Abstract
Background
The aim of our study was to assess the effect of pretreatment with cilostazol and rosuvastatin combination before elective percutaneous coronary intervention (PCI) on peri-procedural myocardial injury (PPMIJ).
Methods
We randomly assigned 172 patients with stable angina pectoris scheduled for elective PCI to pre- treatment with Cilostazol 200mg and Rosuvastatin 40 mg (group 1), or to pretreatment with Rosuvastatin 40 mg group (group 2). The primary end-point was the occurrence of PPMIJ defined as any cardiac troponin I (Tn I) level elevated above the upper normal limit (UNL). The occurrence of peri-procedural myocardial infarction (PPMIN) was defined as a post-procedural increase in cTnI level ≥ 5 times above the UNL.
Results
There was no significant difference in baseline characteristics between group 1 (n = 86) and group 2 (n = 86). The rate of PPMIJ (21% vs. 24%, p = 0.58) and PPMIN (2.3% vs. 7%, p = 0.27) were similar between the two study groups. Subgroup analysis performed on those patients without statin therapy before PCI (53 patients in group 1 and 50 patients in group 2) showed that the incidence of PPMIJ was significantly lower in the group 1 patients without chronic statin treatment [17% (9/53) versus 34% (17/50); p = 0.04], but the rate of PPMIN was similar between the two groups for those patients without chronic statin treatment [1.9% (1/53) versus 10% (5/50); p = 0.07].
Conclusions
We found that adjunct cilostazol and rosuvastatin pre-treatment did not significantly reduce PPMIJ after elective PCI in patients with stable angina pectoris. However, adjunct cilostazol pre-treatment could reduce PPMIJ in patients without chronic statin therapy before elective PCI.
Keywords: Cilostazol, Myocardial injury, Percutaneous coronary intervention, Statin
INTRODUCTION
Percutaneous coronary intervention (PCI) is a common method for the treatment of coronary artery disease. During PCI, activation of inflammatory processes, thrombus formation and microembolization may result in myocardial injury. Previous cardiovascular magnetic resonance imaging studies had shown that post-PCI elevation of TnI had shown evidence of new irreversible myocardial injury.1 Furthermore, mortality and non-fatal myocardial infarction were frequently reported in patients with elevated post-procedural troponin levels.2,3
Several treatment modalities were established for preventing possible peri-procedural myocardial injury (PPMIJ) during PCI. Aspirin and clopidogrel prevent PPMIJ by inhibiting platelet aggregation. Statins were shown to inhibit PPMIJ with their pleiotropic effects and increase nitric oxide synthesis.4-6 Despite these available treatments, no modality has been developed that completely prevents PPMIJ. Cilostazol is a phospodiesterase III enzyme inhibitor, which has antiaggregant, antiproliferatative, anti-inflammatory and vasodilatatory effects. Previously, experimental studies reported that cilostazol may prevent PPMIJ.7 Cilostazol with statin combination has synergistic effects on endothelial nitric oxide (NO) synthesis8 and pleiotrophic effects of statins increases with cilastazol’s synergistic feature. However, the effects of cilostazol and cilostazol-statin combination on PPMIJ has not yet been investigated in patients undergoing PCI.
In this study, our aim was to evaluate the effectiveness of pretreatment with cilostazol and cilostazol-statin combination before undertaking an elective PCI procedure to prevent PPMIJ.
METHODS
Patient selection
In this randomized, prospective and single-center study, we evaluated patients treated with elective PCI. Inclusion criteria were; 1) patients ranging from 18 to 80 years of age; 2) ≥ 70% stenosis at the native coronary artery; 3) absence of visible thrombus at coronary arteries in angiography; and 4) patients planned for elective PCI and patients that had informed consent. Exclusion criteria were: 1) a history of aspirin; 2) allergic reaction to clopidogrel, cilostazol or rosuvastatin; 3) patients with acute coronary syndromes; 4) myocardial infarction within 2-weeks; 5) elevated preprocedural troponin I (Tn-I) and creatine kinase – myocardial band (CK-MB) levels; 6) history of warfarin use, bleeding disorders or patients with high bleeding risk.
