Abstract
A 74-year-old woman who was diagnosed with right breast cancer at age 39 had been treated with mastectomy, and repeated cycles of chemotherapy and radiotherapy. She also had a history of coronary artery disease, wherein two coronary artery bypass grafts were performed 3 years ago. At that time, porcelain aorta was detected during surgery. In the year prior to admission, the patient presented with severe symptomatic critical aortic stenosis. Due to the prohibitively high surgical risk and need for aortic valve replacement, she underwent successful transcatheter aortic valve implantation with transfemoral implantation of a 29 mm Medtronic CoreValve prosthesis. The patient experienced a good result with reduction of the transaortic gradient and mild residual aortic regurgitation.
Keywords: Aortic stenosis, Coronary artery bypass grafting, Porcelain aorta, Radiation, Transcatheter aortic valve implantation
INTRODUCTION
A growing body of evidence has confirmed that transcatheter aortic valve implantation (TAVI) is an effective and useful treatment for high-risk patients with critical valvular aortic stenosis (AS).1 Porcelain aorta is a condition that increases the risk of surgery due to technical impossibility and/or hazards related to clamping of the ascending aorta.2,3 In this context, the patient outcome is expected to be good, provided intervention on the aortic valve can be carried out without aortic clamping, as is the case with TAVI.4 Good midterm results after TAVI in patients with porcelain aorta has been reported in patients where either the transfemoral or transapical approach was utilized.4 We herein report a 74-year-old woman with porcelain aorta, with a history of previous stenotomy for coronary artery bypass grafting (CABG) and critical AS who underwent successful transfemoral TAVI. To the best of our knowledge, this may be the first reported case with this particular indication of TAVI in Taiwan.
CASE REPORT
A 74-year-old woman was admitted to our facility arising from a gradual onset of exertional dyspnea which occurred over the course of one year. Thirty-five years prior to admission, she was diagnosed with right breast cancer and received mastectomy and repeated cycles of chemotherapy and mediastinal and chest radiotherapy. Thereafter the patient’s post-surgical condition was unremarkable until three years prior to admission, when she began to suffer from occasional episodes of chest tightness and exertional dyspnea on exertion. She was admitted to our hospital where a grade 3/6 systolic ejection murmur which radiated to the neck was detected and echocardiographic study revealed severe AS (aortic valve area: 0.40 cm2; mean gradient: 52 mmHg). Cardiac catheterization performed at that time showed severe three-vessel coronary artery disease in the patient’s native coronary arteries. At the time of surgery, the aorta was found to be heavily calcified leaving no place for safe aortic clamping. It was therefore decided to treat the patient with a no-touch aortic approach with off-pump CABG using sequential Y grafts to the left anterior descending artery and right coronary artery, and deferred aortic valve replacement until later. Fortunately, the patient’s symptoms subsequently improved after the operation and she was discharged without any significant complication.
During the preceding two years, the patient was reported to be in good health condition. One year before admission, the patient presented with progressive recurrence of shortness of breath and dyspnea on exertion during ordinary daily physical activity [New York Heart Association (NYHA) functional class III]. A multi-slice computed tomographic (CT) scan was performed, which revealed that the saphenous-vein grafts that had been placed during CABG three years earlier were patent (Figure 1C). The patient was then admitted for TAVI. The patient had a past medical history of hypertension and hypercholesterolemia and was taking amlodipine and atorvastatin. On admission the patient’s blood pressure and pulse rate were 148/71 mmHg and 78/min, respectively, while body weight and height were respectively 55 kg and 160 cm. The 12-lead electrocardiogram (ECG) on admission showed sinus rhythm and findings suggestive of left ventricular hypertrophy with strain. Cardiomegaly and calcification of ascending aorta were noted on the chest X-ray. Additionally, transthoracic and transesophageal echocardiography revealed severe degenerative change of aortic valve with heavy calcification. The aortic valve area was 0.30 cm2 with a mean systolic pressure gradient of 104 mmHg and peak systolic pressure gradient of 177 mmHg, which was compatible with critical AS. Left ventricular end diastolic diameter was 45 mm and ejection fraction was 50%. There was moderate pulmonary hypertension with an estimated right ventricular systolic pressure of 60 mmHg. The aortic diameter measured by CT scan was 23.8 mm (20 × 26 mm) and the perimeter was 74.8 mm at the aortic annulus. Her CT also confirmed a diffuse atherosclerotic change of aorta and both external iliac arteries with calcification, especially the ascending aorta (Figure 1A and B). The aortic root angulation was 42° and the minimal lumen diameter of the right and left external iliac arteries was 6.5 mm and 5.2 mm, respectively. The logistic EuroSCORE and the Society of Thoracic Surgeons (STS) risk score were calculated to be 42% and 30.86%, respectively.
