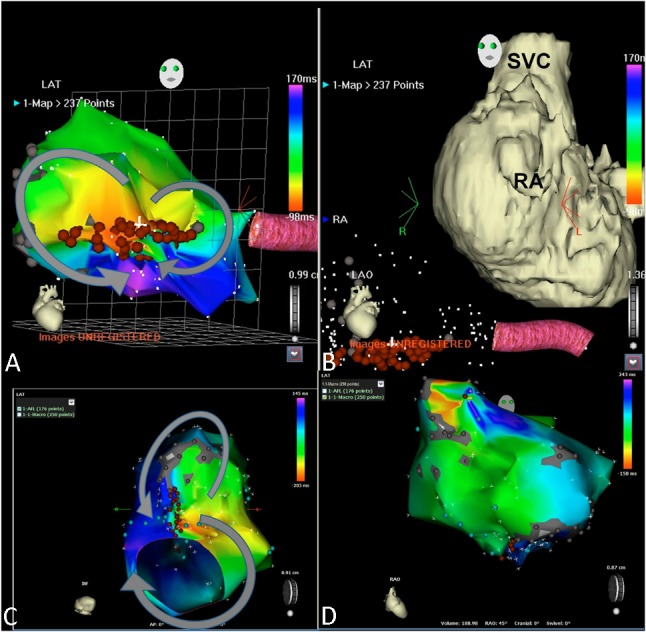
This 31 years-old female is a patient of double inlet left ventricle and pulmonary atresia received classical Fontan operation 23 years ago. She suffered from persistent atrial flutter (2:1 conduction) with episodes of tachycardia (1:1 conduction) presenting as syncope for the last 3 years. (A and B) During the initial electrophysiological examination, we documented the dual loop IART (intra-atrial reentry tachycardia) surrounding the lateral atriotomy scar and medial tricuspid suture scar (left anterior oblique view). Tachycardia cycle length was 276 ms. The arrhythmia ceased after linear ablation line connecting this two scar area. (C) Three months later, arrhythmia recurred with different p wave morphology. Remapping showed a different kind of dual loop IART surrounding the lateral atriotomy scar and inferior vena cava (viewed from inferior, tachycardia cycle length 350 ms). After linear line ablation of the slow conducting zone, another arrhythmia attacked originating from the superior anterior right atrium, suggesting ectopic atrial tachycardia (tachycardia cycle length variation from 375 to 400 ms) (D). The arrhythmia ceased after ablating the earliest atrial activation site. RA, right atrium; SVC, superior vena cava.
New atrial tachyarrhythmias
Among the patients with new atrial tachyarrythmias ture scar (left anterior oblique view). Tachycardia cycle length was 27that were documented during the subsequent electrophysiology study, 9 (60%) had EAT, 7 (47%) had IART, and 1 (7%) had isthmus-dependent AFL. The mechanisms of the new atrial tachyarrhythmias differed significantly from those of the initial atrial tachyarrhythmias. Among the new atrial tachyarrhythmias, EAT and IART predominated, and isthmus-dependent AFL was the least common type of atrial tachyarrhythmia.
DISCUSSION
This study, in which CHD patients received RFCA for atrial tachyarrhythmias, had three primary findings: (1) multiple atrial tachyarrhythmias, which may be present at the initial electrophysiology study or during follow-ups, are common in adults with repaired CHD; (2) isthmus-dependent AFL is the most common type of initial atrial tachyarrhythmia, and EAT and IART are the most common new atrial tachyarrhythmias in repeated RFCA recurrences; and (3) the application of modern electroanatomical mapping, such as CARTO for ablation, can increase success rates, but not reduce recurrence rates, possibly because of the constraint of developing new atrial tachyarrhythmias. Short-term antiarrhythmic agents following ablation might be helpful to decrease arrhythmic recurrence or new arrhythmias after successful RFCA.
