Abstract
Background
This study aimed to explore the feasibility of guiding the application of metoprolol succinate in patients with moderate to severe heart failure (HF) through monitoring plasma brain natriuretic peptide (BNP) levels.
Methods
A total of 195 patients with moderate to severe HF (NYHA Functional Class III to IV) were selected and randomized into two groups: an observation group and a BNP group. The groups were established to observe the clinical conditions and establish plasma BNP levels to guide the application of metoprolol succinate. The average start-up of metoprolol succinate and average dose of metoprolol succinate after one month, as well as the recurrence rate and mortality of HF during hospital stay were compared between the two groups.
Results
Start-up of metoprolol succinate was shorter in the BNP group than in the observation group [(5.89 ± 1.76) d vs. (7.03 ± 2.08) d, p < 0.01], but no significant differences in recurrence rate (26.60% vs. 23.91%, p > 0.05) and mortality (6.38% vs. 5.43%, p > 0.05) of HF were observed between the two groups. The average dose of metoprolol succinate after one month was higher in the BNP group compared with that of the observation group [(47.65 ± 13.09) mg/d vs. (35.08 ± 11.08) mg/d, p < 0.01].
Conclusions
Although monitoring plasma BNP might have limited the clinical impact on the change of left ventricular ejection fraction, recurrence of HF or mortality within 1 month, it could safely facilitate early use and up-titration of the metoprolol succinate in patients with moderate to severe HF.
Keywords: BNP, Heart failure, β receptor blocker
INTRODUCTION
Moderate to severe heart failure (HF) is not only characterized by dyspnea, but also by abnormal hemodynamics and neuroendocrine system activation.1 The customary treatment for HF includes symptom improvement, correction of hemodynamic abnormalities and prevention of excessive neuroendocrine system activation.2 However, the assessment of these curative effects on HF is mainly based on the subjective judgment of clinicians instead of reference to a quantitative standard. The assessment of plasma brain natriuretic peptide (BNP) level in patients facilitates HF diagnosis and prognosis, and reflects drug efficacy as well.3-5 Researchers have observed that BNP levels significantly decrease as HF symptoms improve. Moreover, the decreased amplitude of BNP correlates with the degree of heart function improvement.6,7 In recent years, monitoring plasma BNP level in patients has been extensively studied to guide the drug treatment for chronic HF,8-12 but rarely for moderate to severe HF.
β1 receptor blocker is the basis for the treatment of HF.13-15 However, its application in patients with moderate to severe HF is always late with a typically insufficient dose.16 How to treat HF patients earlier and more reasonably with β1 receptor blocker has become a challenge for many clinicians. We aimed to explore the feasibility and safety of guiding metoprolol succinate application in patients with moderate to severe HF through monitoring the plasma BNP level.
MATERIALS AND METHODS
Subjects
A total of 195 patients with moderate to severe HF (NYHA Functional Class III to IV), including 44 cases of hypertension, 112 cases of coronary heart disease, 22 cases of valvular heart disease and 17 cases of dilated cardiomyopathy, were selected from the Department of Cardiology in our hospital between March 2008 and March 2012. The subjects were observed in a hospital environment for at least one month. The patients with severe renal function damage (serum creatinine > 265 umol/l), bronchial asthma or chronic obstructive pulmonary disease were excluded, as well as end-stage HF patients without response to intravenous drug treatment. This study was performed in accordance with the declaration of Helsinki. Additionally, this study was conducted with approval from the Ethics Committee of Huai’an First People’s Hospital, Nanjing Medical University. Written informed consent was obtained from all participants.
Grouping
The subjects were randomized into an observation group and a BNP group. All the patients were intravenously treated with diuretic (furosemide), vasodilator (nitroglycerin or sodium nitroprusside) and cardiotonic (cedilanid, dobamine, dobutamine, milrinone), followed by metoprolol succinate treatment according to BNP level or clinical conditions.