Figure 1.
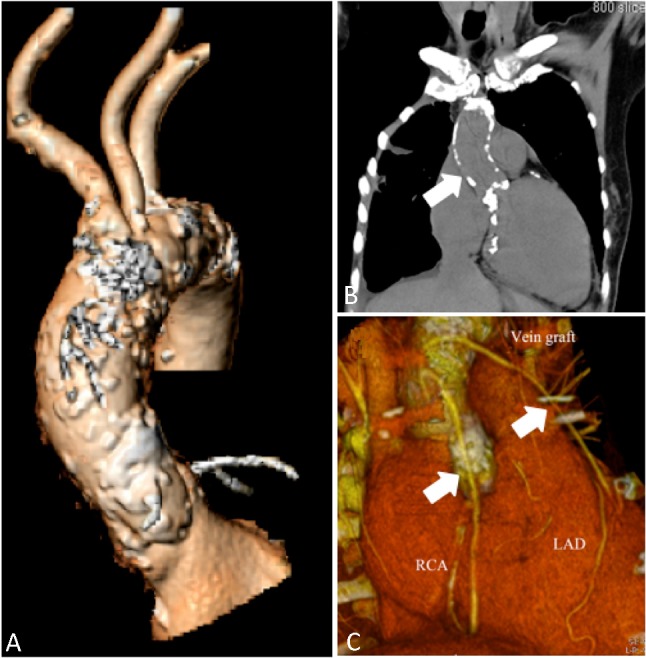
A multi-slice computed tomographic scan shows diffuse atherosclerotic changes with heavy calcifications of the ascending thoracic aorta (A and B, arrow). The saphenous-vein grafts to left anterior descending artery (LAD) and right coronary artery (RCA) that had been placed during coronary artery bypass grafting three years earlier were patent (C, arrows).
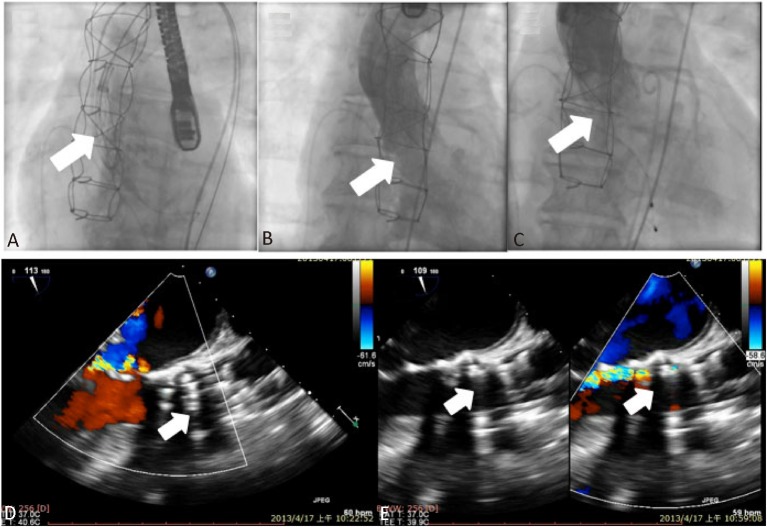
The TAVI procedure was performed under general anesthesia. Vascular access for the CoreValve delivery catheter was obtained at the right common femoral artery (CFA) with standard surgical cut-down techniques. An 18 Fr introducer sheath was inserted through the right CFA, and a pig tail catheter was inserted through the left CFA and positioned at the aortic root for the aortography during the procedure. Then, a temporary pacemaker was placed in the right ventricle via the left femoral vein. A 0.035 inch Amplatz Super Stiff wire (Boston Scientific, Natick, MA, USA) was inserted into the left ventricle through the 18 Fr sheath. Balloon dilation of the stenotic aortic valve was performed with a balloon (diameter 22 mm, length 40 mm, Tyshak, NuMED Canada Inc., Cornwall, CN) under rapid pacing using a temporary pacemaker. Then, a 29 mm CoreValve was deployed at the aortic annulus under angiographic guidance (Figure 2A). An immediate post-procedural aortogram showed the CoreValve had achieved a good position, but severe aortic regurgitation continued (Figure 2B). Intra-operative post-procedural transesophageal echocardiography demonstrated an under-expanded and malopposed CoreValve due to interference of the nodular calcification at the level of the aortic valve ring, leading to both severe valvular and paravalvular aortic regurgitation (Figure 2D). So we decided to post-dilatate the prosthesis using another undersized non-compliant balloon (diameter 25 mm, length 40 mm, Nucleus, NuMED Canada Inc., Cornwall, CN) to improve the hemodynamic parameters. After post-dilatation, both the immediate post-procedural aortogram (Figure 2C) and transesophageal echocardiography (Figure 2E) demonstrated a well-functioning CoreValve with a mean systolic pressure gradient of 8 mm Hg, a peak systolic pressure gradient of 16 mm Hg, and mild paravalvular aortic regurgitation. The aortic regurgitation index before and after balloon post-dilatation were 35% and 18%, respectively. After the procedure, the patient remained under observation in an intensive care unit for 36 hours with continuous ECG monitoring and temporary pacemaker back-up. No significant conduction abnormalities were observed. The patient’s symptoms subsequently improved from NYHA class III to class I, and she was discharged from the hospital on day 12 post-procedure without any significant complication. A 3-month follow-up echocardiogram revealed no significant change from the immediate post-procedure echocardiographic findings. The patient remained free from any symptom or any major cardiovascular event for a total follow-up period of 10 months.
