Abstract
Background
Arterial stiffness is a physiologic quantitative value used to measure arterial compliance. It is predictive of coronary atherosclerosis in patients with intermediate to high cardiovascular risk. However, a correlation between arterial stiffness and subclinical coronary atherosclerosis has yet to be established. Therefore, the purpose of this study was to evaluate arterial stiffness using an arterial stiffness index (ASI) and investigate its association with coronary artery plaque in patients with subclinical coronary atherosclerosis.
Methods
Our study enrolled 156 consecutive subjects who underwent health screening using a 64-slice cardiac computed tomography angiography (CCTA). Their arterial stiffness index was assessed noninvasively by CardioVision® MS-2000. The atheroma on the coronary vessel walls was analyzed.
Results
Of the 156 patients, 53 displayed at least one > 50% stenotic lesion over the coronary arteries in CCTA images. The patients with at least one > 50% coronary stenotic plaque were older and had higher systolic blood pressure and ASI values than patients without > 50% coronary stenotic plaque. After dividing the study population into 2 groups by those patients over and under 50 years of age, the ASI positively correlated with the presentation of at least one > 50% coronary stenotic plaque in patients aged ≥ 50 years (odds ratio = 1.02, 95% confidence interval: 1.00-1.04, p = 0.03).
Conclusions
The ASI could play a role in risk stratification systems for coronary artery disease in patients with subclinical coronary atherosclerosis, and is a useful clinical marker for the correlation of early coronary plaque.
Keywords: Arterial stiffness, Arterial stiffness index, Atherosclerosis, Coronary artery plaque
INTRODUCTION
Atherosclerosis is a long-term pathogenic process. Subclinical plaque can exist for several years before major stenosis or rupture occurs. The measurement of arterial compliance could provide a screening tool for potential subclinical coronary artery disease (CAD) because atherosclerosis typically occurs in the abdominal aorta earlier than it does in other vascular territories, such as the coronary arteries.1 Stiffness of the arterial tree, which increases the systolic blood pressure (BP) and reduces the diastolic BP, is a well-known risk factor for atherosclerosis and, thus, for cardiovascular complications.2-4
Arterial compliance can be assessed according to the ratio of stroke volume to pulse pressure.5 Arterial stiffness is also a dynamic physiologic quantitative value used to measure arterial compliance and a predictor of the extent of aortic atherosclerosis.6,7 Previous studies have shown that arterial compliance is predictive of coronary atherosclerosis in intermediate- to high-cardiovascular-risk patients undergoing coronary angiography.8 However, according to a review of the literature, no previous study has shown a correlation between subclinical coronary atherosclerosis and arterial stiffness in asymptomatic subjects. Therefore, the purpose of this study was to evaluate arterial stiffness referencing the arterial stiffness index (ASI) and investigate its association with coronary artery plaque in patients with subclinical coronary atherosclerosis.
MATERIALS AND METHODS
Patient enrollment
Between January, 2010 and November, 2010, 180 consecutive adults who underwent health screening using a 64-slice cardiac computed tomography angiography (CCTA) at Taipei Medical University Hospital were enrolled. Their detailed medical histories (including CAD, diabetes, hypertension, or hypercholesterolemia records) and medication information were recorded, if available. Blood samples were taken the morning after patients fasted since the previous midnight for at least eight hours. We analyzed blood urea nitrogen (BUN), creatinine (Cr), fasting plasma glucose, total cholesterol (TC), total triglyceride (TG), high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and high-sensitivity C-reactive protein (hs-CRP). Serum concentrations of BUN, Cr, TC and TG were measured using a dry multilayer and analytic slide method in the Fuji Dri-Chem 3000 analyzer (Fuji Photo Film Corporation, Minato-Ku, Tokyo, Japan). The plasma glucose concentration was measured by glucose oxidase method, using a Beckman Glucose Analyzer II (Beckman Instruments, Fullerton, CA, USA). Serum high-density lipoprotein cholesterol levels were determined with an enzymatic cholesterol assay method after dextran sulfate precipitation. The hs-CRP was measured by enzyme-linked immunosorbent assay.
In order to focus on patients with subclinical coronary atherosclerosis, 24 patients with a history of symptomatic CAD confirmed by prior cardiac catheterization were excluded. Thus, a total of 156 patients were recruited. All screened patients provided informed consent to undergoing 64-slice CCTA and data collection, and this study was approved by the human investigations committee.