Experiment protocol
Start-up and incremental use of metoprolol succinate in the observation group patients were according to their clinical manifestation as assessed by our deputy chief physician, or another more highly placed medical professional in cardiology. The patients stopped using intravenous cardiotonic, vasodilator and diuretic, without resting or recumbent asthma, and started to take metoprolol succinate in an initial dose of 6.25 mg bid after their body weight was stable for three days. The clinical manifestation of patients was evaluated; the dose of metoprolol succinate was doubled every week until the maximum tolerated dose or target dose if no HF signs and symptoms were observed. Otherwise, metoprolol succinate was reduced and intravenous cardiotonic, vasodilator or diuretic was applied until HF signs and symptoms improved, and then metoprolol was gradually applied again in patients. Start-up of metoprolol succinate in the patients of BNP group was according to their plasma BNP level. The basal BNP level of patients was checked prior to hospital admission, and plasma BNP level was controlled every 3-5 days during the application of intravenous cardiotonic, vasodilator and diuretic. The threshold of metoprolol succinate start-up was defined as a more than 50% reduction of basal BNP level, or a BNP value that was less than 300 pg/ml,6,17,18 and then the dose of metoprolol succinate was enhanced. The dose was doubled every time and the BNP level was tested every week; when the BNP level did not decrease but was elevated more than 10%, metoprolol succinate was stopped or reduced and intravenous cardiotonic, vasodilator or diuretic was applied until the BNP level reduced to 50% of the basal level or less than 300 pg/ml. Then, metoprolol was again gradually applied in patients.
Observation index
The general data of patients were collected, including but not limited to medical history, age, gender, blood pressure, blood sugar, and blood lipids. Additionally, patient heart rate, physical examination, electrocardiogram (ECG), left ventricular ejection fraction (LVEF) by echocardiograph, renal function and electrolytes were also observed during the treatment.
BNP was measured with immunofluorescence. Then, 2 ml of venous blood was collected from the patients at resting state and put into the tube with EDTA anticoagulant. Plasma was isolated after centrifugation and placed into a Triage Instrument (Biosite, Inc., San Diego, CA, USA), which quantified BNP concentration according to the intensity of fluorescence that was generated by the reaction of the plasma sample and BNP rat-anti-human monoclonal and polyclonal antibody.
We thereafter observed the average start-up of metoprolol succinate and the maximum dose of metoprolol succinate after one month, as well as the recurrence rate (refers to patients who need increase dose of diuretics or intravenous inotrophic agents again) and mortality of HF during one month follow-up observation.
Statistical analysis
Statistical analysis was performed with SPSS v.19 for Windows (SPSS Inc, Chicago, IL) and measurement data was presented as mean and standard deviation. Comparison of mean values between the two groups used the two-sample t test, while enumeration data employed Chi-square test or Fisher’s test. A result of p < 0.05 was considered statistically significant.
RESULTS
Patient group
Our study involved a total of 195 patients from 38-81 years of age (61 ± 12 y), which included 99 cases in the observation group and 96 cases in the BNP group. No significant difference in general data was observed (Table 1). Five patients in the observation group and four in the BNP group quit the clinical trial due to severe bradycardia. There were no marked changes of blood glucose and renal function and other data in patients during hospital stay.
Table 1. Comparison of general data between BNP and observation groups.
| BNP group (N = 94) | Observation group (N = 92) | p value | |
| Average age | 57 (40-78) | 58 (38-81) | NS |
| Gender (male/female) | 53/41 | 51/41 | NS |
| Coronary heart disease | 58 | 54 | |
| Hypertension | 22 | 22 | |
| Dilated cardiomyopathy | 8 | 9 | |
| Valvular heart disease | 13 | 9 | |
| Diabetes mellitus | 30 | 33 | |
| Hyperlipidemia | 29 | 27 | |
| LV ejection fraction | 30 ± 8.1 | 28 ± 7.9 | 0.55 |
| Revascularization therapy | 10 | 12 | |
| ACE inhibitors | 78 | 75 | |
| Angiotensin receptor blockers | 11 | 8 | |
| Loop diuretic agents | 94 | 92 | |
| Aldosterone antagonists | 81 | 79 | |
| Thiazide diuretic agents | 5 | 7 | |
| Nitrates | 67 | 70 | |
| Basal BNP value (pg/ml) | 1167.8 ± 219.9 | 1145.8 ± 224.9 | 0.61 |
Values are expressed as mean and SD or as indicated, p < 0.05 was considered statistically significant.