CARTO-guided electroanatomical mapping and ablation
The outcomes for transcatheter ablations involving postoperative atrial tachyarrhythmia are not satisfactory; previous reports have shown that the success rate for ablation is approximately 71%-93%, and the recurrence rate is as high as 46%-53%.9-11 The potential difficulties of ablation in CHD patients include: (1) mapping difficulties from hemodynamically intolerable arrhythmia; (2) complex and distorted cardiac anatomy with diverse scar locations; (3) atrial wall thickening from atrial fibrosis because of long-term high atrial pressure; and (4) multiple arrhythmias. The application of new mapping methods, such as CARTO-guided electroanatomical mapping with or without computed tomography merge, may improve ablation results.14,15 CARTO-guided electroanatomical mapping enables concomitant electrical signal and anatomical location recording. In addition, it has the advantage of increasing the accuracy of cardiac anatomy and scar location mapping to better understand activation sequences. Because the anatomy of postoperative CHD is frequently distorted, mapping using CARTO-guiding helps delineate arrhythmia substrates. This study shows the benefits of electroanatomical mapping on acute outcomes for ablation. In addition to novel mapping techniques, high-powered ablation tools, such as saline-irrigated cooling catheters or 8-mm tipped ablation catheters, can overcome problems arising from thickened atrial walls to improve outcomes.16 We used these new ablation tools when the voltage of the local electrogram persisted despite repeated ablation, which was effective in most of the patients.
In this study, the most common type of initial atrial tachyarrhythmia was isthmus-dependent AFL, followed by IART and EAT. This was similar to previous reports in which isthmus-dependent AFL was the most common type of atrial tachyarrhythmia following CHD surgery.5,16 For IART, the most common arrhythmia substrates were lateral atriotomy scars, which corresponded with the results of a previous study.5 However, we found that inferior right atrial scars, coronary sinus ostium, and superior right atrial scars were also important re-entry central obstacles, and that the variable re-entry circuit further increased the difficulty of ablation.
Multiple atrial tachyarrhythmias
In addition to these factors, we found that multiple atrial tachyarrhythmias were common in the CHD patients in this study, and that the incidence could be as high as 53%. The complex surgical scars in these adult CHD patients may have caused the development of multiple atrial tachyarrhythmias or multiple mechanisms of atrial tachyarrhythmia.17 Among the patients, 35% had concomitant multiple atrial tachyarrhythmia at the initial electrophysiological study (Figure 2), which corresponds to previous reports (the incidence ranged from 20% to 40%).18,19 In addition, we found that the incidence of multiple atrial tachyarrhythmias in patients with complex cardiac surgery was as high as 71%. In this situation, because one type of atrial tachyarrhythmia frequently dominates, it can be difficult to detect multiple atrial tachyarrhythmias during an initial EKG. In this circumstance, ablation may be difficult because multiple re-entry circuits and EAT may interchange frequently. In these cases, we frequently ablate the dominant reentry atrial tachyarrhythmia first by using linear ablation between two anatomical obstacles. After successful ablation of the dominant arrhythmia, additional arrhythmias may appear. These can directly shift to other arrhythmias during ablation (Figure 2). Tachycardia cycle lengths change in this situation. The atrial tachyarrhythmia may become simpler after successfully ablating the dominant arrhythmia. We can then focus on additional re-entry atrial arrhythmias or ectopic atrial foci without interference from the dominant reentry arrhythmia. In this study, using this principle combined with the use of Carto-guided electroanatomical ablation, the success rate of the ablation of multiple arrhythmias increased to 79%. Therefore, the novel mapping strategy is beneficial to CHD patients.15
The mechanism of arrhythmia recurrence
In addition to concomitant atrial tachyarrhythmia, 31% of patients had new atrial tachyarrhythmias after successful ablation of the initial atrial arrhythmia. These may present as palpitations or near syncope recurrences. However, they can be misinterpreted as recurrences of previous arrhythmias. Reports regarding new tachyarrhythmia in postoperative CHD patients are limited.3,16,18 We found that the mechanism of these new atrial tachyarrhythmias differed from that of the initial atrial tachyarrhythmia. The most common type of new atrial tachyarrhythmias following ablation was EAT, followed by IART, rather than isthmus-dependent AFL. Some of the foci (2 of 9) of these new arrhythmias were near the previous ablation line, but others were located farther from the previous ablation sites. Electrical instability during previous ablation foci may play some role in the development of new arrhythmia in certain patients. Another possible mechanism is that these foci have existed since the beginning. Surgical scars related ectopic foci can trigger IART or isthmus-dependent AFL, which becomes the dominant atrial tachyarrhythmia. After ablating the dominant re-entry tachycardia, these atrial tachyarrhythmias become manifest. This may explain why the recurrence of new atrial tachyarrhythmia is high, even after application of CARTO-guided electroanatomical mapping. In addition, because long-term atrial tachyarrhythmias and high atrial pressure can cause atrial electrical remodeling, these foci may involve not only scars, but non-scar tissue.20,21 Short-term pharmacological manipulation can reverse this electrical remodeling. This may explain why the use of antiarrhythmic agents reduces atrial arrhythmia recurrences and new arrhythmias in these patients. Additional prospective large-scale clinical trials may be necessary to confirm these observations.