ASI measurement
Assessments of ASI were performed noninvasively by using a CardioVision® MS-2000 model (International Medical Devices Partners, Las Vegas, NV, USA). The device uses a computerized oscillometric method for BP measurement through a pressure sensor attached to the BP cuff. The cuff is filled with air, and the change of cuff volume can be measured as a change in the inner pressure. Because of the arterial pressure/volume properties, the elastic modulus of the brachial artery is at its greatest when the cuff pressure is equal to the mean BP. The elasticity of the artery increases when the cuff pressure falls below the mean BP, causing the change of the arterial volume. Therefore, the device provides data on the arterial stiffness index of the brachial artery, the systolic and diastolic BP, and the pulse pressure at rest, according to the arterial volume pattern changes caused by the steady decreasing cuff pressure.
The 64-slice CT technique
CCTA was performed using an electrocardiographic gated 64-slice computed tomography (CT) scanner (GE LightSpeed VCT, GE Healthcare, Milwaukee, WI, USA). The coronary artery calcification was detected and quantified using a prospectively gated low-dose sequential CT scan of the heart.9 A contrast-enhanced retrospectively gated spiral CT scan, covering the distance from the tracheal bifurcation to the diaphragm during a single inspiratory breath hold (6-10 s), was performed. A timing bolus sequence was used to detect the arrival of contrast material in the coronary artery, and a bolus of contrast agent (Optiray 350, 350 mg/mL, Montreal, Quebec, Canada) was injected into an antecubital vein at a flow rate of 4 mL/s followed by a saline chaser bolus. The patients with heart rates > 70 beats per minute 30 minutes prior to CT scanning were administered oral beta-blocker therapy (10-50 mg propranolol) if this was not contraindicated. The images were retrospectively reconstructed from the mid- to end-diastolic phases according to electrocardiographic gating. Other reconstruction parameters for slice thickness, field of view, and convolution kernel were set as described previously.10,11 Thereafter, the atheroma on the vessel wall was analyzed.
Analysis of plaque on a 64-slice CT scan
All scans were analyzed independently by 2 experienced radiologists, who were blind to the clinical information (including the ASI), using a 3D workstation (Brilliance; Philips Medical Systems, Best, The Netherlands). After the 2 radiologists made independent evaluations, a consensus interpretation was determined to obtain a final CCTA diagnosis. For plaque differentiation, an optimal image display setting was selected at a window between 600 and 900 HU and at a level between 40 and 250 HU.12 Plaque analyses were performed on longitudinal sections of straight multiplanar reconstructions (along the vessel center line) and axial crosssections (perpendicular to the vessel center line), with a thickness of 1 mm, using the Coronary Vessel Analysis protocol software on an Advantage Workstation 4.3 (GE Healthcare, Milwaukee, WI). Coronary plaques were defined as structures ≥ 1 mm2 (visible in at least one of the crosssections) on the vessel wall, which could be clearly distinguished from the vessel lumen and the surrounding tissue.
Statistical analysis
Data are presented as the mean ± the standard deviation (SD) if normally distributed or otherwise as the median (range). The means of the variables were compared using an analysis of variance for continuous variables and using a chi-squared test for categorical variables. The numerical variables and frequencies of the groups were compared using Student’s t tests, chi-squared tests and/or Mann-Whitney U tests as appropriate. A binary logistic regression analysis was used to determine the independent predictors of the end point in each group. A receiver operator characteristic (ROC) curve analysis13 was performed to determine the cutoff value of the ASI for distinguishing between coronary plaques with and without > 50% stenosis at the highest possible sensitivity and specificity levels. All analyses were performed using SPSS, version 15.1 (SPSS Inc., Chicago, Illinois, USA). A p-value < .05 was considered statistically significant.
RESULTS
We recruited 156 asymptomatic patients (mean age, 56.7 ± 11.4 y) and observed that 53 of the 156 patients had at least one > 50% stenotic lesion over the coronary arteries on CCTA images. Table 1 shows the baseline characteristics of patients with and without at least one > 50% coronary stenotic plaque. The patients with at least one > 50% coronary stenotic plaque were older and had higher systolic BP and ASI values than the patients without a > 50% coronary stenotic plaque. A Pearson’s correlation analysis showed that the ASI correlated with age (r = 0.37, p < .001), systolic BP (r = 0.54, p < .001), and baseline serum creatinine levels (r = 0.23, p = .009). However, the association between ASI and high-sensitivity C-reactive protein was non-significant (r = -0.04, p = .60).