ACE, angiotensin-converting-enzyme; BNP, brain natriuretic peptide; LV, left ventricular.
Analysis of drug combination with diuretic and vasodilator and cardiotonic
There was no difference in the drug combination involving necessary vasodilator and cardiotonic between the two patient groups. However, the BNP group patients medicated considerably more intravenous diuretic than the observation group patients [(39.03 ± 11.08) mg/d vs. (28 ± 9.11) mg/d, p < 0.01, Table 2].
Table 2. Medication of diuretic agents and inotropic administration.
| Medication | BNP group (N = 94) | Observation group (N = 92) | p |
| Dose of furosemide per day | |||
| Intraveneous dose (mg/d) | 39.03 ± 11.08 | 0.28 ± 9.11 | < 0.01 |
| Oral dose (mg/d) | 20.30 ± 2.08 | 20.65 ± 3.09 | NS |
| Dobamine | |||
| Frequency | 10/84 | 12/80 | NS |
| Dose (ug/mg/min) | 4.3 ± 1.8 | 4.5 ± 1.7 | NS |
| Administration period (days) | 2.0 ± 1.1 | 1.9 ± 1.1 | NS |
| Dobutamine | |||
| Frequency (%) | 03/91 | 05/87 | NS |
| Dose (ug/mg/min) | 2.3 ± 0.8 | 2.5 ± 0.6 | NS |
| Administration period (days) | 1.0 ± 0.5 | 1.0 ± 0.5 | NS |
| Milrinone Frequency (%) | 25/69 | 23/69 | NS |
| Dose (ug/mg/min) | 0.3 | 0.4 | NS |
| Administration period (days) | 4.0 ± 1.2 | 3.9 ± 1.1 | NS |
| Cedilanid dose (mg/d) | 4.0 ± 1.0 | 3.9 ± 0.9 | NS |
Values are expressed as mean and SD or as indicated, p < 0.05 was considered statistically significant.
BNP, brain natriuretic peptide; SD, standard deviation.
Follow-up outcome about LVEF and BNP value
The left ventricular ejection fraction showed no difference between the two groups, regardless of whether the LVEF was a baseline or follow-up observation. The study indicated that the left ventricular ejection fraction was increased after one month of medication (Figure 1). Meanwhile, the BNP value decreased dramatically over the duration of medication, but there was no difference between the two groups (Figure 2). This showed that reasonable medication could improve the heart function.
Figure 1.
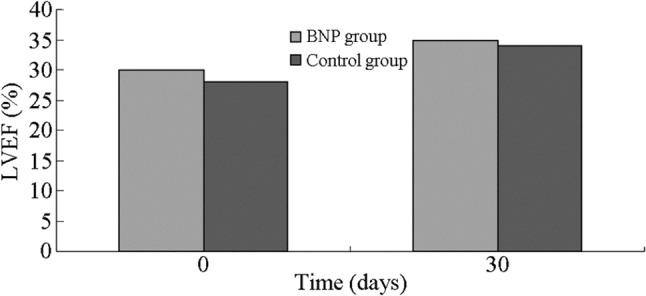
The change of left ventricular ejection fraction after one-month medication. The left ventricular ejection fraction were ameliorated after medication, but on deference between two groups. BNP, brain natriuretic peptide; LVEF, left ventricular ejection fraction.
Figure 2.
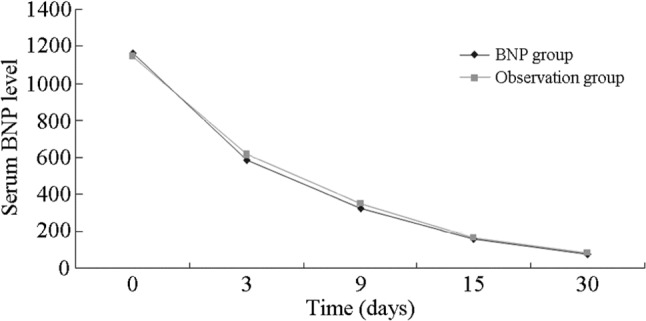
The change of serum BNP level duration of one month medication between BNP group and observation group. The BNP level decreased dramatically, but no difference was detected between the two groups. BNP, brain natriuretic peptide.