Study limitation
Because this was a retrospective study, the eras of conventional ablation and CARTO-guided ablation have somewhat different legacies. This may have caused bias when comparing the success rates and recurrences between the conventional and CARTO-guided ablation.
In this study, we found that short-term antiarrhythmia agents following catheter ablation can reduce arrhythmia recurrences and new arrhythmias. However, as a retrospective study, we were unable to draw firm conclusions from our findings. We suggest further prospective clinical trials to clarify this issue.
CONCLUSIONS
Electroanatomic mapping can enhance the acute results of RFCA in atrial tachyarrhythmias that have high recurrence rates and are frequently caused by multiple and new atrial tachyarrhythmias. The dominant mechanism of atrial tachyarrhythmias is isthmus-dependent AFL, but this shifted to EAT for the recurrences. Short-term pharmacological therapy following RFCA to foster positive remodeling may be an alternative and should be studied further.
CONFLICT OF INTEREST
None.
REFERENCES
- 1.Marelli AJ, Mackie AS, Ionescu-Ittu R, et al. Congenital heart disease in the general population: Changing prevalence and age distribution. Circulation. 2007;115:163–172. doi: 10.1161/CIRCULATIONAHA.106.627224. [DOI] [PubMed] [Google Scholar]
- 2.Koyak Z, de Groot JR, Mulder BJ. Interventional and surgical treatment of cardiac arrhythmias in adults with congenital heart disease. Expert Rev Cardiovasc Ther. 2010;8:1753–1766. doi: 10.1586/erc.10.152. [DOI] [PubMed] [Google Scholar]
- 3.de Groot NM, Zeppenfeld K, Wijffels MC, et al. Ablation of focal atrial arrhythmia in patients with congenital heart defects after surgery: role of circumscribed areas with heterogeneous conduction. Heart Rhythm. 2006;3:526–535. doi: 10.1016/j.hrthm.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Deal BJ, Mavroudis C, Jacobs JP, et al. Arrhythmic complications associated with the treatment of patients with congenital cardiac disease: consensus definitions from the multi-societal database committee for pediatric and congenital heart disease. Cardiol Young. 2008;18:202–205. doi: 10.1017/S104795110800293X. [DOI] [PubMed] [Google Scholar]
- 5.Lukac P, Pedersen AK, Mortensen PT, et al. Ablation of atrial tachycardia after surgery for congenital and acquired heart disease using an electroanatomic mapping system: which circuits to expect in which substrate? Heart Rhythm. 2005;2:64–72. doi: 10.1016/j.hrthm.2004.10.034. [DOI] [PubMed] [Google Scholar]
- 6.Bouchardy J, Therrien J, Pilote L, et al. Atrial arrhythmias in adults with congenital heart disease. Circulation. 2009;120:1679–1686. doi: 10.1161/CIRCULATIONAHA.109.866319. [DOI] [PubMed] [Google Scholar]
- 7.Verheugt CL, Uiterwaal CS, van der Velde ET, et al. Mortality in adult congenital heart disease. Eur Heart J. 2010;31:1220–1229. doi: 10.1093/eurheartj/ehq032. [DOI] [PubMed] [Google Scholar]
- 8.Saul JP. Role of catheter ablation in postoperative arrhythmias. Pacing Clin Electrophysiol. 2008;31:S7–S12. doi: 10.1111/j.1540-8159.2008.00946.x. [DOI] [PubMed] [Google Scholar]
- 9.Baker BM, Lindsay BD, Bromberg BI, et al. Catheter ablation of clinical intraatrial reentrant tachycardias resulting from previous atrial surgery: localizing and transecting the critical isthmus. J Am Coll Cardiol. 1996;28:411–417. doi: 10.1016/0735-1097(96)00154-4. [DOI] [PubMed] [Google Scholar]