Table 1. Baseline characteristics of patients with or without at least one > 50% coronary stenotic plaque according to CCTA.
| Without > 50% stenotic lesion | With > 50% stenotic lesion | ||
| Variables | (n = 103) | (n = 53) | p-value |
| Variables | (n = 103) | (n = 53) | p-value |
| Age (y) | 54.4 ± 10.6 | 61.2 ± 11.8 | < .001 |
| Men/women | 62/41 | 40/13 | 0.08 |
| Hypertension (%) | 29 (%) | 17 (%) | 0.71 |
| Dyslipidemia (%) | 37 (%) | 19 (%) | 1 |
| Type 2 diabetes mellitus (%) | 9 (%) | 6 (%) | 0.06 |
| Smoking (%) | 12 (%) | 11 (%) | 0.17 |
| Body mass index (kg/m2) | 24.5 ± 3.5 | 25.2 ± 2.7 | 0.26 |
| Systolic blood pressure (mmHg) | 122.6 ± 13.8 | 132.2 ± 13.3 | < .001 |
| Diastolic blood pressure (mmHg) | 70.8 ± 10.7 | 73.2 ± 9.6 | 0.18 |
| Fasting blood glucose (mg/dL) | 82.0 ± 16.5 | 80.7 ± 20.9 | 0.7 |
| Total cholesterol (mg/dL) | 218.1 ± 34.2 | 217.5 ± 38.1 | 0.92 |
| Total triglyceride (mg/dL) | 150.1 ± 93.6 | 174.6 ± 98.9 | 0.15 |
| Low-density lipoprotein cholesterol (mg/dL) | 146.0 ± 35.9 | 140.3 ± 38.0 | 0.37 |
| High-density lipoprotein cholesterol (mg/dL) | 42.4 ± 13.4 | 42.3 ± 12.9 | 0.98 |
| Statin usage (%) | 31 (%) | 16 (%) | 1 |
| Creatinine (mg/dL) | 1.1 ± 0.2 | 1.1 ± 0.2 | 0.92 |
| High sensitive C-reactive protein (mg/dL) | 0.2 ± 0.3 | 0.2 ± 0.3 | 0.71 |
| ASI | 59.3 ± 22.6 | 74.8 ± 39.6 | 0.002 |
Data are presented as the mean ± SD or numbers of patients (%).
ASI, arterial stiffness index; CCTA, cardiac computed tomography angiography; SD, standard deviation.
Table 2 shows the characteristics of patients aged ≥ 50 years and < 50 years. The patients over 50 years of age had a higher prevalence of coronary stenotic plaque and a higher ASI than the patients less than 50 years of age. When we evaluated the differences between patients with or without at least one > 50% coronary stenotic plaque in the 2 subgroups (Table 3), we observed that the ASI was significantly higher in the patients with at least one > 50% coronary stenotic plaque than in those without a > 50% coronary stenotic plaque in the ≥ 50 years subgroup (74.1 ± 35.7 vs. 58.7 ± 20.3, p = .005). However, in the < 50 years subgroup, the difference in the ASI between the patients with or without at least one > 50% coronary stenotic plaque was non-significant (53.2 ± 14.2 vs. 54.9 ± 16.4, p = .74).
Table 2. Characteristics of patients aged ≥ 50 years and < 50 years.