Start-up of metoprolol succinate and average dose of metoprolol succinate
The start-up of metoprolol succinate was shorter in the BNP group than that of the observation group [(5.89 ± 1.76) d vs. (7.03 ± 2.08) d, p < 0.01]. The average dose of metoprolol succinate per day after one month was higher in the BNP group compared to the observation group (47.65 ± 13.09) mg/d vs. (35.08 ± 11.08) mg/d, p < 0.01, Table 3]. This indicated that monitoring BNP level allowed patients with moderate to severe HF to use a relatively high dose of metoprolol succinate earlier and easier compared with clinical observation (Table 3).
Table 3. Comparison of time of start-up of metoprolol succinate (time of start-up) and average dose of metoprolol succinate after one month(target dosage) between BNP and observation groups (mean and SD).
| Outcome | BNP group (N = 94) | Observation group (N = 92) | t value | p value |
| Time of start-up (days) | 5.89 ± 1.76 | 7.03 ± 2.08 | 4.409 | 0.01 |
| Target dosage (mg/d) | 47.65 ± 13.09 | 35.08 ± 11.08 | 5.414 | 0.009 |
Values are expressed as mean and SD or as indicated. Statistical analysis was performed to compare the two groups. p < 0.05 was considered statistically significant.
BNP, brain natriuretic peptide; SD, standard deviation.
Recurrence rate and mortality of HF
There were no distinct differences in the recurrence rate (26.60% vs. 23.91%, p > 0.05) and mortality rate (6.38% vs. 5.43%, p > 0.05) of HF between the two groups (Table 4). In ischemic subgroup analysis, there were no differences in the recurrence rate and mortality of HF. In the BNP group, dosages of metoprolol succinate had to be adjusted more frequently. For patients with HF recurrence that were sensitive to diuretic, cessation of metoprolol succinate use was not necessary. While the patients with recurrence of HF in observation group were also sensitive to diuretic, 28% of them had to stop using metoprolol succinate.
Table 4. Comparison of recurrence rate and mortality of HF between BNP and observation groups.
| Group | CasesN | Recurrence rate of HF | Mortalityof HF |
| Cases (%) | Cases (%) | ||
| Observation group | 92 | 22 (23.91) | 5 (5.43) |
| BNP group | 94 | 25 (26.60) | 6 (6.38) |
| x2 value | 0.18 | 0.08 | |
| p value | 0.67 | 0.78 | |
| Ischemic (subgroup) | |||
| Observation group | 54 | 13 | 3 |
| BNP group | 58 | 16 | 3 |
| x2 value | 0.18 | 0.008 | |
| p value | 0.67 | 0.93 |
Values are expressed as a percentage of recurrence and mortality of the two groups. p < 0.05 was considered statistically significant.
BNP, brain natriuretic peptide; HF, heart failure.
DISCUSSION
Sympathetic activation during decompensated HF serves as an important short-term compensatory mechanism to maintain stable hemodynamics, but excessive or persistent sympathetic activation is also a precipitating factor in worsening ventricular remodeling and heart failure. β1 receptor blocker ameliorates ventricular remodeling and the long-term prognosis of patients with HF by inhibiting excessive or persistent sympathetic activation. Clinicians always worry that early application of β1 receptor blocker suppresses the compensatory effect of the sympathetic nerve, which deteriorates cardiac function or aggravates clinical symptoms of HF. And late application of β1 receptor blocker typically fails to prevent excessive activation of neuroendocrine system and ventricular remodeling in sufficient time. CIBIS II, MERIT-HF and COPERNICUS13-15 showed that β1 receptor blocker could reduce all-cause mortality, cardiovascular mortality and hospitalization rate in patients with moderate to severe HF. IMPACT-HF15 confirmed that hospitalized HF patients might benefit more from early use of β1 receptor blocker. The COMET16 trial demonstrated that an insufficient dose of β1 receptor blocker probably influenced the protective effect on HF. Therefore, early start-up of adequate β1 receptor blocker may produce an added benefit for hospitalized patients with HF. However, start-up and incremental use of β1 receptor blocker in patients with moderate to severe HF mainly is often based on the patients’ clinical manifestations, and not any objective standard, and β1 receptor blocker is frequently initiated late with a slowly increasing dose arising from excessive clinician concerns about potential negative inotropic action. Currently, there is no objective and reliable standard for the use of β1 receptor blocker in patient with moderate to severe HF.