In our investigation, 212 patients were evaluated and 172 eligible patients were ultimately included in the study. All patients gave written informed consent that was approved by the Bursa Postgraduate Hospital Ethics Committee protocol.
Study design
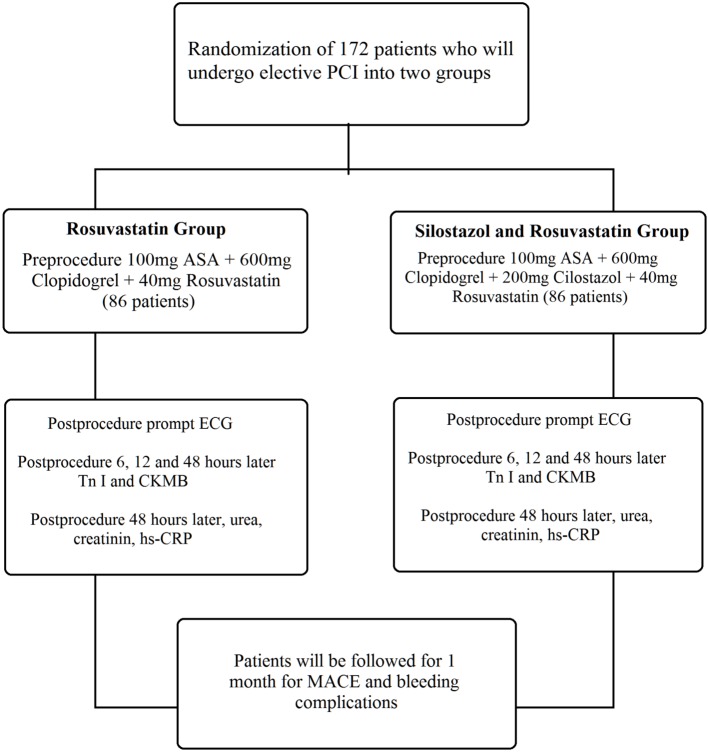
Between 2010 and 2012, 172 patients that met the inclusion criteria were recruited, and were randomized into two groups (Figure 1). The process of patient randomization was accomplished automatically by computer program. There were 86 patients pretreated with cilostazol 200 mg, clopidogrel 600 mg and rosuvastatin 40 mg (group 1). The remaining 86 patients were pretreated with clopidogrel 600 mg and rosuvastatin 40 mg (group 2). For equalizing the number of pills, one pill of rosuvastatin 40 mg was given to the patients in group 1, and 2 tablets of 10 mg and one tablet of rosuvastatin 20 mg were given to the study subjects in group 2. We also recorded patient histories, physical examination findings, 12-lead electrocardiography (ECG), transthoracic echocardiography and coronary angiography findings, medications and systemic diseases. Additionally, patients in our study were evaluated for cardiovascular risk factors. After 12 hours of fasting, blood samples were taken from the antecubital vein, and biochemical measurements were determined by standard laboratory methods. Hematologic indices were evaluated by complete blood count analysis. Hyperlipidemia was defined as low density lipoprotein (LDL) value > 100 mg/dl or use of lipid-lowering drugs. Diabetes was established by physician report and was based on a fasting blood sugar level ≥ 126 mg/dl or use of an antidiabetic medication. Hypertension was physician-reported with a systolic blood pressure ≥ 140 mm Hg, diastolic blood pressure ≥ 90 mm Hg, or use of antihypertensive agents.
Figure 1.
Study design. ASA, asetyl salisilic acid; CK-MB, creatine kinase – myocardial band; ECG, electrocardiography; hs-CRP, high sensitivity C-Reactive Protein; MACE, major adverse cardiac events; Tn I, troponin I; PCL, percutaneous coronary intervention.