| Variables | < 50 years (n = 46) | ≥ 50 years (n = 110) | p-value |
| Men/women | 13424 | 66/44 | 0.04 |
| Hypertension (%) | 7 (%) | 39 (%) | 0.01 |
| Dyslipidemia (%) | 18 (%) | 38 (%) | 0.59 |
| Type 2 diabetes mellitus (%) | 6 (%) | 9 (%) | 0.38 |
| Smoking (%) | 11 (%) | 12 (%) | 0.05 |
| Body mass index (kg/m2) | 24.9 ± 3.7 | 24.6 ± 3.1 | 0.66 |
| Systolic blood pressure (mmHg) | 119.5 ± 12.7 | 128.1 ± 14.3 | 0.001 |
| Diastolic blood pressure (mmHg) | 70.3 ± 10.2 | 72.1 ± 10.5 | 0.36 |
| Fasting blood glucose (mg/dL) | 85.4 ± 25.2 | 80.3 ± 15.3 | 0.18 |
| Total cholesterol (mg/dL) | 220.3 ± 28.5 | 217.2 ± 37.4 | 0.67 |
| Total triglyceride (mg/dL) | 181.4 ± 110.9 | 152.9 ± 90.9 | 0.15 |
| Low-density lipoprotein cholesterol (mg/dL) | 140.4 ± 30.9 | 144.8 ± 38.2 | 0.55 |
| High-density lipoprotein cholesterol (mg/dL) | 42.6 ± 13.9 | 42.3 ± 13.0 | 0.9 |
| Statin usage (%) | 7 (%) | 40 (%) | 0.01 |
| Creatinine (mg/dL) | 1.1 ± 0.2 | 1.1 ± 0.2 | 0.61 |
| High sensitive C-reactive protein (mg/dL) | 0.1 ± 0.2 | 0.2 ± 0.4 | 0.51 |
| ASI | 52.9 ± 12.7 | 64.7 ± 28.1 | < .001 |
| Presence of a > 50% stenotic lesion (%) | 10 (%) | 43 (%) | 0.04 |
Data are presented as the mean ± SD or numbers of patients (%).
ASI, arterial stiffness index; SD, standard deviation.
Table 3. Characteristics of patients aged < 50 years and ≥ 50 years with or without at least one > 50% coronary stenotic plaque according to CCTA.
| < 50 years old | ≥ 50 years old | |||||
| Without > 50% stenosis | With > 50% stenosis | Without > 50% stenosis | With > 50% stenosis | |||
| (n = 36) | (n = 10) | p-value | (n = 67) | (n = 43) | p-value | |
| Age (y) | 43.6 ± 6.2 | 47.5 ± 2.5 | 0.06 | 60.2 ± 7.5 | 64.3 ± 10.7 | 0.03 |
| Men/women | 42610 | 42584 | 1 | 34/33 | 11994 | 0.02 |
| Hypertension (%) | 06 (%) | 1 (10%) | 1 | 23 (%) | 16 (%) | 0.84 |
| Dyslipidemia (%) | 14 (%) | 4 (40%) | 1 | 23 (%) | 15 (%) | 1 |
| Type 2 diabetes mellitus (%) | 4 (%) | 2 (20%) | 0.6 | 5 (%) | 4 (%) | 0.74 |
| Smoking (%) | 7 (%) | 4 (40%) | 0.22 | 7 (15.6%) | 16 (18.8%) | 0.21 |
| Statin usage (%) | 6 (%) | 1 (10%) | 1 | 25 (%) | 15 (%) | 0.84 |
| Body mass index (kg/m2) | 24.8 ± 3.8 | 25.4 ± 2.6 | < .001 | 24.3 ± 3.3 | 25.2 ± 2.8 | 0.25 |
| Pulse pressure (mmHg) | 49.3 ± 9.4 | 51.8 ± 9.3 | 0.45 | 53.8 ± 11.1 | 61.2 ± 12.2 | 0.002 |
| Fasting blood glucose (mg/dL) | 82.3 ± 18.7 | 96.4 ± 37.1 | 0.16 | 81.8 ± 15.4 | 78.0 ± 15.8 | 0.23 |
| Total cholesterol (mg/dL) | 218.1 ± 34.2 | 217.5 ± 38.1 | 0.3 | 219.3 ± 37.7 | 215.6 ± 39.7 | 0.63 |
| Total triglyceride (mg/dL) | 180.7 ± 114.7 | 160.8 ± 37.4 | 0.61 | 135.1 ± 78.1 | 177.5 ± 107.5 | 0.02 |
| Low-density lipoprotein cholesterol (mg/dL) | 137.3 ± 29.1 | 145.2 ± 31.3 | 0.49 | 150.4 ± 38.3 | 139.2 ± 39.5 | 0.16 |
| High-density lipoprotein cholesterol (mg/dL) | 41.5 ± 13.3 | 48.9 ± 11.1 | 0.14 | 42.8 ± 13.6 | 40.9 ± 13.0 | 0.48 |
| Creatinine (mg/dL) | 1.1 ± 0.2 | 0.9 ± 0.2 | 0.01 | 1.1 ± 0.2 | 1.1 ± 0.2 | 0.25 |
| High sensitive C-reactive protein (mg/dL) | 0.2 ± 0.2 | 0.1 ± 0.1 | 0.67 | 0.1 ± 0.4 | 0.2 ± 0.3 | 0.62 |
| ASI | 54.9 ± 16.4 | 53.2 ± 14.2 | 0.74 | 58.7 ± 20.3 | 74.1 ± 35.7 | 0.005 |
| Calcium artery score | 8.7 ± 35.1 | 122.3 ± 140 | < .001 | 22.4 ± 53.7 | 328.7 ± 466.3 | < .001 |
ASI, arterial stiffness index; CCTA, cardiac computed tomography angiography.