Ventricular myocytes synthesize and secrete most BNP that is upregulated in right ventricular volume and pressure overload and ventricular remodeling.4-7 BNP not only reflects early volume and pressure loading conditions in patients with HF, but also shows the evolution of ventricular remodeling that induces progressive and worsening HF. Prior research17,18 has demonstrated that the short-term use of intravenous cardiotonic, diuretic and vasodilator effectively reduced BNP level by more than 50% in patients with decompensated HF, whose hemodynamics also significantly improved to stable state (cardiac index increased by 30%, pulmonary artery wedge pressure reduced by 30%). We also observed that plasma BNP level dramatically declined in patients with moderate to severe HF after using intravenous diuretic and vasodilator, while a slight change of the plasma BNP level in the patients with acute HF corresponded to the negative response to intravenous drugs. This indicated that apparent BNP reduction reflected a reduction in left ventricular volume loading and improved cardiac function, which was consistent with previous results.17,18 At the same time, a significant number of studies on BNP guiding drug treatment for HF were performed, but primarily focused on chronic HF or outpatient follow-up.8-12 Some research showed that monitoring BNP level facilitated the diagnosis of volume loading condition in patients with acute HF and the treatment for HF.19-22 However, the significance of BNP guiding drug treatment has not been fully reported. We explored the neuroendocrine activation mechanism and protective effect of β1 receptor blocker to clarify the importance of using BNP to guide the use of β1 receptor blocker in patients with moderate to severe HF.
Bayés G et al.6 proved that the decrease in magnitude of plasma BNP level in patients with HF before and after treatment was more valuable in prognosis than the actual BNP value itself. In our research, a more than 50% reduction of BNP level or less than 300 pg/ml were considered as threshold to instruct the application of metoprolol succinate.6,17,18 The BNP level in plasma of moderate or severe cardiac failure patients was monitored to determine the start-up of metoprolol succinate, where the average administration time was (5.89 ± 1.76) d, shorter than (7.03 ± 2.08) d in the observation group (p < 0.01). Consistent with previous studies, a decrease of BNP level after effective treatment was observed earlier than the improvement of clinical symptoms in these patients, indicating that the plasma BNP level might actually reflect volume load condition in patients, rather than clinical observation.19,20 Meanwhile, we observed that the intravenous diuretic dosages in the BNP group were much higher than in the observation group [(39.03 ± 11.08) mg/d vs. (28 ± 9.11) mg/d, p < 0.01]. This suggested that monitoring serum BNP level could contribute to better achieve the dry weight and safe transition from intensive drug treatment such as cardiac stimulant, vasodilator and diuretic to the use of β1 receptor blocker in these patients. This fact further demonstrated that monitoring plasma BNP level in patients would facilitate earlier use of β1 receptor blocker, which maybe would lead to long-term amelioration of heart function by earlier and potentially inhibiting the excessive activation of neuroendocrine. It was also observed that although the use of metoprolol succinate was initiated earlier in the BNP group, the recurrence rate of HF during hospital stay was higher (26.60% vs. 23.91%), but the difference between the two groups was not statistically significant, as well as the mortality (6.38% vs. 5.43%, p > 0.05) and the left ventricular ejection fraction based on echocardiograph, even in the ischemic subgroup. This could be due to shorter observation duration and fewer enrolled revascularization patients. However these results indicated that the early start-up of metoprolol succinate was safe for patients, and would not increase the recurrence of HF and related events during the one month study. Moreover, although there was no significant difference in the recurrence rate of HF between the BNP and observation groups, the incremental use of metoprolol succinate was more frequent in the BNP group than that of the observation group. Even for patients with recurring HF, the metoprolol succinate treatment could be continued if they were regulated by intravenous drugs (mostly with diuretic), and/or the amount of metoprolol succinate was reduced when the BNP level was under-monitored. On the other hand, the incremental use of metoprolol succinate in the observation group was slow, and 28% of the recurring patients required a pause before reusing it, suggesting the clinical observation might overstate the negative inotropic action of metoprolol succinate or it failed to reflect ventricular volume load. Therefore, it was also safe to use metoprolol succinate in patients with aggravated recurrent HF by monitoring their BNP level. Monitoring BNP level is necessary to ensure the application of metoprolol succinate to those patients with moderate to severe HF was speculated to be based on the obvious improvement of ventricular volume load in patients, which not only facilitated early start-up of metoprolol succinate, but also certified its safety.