Figure 2.
Fluoroscopic images during the implantation of the CoreValve (A, arrow) and the immediate post-procedure aortogram showing severe aortic regurgitation (B, arrow). Intra-operative post-procedural transesophageal echocardiography demonstrated an under-expanded and mal-apposed CoreValve due to interference of the nodular calcification at the level of the aortic valve ring, leading to both severe valvular and paravalvular aortic regurgitation (D). After post-dilatation of the implanted CoreValve, both the immediate post-procedural aortogram (C, arrow) and transesophageal echocardiography (E, arrows) demonstrated a well-functioning CoreValve and mild aortic regurgitation.
DISCUSSION
The incidence of porcelain aorta is elevated in elderly patients with severe AS and coronary artery disease, and varies from 1.2% to 28%.2,3 In patients with a porcelain aorta, aortic valve replacement and CABG are technically demanding and associated with a higher perioperative risk due to the high risk of cerebral embolism and the impossibility of safe aortic dissection and clamping.2,3 Although different surgical approaches have been described, almost all of these techniques require cardiopulmonary bypass and manipulations at the calcified aorta, to some extent.2,3 Hypothermic circulatory arrest or apico-aortic bypass is an alternative bailout procedure for patients with unclampable aorta. However, these procedures also carry extreme high risks, including stroke, respiratory failure, and even death.
With the introduction of TAVI, high-risk elderly patients with porcelain aorta and AS can now be treated without aortic cross-clamping and cardiopulmonary bypass.4-6 In patients presenting with porcelain aorta, peripheral vascular disease is a frequent concomitant condition. Therefore, the transapical technique was considered to be more appropriate in most of these patients to avoid the often heavily calcified femoral vessels, the abdominal aorta, and most importantly the aortic arch to provide maximal patient safety.4 However, recent studies have demonstrated that both transapical and transfemoral delivery routes and both approved devices, the Edwards Sapien prosthesis and the Medtronic CoreValve prosthesis, are equally feasible and safe in such patients.4-6
Approximately 20% of the patients undergoing TAVI have porcelain aorta.4-6 In the 29 patients with porcelain aorta who underwent transapical TAVI reported by Kempfert et al., all valves were implanted successfully and all procedures were primarily performed off-pump, but 4 patients required secondary cardiopulmonary bypass as a result of complications.4 Stroke and 30-day mortality rates were 3.4% and 17.2%, respectively.4 In another series of 61 patients with porcelain aorta who underwent TAVI (54% of them were done transapically) reported by Rodés-Cabau et al., all valves except one were implanted successfully.5 Stroke and 30-day mortality were 1.6% and 11.5%, respectively, with no differences compared with patients without porcelain aorta.7 Moreover, patients with porcelain aorta were younger, exhibited a lower surgical risk, and had a lower prevalence of cerebrovascular disease, pulmonary hypertension, and severe mitral regurgitation. Those patients who survived the procedure had a lower late mortality rate of 86% at 1-year follow-up.5
The presented case had breast cancer diagnosed at age 39, and treated with repeated cycles of chemotherapy and radiotherapy, which was probably the underlying cause of her porcelain aorta and aortic stenosis.7,8 Three years prior to admission, she was diagnosed with coronary artery disease and critical AS after complaining of intermittent typical angina and exercise intolerance. At that time, only two coronary artery bypass grafts were done and aortic valve replacement was deferred because extensive calcification of the aorta was omitted preoperatively due to an oversight and was detected at surgery. Fortunately, the patient’s symptoms of effort angina much improved after successful CABG and there had been uneventful 2-year period after the operation. This time, when she presented with progressive exertional dyspnea, which had been ongoing for the last year prior to admission, less invasive TAVI was recommended due to the prohibitively high surgical risk and the need for aortic valve replacement. Finally, she underwent successful TAVI with transfemoral implantation of a 29 mm Medtronic CoreValve prosthesis. The patient has been followed for 10 months and is still doing very well.