As shown in the Figure 1, the area under the ROC curve of the ASI for the prediction of at least one > 50% coronary stenotic plaque in CCTA in patients aged ≥ 50 years was 0.64 (p < .01). We determined a cutoff ASI value of 80 by using Youden Index. We performed a logistic regression analysis to determine whether an ASI > 80 correlated with at least one > 50% coronary stenotic plaque in CCTA. After adjusting for age, sex, and the baseline lipid profiles, the ASI correlated with at least one > 50% coronary stenotic plaque in patients ≥ 50 years old (odds ratio = 1.02, 95% confidence interval = 1.00-1.04, p = .03).
Figure 1.
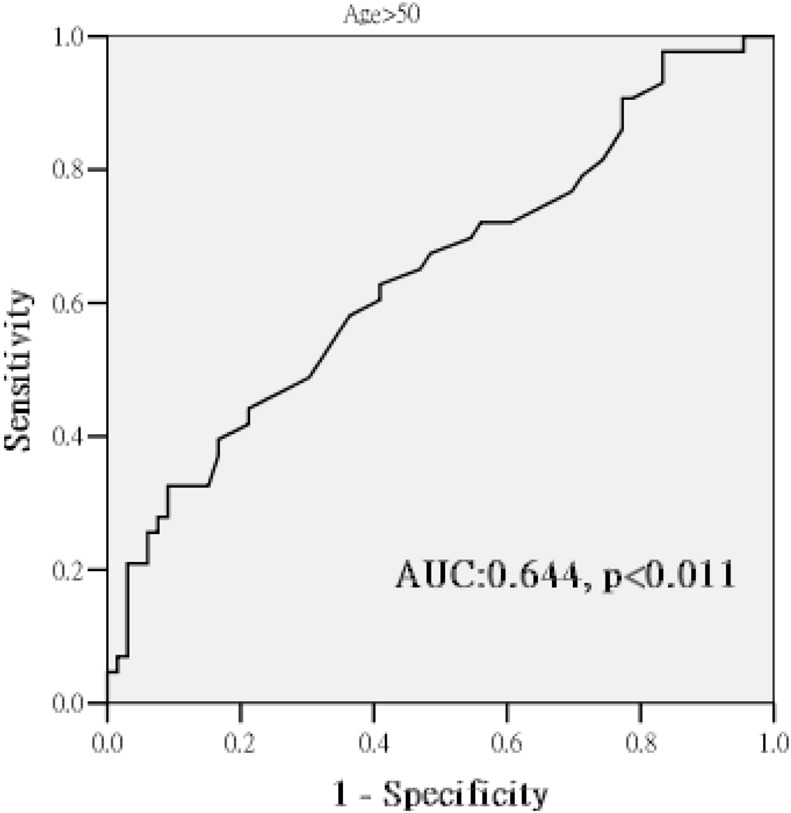
Receiver operating curve for the ASI for predicting at least one > 50% stenotic coronary plaque in patients > 50 years old. ASI, arterial stiffness index; AUC, area under curve.
DISCUSSION
Our study results showed a correlation between the ASI and a > 50% coronary stenotic plaque according to CCTA images in patients with subclinical coronary atherosclerosis ≥ 50 years of age. The area under curve was 0.64, which indicated a less-than-satisfactory discriminatory power. However, it was very close to 0.7 and could be interpreted as a trend. Though the value 0.64 was derived from a selected population with subclinical coronary atherosclerosis with age ≥ 50 years, ASI is noninvasive, easily applied, and free from radiation exposure, which makes it a convenient tool in selected clinical application.