The average dose of metoprolol succinate after one month was higher in the BNP group compared with that of the observation group [(47.65 ± 13.09) mg/d vs. (35.08 ± 11.08) mg/d, p < 0.01]. This correlated with early start-up and frequent incremental use of metoprolol succinate, as well as the results when less patients who stopped using metoprolol succinate during HF recurrence in BNP group. These results indicated that monitoring the BNP level allowed patients with moderate to severe HF to use a relatively high dose of metoprolol succinate earlier compared with mere clinical observation.
We investigated the efficacy and safety of monitoring BNP level to guide the application of β1 receptor blocker (metoprolol succinate) to the patients with moderate to severe HF. Compared with clinical observation, monitoring BNP level facilitated earlier start-up and incremental use of metoprolol succinate, which provided an improved benefit to HF patients. Due to the small size of the sample population and shorter observation duration, although angiotensin-converting-enzyme inhibitor or angiotensin receptor blocker was combined in the treatment for HF. It was later than the start-up and incremental use of metoprolol succinate and did not return to the normative and reasonable treatment. Additional investigation is necessary to determine what level of plasma BNP is more suitable for the application of metoprolol succinate in the treatment for HF.
CONCLUSIONS
Although monitoring plasma BNP might have limited the clinical impact on the change of left ventricular ejection fraction, recurrence of HF or mortality within 1 month, it could safely facilitate early use and up-titration of the metoprolol succinate in patients with moderate to severe HF.
REFERENCES
- 1.Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol. 2009;53:557–573. doi: 10.1016/j.jacc.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 2.Dobrek Ł, Thor P. Neuroendocrine activation as a target of modern chronic heart failure pharmacotherapy. Acta Pol Pharm. 2011;68:307–316. [PubMed] [Google Scholar]
- 3.Aleksandrova EB, Sidorenko BA. Brain natriuretic peptide for early diagnosis of chronic heart failure in patients with preserved left ventricular ejection fraction. Kardiologiia. 2012;52:27–32. [PubMed] [Google Scholar]
- 4.Shaikh K, Hanif B, Siddique AA, et al. Pro-brain natriuretic peptide plasma levels,left ventricular dimensions and ejection fraction in acute dyspnoea. J Coll Physicians Surg Pak. 2012;22:751–755. [PubMed] [Google Scholar]
- 5.Doust JA, Pietrzak E, Dobson A, Glasziou P. How well does B-type-natriuretic peptide predict death and cardiac events in patients with heart failure:a systematic review. BMJ. 2005;330:625. doi: 10.1136/bmj.330.7492.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bayés-Genís A, Lopez L, Zapico E, et al. NT-ProBNP reduction percentage during admission for acutely decompensated heart failure predicts long-term cardiovascular mortality. J Card Fail. 2005;11:S3–S8. doi: 10.1016/j.cardfail.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Kazanegra R, Cheng V, Garcia A, et al. A rapid test for B-type natriuretic peptide correlates with falling wedge pressures in patients treated for decompensated heart failure:a pilot study. J Card Fail. 2001;7:21–29. doi: 10.1054/jcaf.2001.23355. [DOI] [PubMed] [Google Scholar]
- 8.Valle R, Aspromonte N. Use of brain natriuretic peptide and bioimpedance to guide therapy in heart failure patients. Contrib Nephrol. 2010;164:209–216. doi: 10.1159/000313732. [DOI] [PubMed] [Google Scholar]