Medical treatment
Pharmacological treatments (beta blocker, nitrate, calcium channel blocker, angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, oral antidiabetic) were administered according to the patients’ clinical status. During and/or after the procedure, glycoprotein (GP) IIb/IIIa inhibitor was used based on the discretion of the operator. The patients received clopidogrel 75 mg/day and aspirin 100 mg/day after the PCI.
Percutenous coronary intervention and angiographic analysis
Coronary angiographies were performed in our clinic using the standard Judkins technique, and coronary lesions were assessed by coronary angiography with multiple views. Quantitative coronary angiography analysis was performed using analysis of ACOM PC Lite version 2.0 program (Siemens, München, Germany). Modified American Heart Association/American College of Cardiology (AHA/ACC) criteria and SYNTAX score was used for angiographic lesion classification. A diseased coronary vessel was defined as the presence of at least one > 50% reduction of intraluminal diameter on major coronary arteries (left main, left anterior descending, left circumflex, right coronary arteries) or their branches with a diameter ≥ 2.0 mm. Balloon angioplasty and stent implantation were performed according to standard clinical practice by the femoral approach. A 6 Fr or 7 Fr standard interventional guiding catheter was used for PCI. During PCI, patients received unfractionated heparin (70-100 IU/kg, maximum 10000 IU). After the passage of the stenosis at the culprit artery with 0.014 inch guidewire, direct stenting or if necessary balloon angioplasty was performed. The procedure was deemed successful if there was residual stenosis < 10% and thrombolysis in myocardial infarction (TIMI) flow reaching grade 3.
Follow-up
After successful PCI, activated clotting time (ACT) levels were measured and femoral sheaths were removed if the ACT was < 150 sec. Subsequent to catheter removal, no closure device was used. Patient ECG was interpreted before PCI, immediately after PCI and before hospital discharge. Blood samples for serum creatine kinase (CK), CK-MB and Tn I were drawn before and 6, 12, and 48 hours after coronary intervention. Serum high sensitivity C-reactive protein (hs-CRP) levels were measured before and 48 hours after the procedure was completed. All patients were followed up clinically at 30 days after hospital discharge. Cardiac TnI assays were performed on an ADVIA Centaur CP Immunoassay System (Siemens Healthcare Diagnostics Inc. Tarrytown, NY, USA) with a cut-off value of 0.04 ng/mL and a lower threshold detection limit of 0.006 ng/mL as recommended by the manufacturer.
Clinical end points
Primary end points were established in this study to be PPMIJ or peri-procedural myocardial infarction (PPMIN), and secondary end points were major adverse cardiac events (MACE) or bleeding complication during the following 30 days. PPMIJ was defined as increasing Tn-I or CK-MB levels over baseline values within 48 hours. PPMIN was defined as a 5-time increase in Tn-I or CK-MB levels over the baseline values within 48 hours, plus either evidence of prolonged ischaemia (≥ 20 min), or ischaemic ST changes or new pathological Q waves, or angiographic evidence of a flow limiting complication, or imaging evidence of new loss of viable myocardium or new regional wall motion abnormality.9 MACE was defined as cardiovascular-related death, acute myocardial infarction or repeated target lesion revascularization. Bleeding complications were classified according to applicable TIMI criteria.10
Statistical analysis
Statistical analysis was performed using SPSS 10.0 (Statistical Package for the Social Sciences ver. 10.0, SPSS Inc, Chicago, Illinois, USA). Continuous variables were presented as mean ± standard deviation and categorical data were expressed as percentage. Variables between the groups were compared by two-sample t-test for normally distributed values. The Mann-Whitney U test was used to compare non-normally distributed variables between groups. Categorical variables were compared by chi-square or Fisher’s exact test. Parameters of periprocedural ischemia were assessed by univariate or multivariate logistic regression tests because of the dichotomous variable. A p-value of < 0.05 was accepted as statistically significant.