When evaluating the pathogenesis of atherosclerosis, its structural and functional changes should be considered. The structural transformation involves concentric hyaline thickening of the arterial and arteriolar walls, as well as smooth muscle cell proliferation, the accumulation of lipids, and the formation of collagen, elastin, and proteoglycans. Functional pathogenesis refers to endothelial dysfunction and the impaired release of nitric oxide, which causes vasoconstriction and reduced vascular compliance. As atherosclerosis progresses, the tunica media thickens and the tunica intima becomes rigid, thus reducing the arterial elasticity.14
The ASI is a functional reflection of systemic arterial stiffness and elasticity, and a quantitative value of dynamic physiology. A study by Park et al. showed its potential use for the detection of atherosclerotic coronary disease.15 Previously, McLeod et al. showed an association between aortic and coronary atherosclerosis by identifying the correlation between central aortic stiffness and coronary artery plaque volume, as measured using intravascular ultrasound in patients with suspected CAD.16 However, according to a review of the literature, no previous study has shown a correlation between the ASI and subclinical coronary plaque in an asymptomatic population.
In this study, we identified that the ASI provides a useful and noninvasive tool for the detection of > 50% coronary stenotic plaque in CCTA images collected from patients with subclinical coronary atherosclerosis, especially those ≥ 50 years of age. Our findings were similar to those of previous studies.2,17,18 For example, Mattace-Raso et al. indicated that arterial stiffness, measured as aortic pulse wave velocity, is a strong predictor of coronary heart disease in apparently healthy patients.2 A study by Alan et al. described that the stiffness index combined with intima-media thickening and arterial distensibility are all noninvasive and easily available parameters that can be used for the early diagnosis of CAD.17 Izuhara et al. showed that the cardio-ankle vascular index, reported as a novel index of aortic stiffness, is associated with carotid and coronary arteriosclerosis progression.18
Atherosclerosis involves an ongoing inflammatory response that might progress to thrombotic complications.19 The development of novel approaches for the detection of atherosclerosis is a crucial research priority. The major pathophysiologic process involved in coronary atherosclerosis is a defect in, or injury to, arterial endothelial function. Previous studies have evaluated arterial stiffness measures as noninvasive methods of evaluating vascular health20 and endothelial function.21 These studies established that a high ASI indicates endothelial dysfunction and early atherosclerosis.
Our study has some limitations. The study population was relatively small and we did not control for patient comorbidity. We also did not distinguish between menopausal and nonmenopausal women. In addition, except for records on the use of statins, our medication information was limited. We did not perform ASI after hyperemia or after taking 0.5 mg nitroglycerin sublingually as done in the previous study.15 We additionally did not measure pulse wave velocity (PWV), which has been a more common measurement of arterial stiffness. However, the ASI was positively correlated to the PWV according to previous studies,22,23 which makes it a useful tool to evaluate arterial stiffness noninvasively.
CONCLUSIONS
Our study results showed that the ASI could play a correlative role in CAD risk stratification systems. We identified that an ASI > 80 correlates with at least one > 50% coronary stenotic plaque in CCTA in patients with subclinical coronary atherosclerosis who were ≥ 50 years of age. The ASI could provide a useful clinical marker of arterial stiffness for the detection of early coronary plaque. Further longitudinal studies measuring the serial changes in ASI to monitor atherosclerosis progression and possible cardiovascular events are warranted.
Acknowledgments
The authors would like to thank Ms. Shih-Chuan Wei for her expert technical assistance.
COMPETING INTEREST STATEMENT
The authors state that they have no conflict of interest.