- 10.Triedman JK, Bergau DM, Saul JP, et al. Efficacy of radiofrequency ablation for control of intraatrial reentrant tachycardia in patients with congenital heart disease. J Am Coll Cardiol. 1997;30:1032–1038. doi: 10.1016/s0735-1097(97)00252-0. [DOI] [PubMed] [Google Scholar]
- 11.Collins KK, Love BA, Walsh EP, et al. Location of acutely successful radiofrequency catheter ablation of intraatrial reentrant tachycardia in patients with congenital heart disease. Am J Cardiol. 2000;86:969–974. doi: 10.1016/s0002-9149(00)01132-2. [DOI] [PubMed] [Google Scholar]
- 12.Chiu SN, Lu CW, Chang CW, et al. Radiofrequency catheter ablation of supraventricular tachycardia in infants and toddlers. Circ J. 2009;73:1717–1721. doi: 10.1253/circj.cj-09-0123. [DOI] [PubMed] [Google Scholar]
- 13.Saoudi N, Cosio F, Waldo A, et al. A classification of atrial flutter and regular atrial tachycardia according to electrophysiological mechanisms and anatomical bases; a statement from a joint expert group from the working group of arrhythmias of the european society of cardiology and the north american society of pacing and electrophysiology. Eur Heart J. 2001;22:1162–1182. doi: 10.1053/euhj.2001.2658. [DOI] [PubMed] [Google Scholar]
- 14.Abrams DJ, Earley MJ, Sporton SC, et al. Comparison of noncontact and electroanatomic mapping to identify scar and arrhythmia late after the fontan procedure. Circulation. 2007;115:1738–1746. doi: 10.1161/CIRCULATIONAHA.106.633982. [DOI] [PubMed] [Google Scholar]
- 15.Triedman JK, Alexander ME, Love BA, et al. Influence of patient factors and ablative technologies on outcomes of radiofrequency ablation of intra-atrial re-entrant tachycardia in patients with congenital heart disease. J Am Coll Cardiol. 2002;39:1827–1835. doi: 10.1016/s0735-1097(02)01858-2. [DOI] [PubMed] [Google Scholar]
- 16.de Groot NM, Atary JZ, Blom NA, et al. Long-term outcome after ablative therapy of postoperative atrial tachyarrhythmia in patients with congenital heart disease and characteristics of atrial tachyarrhythmia recurrences. Circ Arrhythm Electrophysiol. 2010;3:148–154. doi: 10.1161/CIRCEP.109.909838. [DOI] [PubMed] [Google Scholar]
- 17.Nakagawa H, Shah N, Matsudaira K, et al. Characterization of reentrant circuit in macroreentrant right atrial tachycardia after surgical repair of congenital heart disease: isolated channels between scars allow “focal” ablation. Circulation. 2001;103:699–709. doi: 10.1161/01.cir.103.5.699. [DOI] [PubMed] [Google Scholar]
- 18.Seiler J, Schmid DK, Irtel TA, et al. Dual-loop circuits in postoperative atrial macro re-entrant tachycardias. Heart. 2007;93:325–330. doi: 10.1136/hrt.2006.094748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akar JG, Kok LC, Haines DE, et al. Coexistence of type i atrial flutter and intra-atrial re-entrant tachycardia in patients with surgically corrected congenital heart disease. J Am Coll Cardiol. 2001;38:377–384. doi: 10.1016/s0735-1097(01)01392-4. [DOI] [PubMed] [Google Scholar]
- 20.Hu YF, Higa S, Huang JL, et al. Electrophysiologic characteristics and catheter ablation of focal atrial tachycardia with more than one focus. Heart Rhythm. 2009;6:198–203. doi: 10.1016/j.hrthm.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 21.Corradi D, Callegari S, Maestri R, et al. Structural remodeling in atrial fibrillation. Nat Clin Pract Cardiovasc Med. 2008;5:782–796. doi: 10.1038/ncpcardio1370. [DOI] [PubMed] [Google Scholar]