RESULTS
Clinical characteristics were similar between the study groups except for history of smoking, which was significantly higher in the cilostazol group [65.1% (n = 56) vs. 43% (n = 37), where p = 0.004]. Also, there wasn’t any significant difference between the study groups in terms of coronary lesion type, coronary lesion characteristics defined by SYNTAX score, the total number of vessels with lesions, the total number of stents implanted per lesion and PCI characteristics (Table 1). No patient received GPIIb/IIIa inhibitor in both study groups. Tn-I, hs-CRP and CK-MB levels before and after the PCI did not differ between these two groups of patients. There was no significant difference in MACE and bleeding complication incidence between the two groups during one-month follow-up (Table 2).
Table 1. Comparison of demographic, biochemical, angiographic and intervention related parameters of two groups .
| Cilostazol + rosuvastatin group (86 patients) | Rosuvastatin group (86 patients) | p value | |
| Age (years) | 58.86 ± 10.68 | 60.06 ± 9.8 | 0.44 |
| Sex, male/female, n (%) | 67 (77.9)/19 (22.1) | 62 (72.1)/24 (27.9) | 0.37 |
| Diabetes mellitus, n (%) | 15 (17.4) | 24 (27) | 0.10 |
| Hypertension, n (%) | 52 (60.4) | 53 (61.6) | 0.87 |
| Hyperlipidemia, n (%) | 35 (40,7) | 40 (46.5) | 0.44 |
| Smoking, n (%) | 56 (65.1) | 37 (43) | 0.004 |
| Ejection fraction, (%) | 52.41 ± 9.2 | 50.72 ± 11.53 | 0.28 |
| Systolic blood pressure, (mmHg) | 125.37 ± 16.90 | 120.03 ± 21.88 | 0.07 |
| Diastolic blood pressure, (mmHg) | 76.39 ± 15.16 | 73.63 ± 12.76 | 0.20 |
| Heart rate (beats/minute) | 71.18 ± 11.47 | 70.31 ± 13.38 | 0.64 |
| Glucose (mg/dl) | 120.12 ± 53.00 | 128.39 ± 48.68 | 0.28 |
| White blood count (× 103) | 8.33 ± 2.4 | 7.97 ± 1.72 | 0.25 |
| Hemoglobin (gr/dl) | 13.21 ± 1.94 | 12.86 ± 1.79 | 0.22 |
| Thrombocyte(× 103) | 239.43 ± 59.63 | 279.74 ± 306.26 | 0.23 |
| Creatinin (mg/dl) | 0.8 ± 0.7 | 0.9 ± 0.9 | 0.45 |
| LDL (mg/dl) | 112.50 ± 36 | 107.47 ± 45.58 | 0.42 |
| HDL (mg/dl) | 39.54 ± 9.37 | 38.90 ± 8.92 | 0.64 |
| Medications | |||
| ASA, n (%) | 86 (100) | 86 (100) | 1.0 |
| Clopidogrel, n (%) | 32 (37.2) | 32 (37.2) | 1.0 |
| Beta blocker, n (%) | 77 (89.5) | 70 (81.3) | 0.13 |
| CCB, n (%) | 10 (11.6) | 7 (8.1) | 0.44 |
| ACEI, n(%) | 54 (62.7) | 59 (68.6) | 0.42 |
| ARB, n (%) | 14 (16.2) | 10 (11.6) | 0.37 |
| Statin, n (%) | 33 (38.3) | 36 (41.8) | 0.64 |
| OAD, n (%) | 8 (9.3) | 16 (18.6) | 0.07 |
| PPI, n (%) | 28 (32.5) | 27 (31.3) | 0.87 |
| Target vessel n (%) | 0.72 | ||
| LAD | 32 | 32 | |
| CX | 24 | 24 | |
| RCA | 18 | 20 | |
| LAD/CX | 8 | 4 | |
| LAD/RCA | 1 | 3 | |
| RCA/CX | 3 | 2 | |
| LAD/RCA/CX | 0 | 1 | |
| Lesion type, n (%) | 0.43 | ||