REFERENCES
- 1.Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Relationship of atherosclerosis in young men to serum lipoprotein cholesterol concentrations and smoking. A preliminary report from the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. JAMA. 1990;264:3018–3024. doi: 10.1001/jama.1990.03450230054029. [DOI] [PubMed] [Google Scholar]
- 2.Mattace-Raso FU, van der Cammen TJ, Hofman A, et al. Arterial stiffness and risk of coronary heart disease and stroke:the Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 3.Sutton-Tyrrell K, Najjar SS, Boudreau RM, et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness,predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 4.Willum-Hansen T, Staessen JA, Torp-Pedersen C, et al. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson JJ, Julius S, Randall OS. Stroke volume-pulse pressure relationships in borderline hypertension:a possible indicator of decreased arterial compliance. J Hypertens Suppl. 1984;2:S397–S399. [PubMed] [Google Scholar]
- 6.Herrington DM, Brown WV, Mosca L, et al. Relationship between arterial stiffness and subclinical aortic atherosclerosis. Circulation. 2004;110:432–437. doi: 10.1161/01.CIR.0000136582.33493.CC. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell GF, Guo CY, Benjamin EJ, et al. Cross-sectional correlates of increased aortic stiffness in the community:the Framingham Heart Study. Circulation. 2007;115:2628–2636. doi: 10.1161/CIRCULATIONAHA.106.667733. [DOI] [PubMed] [Google Scholar]
- 8.Herrington DM, Kesler K, Reiber JH, et al. Arterial compliance adds to conventional risk factors for prediction of angiographic coronary artery disease. Am Heart J. 2003;146:662–667. doi: 10.1016/S0002-8703(03)00254-0. [DOI] [PubMed] [Google Scholar]
- 9.Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 10.Leschka S, Husmann L, Desbiolles LM, et al. Optimal image reconstruction intervals for non-invasive coronary angiography with 64-slice CT. Eur Radiol. 2006;16:1964–1972. doi: 10.1007/s00330-006-0262-x. [DOI] [PubMed] [Google Scholar]
- 11.Husmann L, Alkadhi H, Boehm T, et al. Influence of cardiac hemodynamic parameters on coronary artery opacification with 64-slice computed tomography. Eur Radiol. 2006;16:1111–1116. doi: 10.1007/s00330-005-0110-4. [DOI] [PubMed] [Google Scholar]
- 12.Leber AW, Knez A, Becker A, et al. Accuracy of multidetector spiral computed tomography in identifying and differentiating the composition of coronary atherosclerotic plaques:a comparative study with intracoronary ultrasound. J Am Coll Cardiol. 2004;43:1241–1247. doi: 10.1016/j.jacc.2003.10.059. [DOI] [PubMed] [Google Scholar]
- 13.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 14.Orlandi A, Bochaton-Piallat ML, Gabbiani G, Spagnoli LG. Aging,smooth muscle cells and vascular pathobiology:implications for atherosclerosis. Atherosclerosis. 2006;188:221–230. doi: 10.1016/j.atherosclerosis.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 15.Park SM, Seo HS, Lim HE, et al. Assessment of arterial stiffness index as a clinical parameter for atherosclerotic coronary artery disease. Circ J. 2005;69:1218–1222. doi: 10.1253/circj.69.1218. [DOI] [PubMed] [Google Scholar]
- 16.McLeod AL, Uren NG, Wilkinson IB, et al. Non-invasive measures of pulse wave velocity correlate with coronary arterial plaque load in humans. J Hypertens. 2004;22:363–368. doi: 10.1097/00004872-200402000-00021. [DOI] [PubMed] [Google Scholar]
- 17.Alan S, Ulgen MS, Ozturk O, et al. Relation between coronary artery disease,risk factors and intima-media thickness of carotid artery,arterial distensibility,and stiffness index. Angiology. 2003;54:261–267. doi: 10.1177/000331970305400301. [DOI] [PubMed] [Google Scholar]
- 18.Izuhara M, Shioji K, Kadota S, et al. Relationship of cardio-ankle vascular index (CAVI) to carotid and coronary arteriosclerosis. Circ J. 2008;72:1762–1767. doi: 10.1253/circj.cj-08-0152. [DOI] [PubMed] [Google Scholar]
- 19.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 20.Duprez D, Cohn JN. Monitoring vascular health beyond blood pressure. Curr Hypertens Rep. 2006;8:287–291. doi: 10.1007/s11906-006-0066-z. [DOI] [PubMed] [Google Scholar]
- 21.Nigam A, Mitchell GF, Lambert J, Tardif JC. Relation between conduit vessel stiffness (assessed by tonometry) and endothelial function (assessed by flow-mediated dilatation) in patients with and without coronary heart disease. Am J Cardiol. 2003;92:395–399. doi: 10.1016/s0002-9149(03)00656-8. [DOI] [PubMed] [Google Scholar]
- 22.Park SM, Seo HS, Lim HE, et al. Assessment of the arterial stiffness index as a clinical parameter for atherosclerotic coronary artery disease. Korean Circulation J. 2004;34:677–683. doi: 10.1253/circj.69.1218. [DOI] [PubMed] [Google Scholar]
- 23.Kaibe M, Ohishi M, Komai N, et al. Arterial stiffness index:a new evaluation for arterial stiffness in elderly patients with essential hypertension. Geriatr Gerontol Int. 2002;2:199–205. [Google Scholar]