Of high clinical relevance, the need for a second valve tended to be more frequent in those patients with porcelain aorta who underwent TAVI, probably reflecting a higher incidence of valve malposition due to either difficulty in valve positioning or valve displacement in the presence of a highly calcified aorta and aortic valve.5 As known, failure to correctly place the prosthesis in the TAVI procedure may be life-threatening. The frequently observed nodular calcifications occurring at the level of the aortic valve ring or at/close to the base of the individual cusp are one of the major causes of malpositioning of the prosthesis. This is the most likely reason for the occurrence of under-expansion and paravalvular regurgitations after TAVI because when the stenotic valve is opened, the pressure is not sufficient to destroy the calcified nodule. Implanting the prosthesis at the level of the nodule then results in an uneven extension which then leads to a paravalvular leak on both sides of the nodule, due to an incomplete seal around the entire ring area.4,5,9 Post-dilatation of the prosthesis after valve implantation may be helpful to improve hemodynamic parameters in cases of distinct calcifications,9,10 such as we had done in the presented case. However, an undersized balloon should be used in this scenario, since if the calcification involves primarily the aortic ring and extends through the entire aortic wall, the bursting of the wall could have disastrous consequences such as a massive bleed and a subsequently fatal pericardial tamponade.10
In conclusion, TAVI is a promising, truly minimally invasive approach to treat elderly high-risk patients with porcelain aorta requiring aortic valve replacement. The outcome is expected to be good in those patients who survive the procedure.
REFERENCES
- 1.Salinas P, Moreno R, Lopez-Sendon JL. Transcatheter aortic valve implantation:current status and future perspectives. World J Cardiol. 2011;3:177–185. doi: 10.4330/wjc.v3.i6.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gillinov AM, Lytle BW, Hoang V, et al. The atherosclerotic aorta at aortic valve replacement:surgical strategies and results. J Thorac Cardiovasc Surg. 2000;120:957–963. doi: 10.1067/mtc.2000.110191. [DOI] [PubMed] [Google Scholar]
- 3.Zingone B, Rauber E, Gatti G, et al. Diagnosis and management of severe atherosclerosis of the ascending aorta and aortic arch during cardiac surgery:focus on aortic replacement. Eur J Cardiothorac Surg. 2007;31:990–997. doi: 10.1016/j.ejcts.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 4.Kempfert J, Van Linden A, Linke A, et al. Transapical aortic valve implantation: therapy of choice for patients with aortic stenosis and porcelain aorta? Ann Thorac Surg. 2010;90:1457–1461. doi: 10.1016/j.athoracsur.2010.06.080. [DOI] [PubMed] [Google Scholar]
- 5.Rodés-Cabau J, Webb JB, Anson C, et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk. Acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol. 2010;55:1080–1090. doi: 10.1016/j.jacc.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Ussia GP, Barbanti M, Petronio AS, et al. Transcatheter aortic valve implantation:3-year outcomes of self-expanding CoreValve prosthesis. Eur Heart J. 2012;33(8):969–976. doi: 10.1093/eurheartj/ehr491. [DOI] [PubMed] [Google Scholar]
- 7.Adams MJ, Lipshultz SE, Schwartz C, et al. Radiation-associated cardiovascular disease:manifestations and management. Seminars in Radiation Oncol. 2003;13:346–356. doi: 10.1016/S1053-4296(03)00026-2. [DOI] [PubMed] [Google Scholar]
- 8.Nadlonek NA, Weyant MJ, Yu JA, et al. Radiation induces osteogenesis in human aortic valve interstitial cells. J Thorac Cardiovasc Surg. 2012;144:1466–1470. doi: 10.1016/j.jtcvs.2012.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loeser H, Wittersheim M, Puetz K, et al. Potential complications of transcatheter aortic valve implantation (TAVI) -- an autopsy perspective. Cardiovasc Pathol. 2013 (in press) doi: 10.1016/j.carpath.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Al Ali AM, Altwegg L, Horlick EM, et al. Prevention and management of transcatheter balloon-expandable aortic valve malposition. Catheter Cardiovasc Interv. 2008;72:573–578. doi: 10.1002/ccd.21667. [DOI] [PubMed] [Google Scholar]