| A | 4 | 3 | |
| B1 | 42 | 36 | |
| B2 | 8 | 15 | |
| C | 32 | 32 | |
| Lesion length, mm (mean ± SD) | 30.89 ± 35.89 | 26.91 ± 14.28 | 0.34 |
| SYNTAX score | 9.62 ± 4.94 | 11.11 ± 6.57 | 0.22 |
| Procedure | 0.44 | ||
| Direct stent, n (%) | 52 (60.5) | 47 (54.7) | |
| PTCA+stent, n (%) | 34 (39.5) | 39 (45.3) | |
| Stent length, mm (mean ± SD) | 30.89 ± 35.89 | 26.91 ± 14.28 | 0.34 |
| Stent diameter, mm (mean ± SD) | 3.03 ± 0.46 | 2.8 ± 0.44 | 0.44 |
| Amount of stent used per procedure | 1.51 ± 0.77 | 1.4 ± 0.71 | 0.47 |
| Procedure time, min (mean ± SD) | 20.16 ± 7.33 | 20.56 ± 6.93 | 0.70 |
| Use of IIb/IIIa inhibitors, n (%) | 0 (0) | 0 (0) | 1.0 |
| Quantitative angiographic analysis | |||
| Pre-procedure | |||
| RLD | 3.02 ± 0.47 | 2.8 ± 0.46 | 0.13 |
| MLD | 0.32 ± 0.25 | 0.34 ± 0.24 | 0.62 |
| % stenosis | 89.23 ± 8.38 | 87.81 ± 9.17 | 0.30 |
| Post-procedure | |||
| RLD | 3.02 ± 0.47 | 2.88 ± 0.48 | 0.11 |
| MLD | 3.02 ± 0.48 | 2.87 ± 0.47 | 0.10 |
| % stenosis | 0.23 ± 2.15 | 0.46 ± 3.03 | 0.56 |
ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; ASA, asetyl salisilic acid; CCB, calcium channel blocker; CX, left circumflex artery; HDL, high density lipoprotein; LAD, left anterior descending artery; LDL, low density lipoprotein; min, minute; MLD, minimal luminal diameter; n, patient count; OAD, oral antidiabetic; PPI, proton pump inhibitor; PTCA, percutaneous transluminal coronary angioplasty; RCA, right coronary artery; RLD, reference lumen diameter; RVD, reference luminal diameter; SD, standard deviation; UFH, unfractionated heparin.
Table 2. Comparison of myocardial enzymes and adverse events of two groups .
| Cilostazol + rosuvastatin group (86 patients) | Rosuvastatin group (86 patients) | p value | |
| Baseline hs-CRP | 9.89 ± 1.12 | 10.93 ± 1.61 | 0.54 |
| Post-procedure hs-CRP | 15.16 ± 1.63 | 15.21 ± 1.49 | 0.56 |
| Baseline Tn I (ng/ml) | 0.023 ± 0.003 | 0.019 ± 0.004 | 0.052 |
| Post-procedure 6 hour Tn I | 0.10 ± 0.02 | 0.12 ± 0.02 | 0.41 |
| Post-procedure 12 hour Tn I | 0.25 ± 0.04 | 0.36 ± 0.08 | 0.48 |
| Post-procedure 48 hour Tn I | 0.25 ± 0.05 | 0.42 ± 0.09 | 0.84 |
| Baseline CK-MB (U/L) | 16.32 ± 0.78 | 21.84 ± 3.83 | 0.77 |
| Post-procedure 6 hour CK-MB | 14.63 ± 0.76 | 17.79 ± 2.55 | 0.97 |
| Post-procedure 12 hour CK-MB | 16.01 ± 0.79 | 20.04 ± 2.75 | 0.71 |
| Post-procedure 48 hour CK-MB | 13.79 ± 0.85 | 17.97 ± 2.75 | 0.46 |
| 1. month MACE n (%) | 1 (1.2) | 1 (1.2) | 1.00 |
| Major bleeding n (%) | 1 (1.2) | 1 (1.2) | 1.00 |
| Minor bleeding n (%) | 2 (2.3) | 1 (1.2) | 0.50 |
CK-MB, creatine kinase – myocardial band; hs-CRP, high sensitivity C-reactive protein; MACE, major adverse cardiac events; Tn I, troponin I.