- 9.Newton PJ, Betihavas V, Macdonald P. The role of b-type natriuretic peptide in heart failure management. Aust Crit Care. 2009;22:117–123. doi: 10.1016/j.aucc.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Januzzi JL, Jr., Rehman SU, Mohammed AA, et al. Use of amino-terminal pro-B-type natriuretic peptide to guide outpatient therapy of patients with chronic left ventricular systolic dys-function. J Am Coll Cardiol. 2011;58:1881–1889. doi: 10.1016/j.jacc.2011.03.072. [DOI] [PubMed] [Google Scholar]
- 11.Kelly NP, Januzzi JL., Jr. The role of B-type natriuretic peptide testing in guiding outpatient heart failure treatment. Curr Treat Options Cardiovasc Med. 2013;15:397–409. doi: 10.1007/s11936-013-0247-4. [DOI] [PubMed] [Google Scholar]
- 12.DeBeradinis B, Januzzi JL., Jr. Use of biomarkers to guide outpatient therapy of heart failure. Curr Opin Cardiol. 2012;27:661–668. doi: 10.1097/HCO.0b013e3283587c4d. [DOI] [PubMed] [Google Scholar]
- 13.The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 14.Packer M, Fowler M, Rouleau J, et al. COPERNICUS (carvedilol prospective randomized cumulative survival trial) A multicenter randomized double-blind, placebo-controlled study to determine the effect of carvedilol on mortality in severe congestive heart failure. Cardilvasc Drugs Ther. 1999;13:2. [Google Scholar]
- 15.Gattis WA, O'Connor CM, Gallup DS, et al. Predischarge initiation of carvedilol in patients hospitalized for decompensated heart failure:results of the Initiation Management Predischarge:Process for Assessment of Carvedilol Therapy in Heart Failure (IMPACT-HF) trial. J Am Coll Cardiol. 2004;43:1534–1541. doi: 10.1016/j.jacc.2003.12.040. [DOI] [PubMed] [Google Scholar]
- 16.Poole-Wilson PA, Swedberg K, Cleland JG, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- 17.Knebel F, Schimke I, Pliet K, et al. BNP in acute heart failure:correlation with invasively measured hemodynamic parameters during recompensation. J Card Fail. 2005;11:S38–S41. doi: 10.1016/j.cardfail.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 18.Michtalik HJ. Acute changes in N-terminal pro-B-type natriuretic peptide during hospitalization and risk of readmission and mortality in patients with heart failure. Am J Cardiol. 2011;107:1191–1195. doi: 10.1016/j.amjcard.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 19.Peacock WF, Soto KM. Current techniques of fluid status assessment. Contrib Nephrol. 2010;164:128–142. doi: 10.1159/000313726. [DOI] [PubMed] [Google Scholar]
- 20.Valle R, Aspromonte N, Milani L, et al. Optimizing fluid management in patients with acute decompensated heart failure (ADHF):the emerging role of combined measurement of body hydration status and brain natriuretic peptide (BNP) levels. Heart Fail Rev. 2011;16:519–529. doi: 10.1007/s10741-011-9244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong DT, George K, Wilson J, et al. Effectiveness of serial increases in amino-terminal pro-B-type natriuretic peptide levels to indicate the need for mechanical circulatory support in children with acute decompensated heart failure. Am J Cardiol. 2011;107:573–578. doi: 10.1016/j.amjcard.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 22.Saremi A, Gopal D, Maisel AS. Brain natriuretic peptide-guided therapy in the inpatient management of decompensated heart failure. Expert Rev Cardiovasc Ther. 2012;10:191–203. doi: 10.1586/erc.11.188. [DOI] [PubMed] [Google Scholar]