The incidence of PPMIJ was 21% (n = 18/86) in group 1 and 24% (n = 21/86) in group 2 (p = 0.58). Furthermore, the incidence of PPMIN was 2.3% (n = 2/86) in group 1 and 7% (n = 6/86) in group 2 which also showed no significant difference (p = 0.27).
After the procedure, > 1-mm ST-segment elevation/depression at consecutive ECG derivations was observed in 4 patients in the group 1 (4.7%) and in 9 patients in group 2 (10.5%) (p = 0.14).
We performed logistic regression analysis factoring in age, diabetes mellitus, hyperlipidemia, chronic statin treatment history, PCI history, multivessel PCI, procedure (angioplasty + stent or direct stent), baseline hs-CRP, SYNTAX score and lesion length (< 28 mm or > 28 mm). We found that SYNTAX score and lesion length are the significant predictors for PPMIJ in univariate [SYNTAX score; odds ratio (OR): 1.11 [95% confidence interval (Cl): 1.04-1.18], p = 0.001, lesion length; OR: 1.06 (95% CI: 1.02-1.09), p < 0.001] and multivariate analysis [SYNTAX score; OR: 1.07 (95% CI: 1.001-1.14), p = 0.04, lesion length; OR: 1.04 (95% CI: 1.01-1.08), p = 0.006].
In the subgroup analysis of patients without statin therapy (103 patients), periprocedural Tn-I values (post-procedural 6th, 12th and 48th hour) were significantly lower in cilostazol-taking individuals (53 patients) than in the cilostazol-free group (50 patients) (Table 3). PPMIJ incidence was significantly lower in the cilostazol-taking group than in the cilostazol-free group [cilostazol group 17% (n = 9/53), cilostazol-free group 34% (n = 17/50); p = 0.04] (Figure 2). On the other hand, the incidence of PPMIN was similar in both cilostazol-free (10%, n = 5/50) and cilostazol-taking (1.9%, n = 1/53) patients in this statin-free group (p = 0.07) (Figure 2).
Table 3. Comparison of myocardial enzymes of patients who were not on chronic statin therapy .
| Cilostazol + rosuvastatin group (53 patients) | Rosuvastatin group (50 patients) | p value | |
| Baseline hs-CRP | 9.60 ± 1.28 | 11.66 ± 2.25 | 0.42 |
| Post-procedure hs-CRP | 15.21 ± 2.19 | 15.69 ± 2.24 | 0.87 |
| Baseline Tn I (ng/ml) | 0.022 ± 0.004 | 0.021 ± 0.007 | 0.98 |
| Post-procedure 6 hour Tn I | 0.088 ± 0.017 | 0.16 ± 0.034 | 0.04 |
| Post-procedure 12 hour Tn I | 0.20 ± 0.044 | 0.32 ± 0.044 | 0.03 |
| Post-procedure 48 hour Tn I | 0.23 ± 0.053 | 0.55 ± 0.14 | 0.03 |
| Baseline CK-MB (U/L) | 16.92 ± 1.11 | 20.54 ± 1.87 | 0.09 |
| Post-procedure 6 hour CK-MB | 14.79 ± 1.04 | 17.14 ± 1.45 | 0.19 |
| Post-procedure 12 hour CK-MB | 16.66 ± 1.03 | 19.54 ± 1.68 | 0.14 |
| Post-procedure 48 hour CK-MB | 14.39 ± 1.14 | 17.14 ± 1.68 | 0.17 |
CK-MB, creatine kinase – myocardial band; hs-CRP, high sensitivity C-reactive protein.
Figure 2.
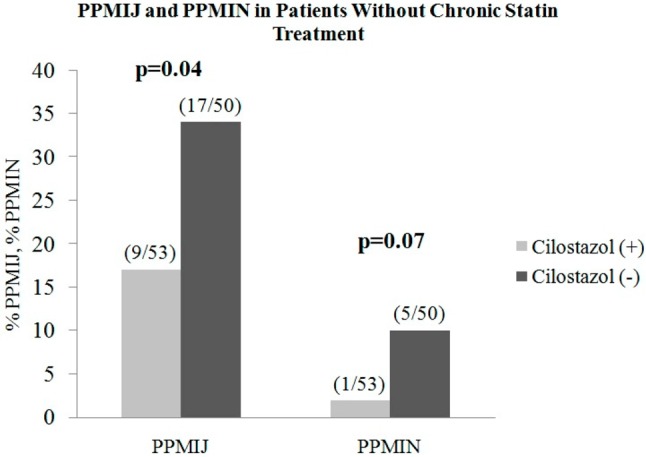
Comparison of PPMIJ in patients who were not on statin therapy before percutaneous coronary intervention (PCI).
DISCUSSION
The primary finding of this prospective study was that a single high dose of cilostazol showed no significant effect on prevention of PPMIJ in patients treated by elective PCI. However, in subgroup analysis, a cilostazol and statin combination was determined as an effective protective strategy for prevention of PPMIJ in patients who were not on statin therapy before elective PCI.
Percutaneous treatment of patients with significant coronary artery lesions became the standard mode of care. The clinical application of PCI with coronary stent implantation has markedly increased, which has significantly improved the outcomes in patients with coronary artery disease. PCI has been the choice of treatment for coronary artery disease in large populations with serious comorbidity, challenging coronary anatomy, development in equipment and operator experiences. However, the extensive use of interventional procedures led to many PCI-related complications. One of those complications is PPMIJ. There are many factors that contribute to post-PCI myocardial injury such as procedure-related complications (such as coronary dissection and no-reflow), lesion characteristics, microembolization, oxidative stress, inflammation, anticoagulant failure and antiaggregant therapy. Plaque rupture and endothelial damage during PCI increase the risk of coronary thrombosis and myocardial necrosis. Also, periprocedural vasospasm and micro-embolisations contribute to these risks.
Several different agents were researched for the purpose of adjustable parameters. There has been research into the possibility of decreasing the risk of myocardial injury in patients undergoing PCI with further inhibition of platelets. It has been reported that further inhibition of platelet function with GpIIb/IIIa inhibitors did not provide additional cardioprotection.11 Cilostazol has been shown to inhibit adrenalin, adenosine diphosphate, arachidonic acid and collagen-induced thrombocyte aggregation.12-14 Cilostazol also shows antiaggregant and anticoagulant effects in patients with aspirin and clopidogrel resistance. Previous studies reported that, in high risk patients such as those with diabetes and long-segment coronary lesions, cilotazol treatment provides additional benefit when added to aspirin and clopidogrel therapy.15,16 Furthermore, previous studies have shown that single high dose statin treatment could reduce the PPMIJ; however, high single dose cilostasol and statin combination pretreatmet strategy had not been tested and reported prior to our study.17 In the NAPLES 2 trial, it was shown that single high dose statin can effect the PPMIJ, so we wanted to test the single high dose effect of a cilostazol and rosuvastatin combination.17 By augmenting NO release, cilostazol provides vasodilatation which would impede procedure-related vasospasm. However, in our study, we could not detect any additional benefit of cilostazol in preventing PPMIJ when added to statin therapy. Our findings were also supported by the results of Byeong-Keuk Kim et al.18 On the other hand, Kim et al. tested the effect of cilostasol, clopidogrel and aspirin combination antiaggregant. However, we tested the high dose clopidogrel and rosuvastatin combination effect, and the pleotrophic effect.18 Our study population decidedly had stable coronary lesions and underwent elective PCI, in which approximately 40% of the study subjects were on chronic statin therapy. That factor might be the reason for the ineffectiveness of cilostazol treatment in our study.
High dose cilostazol has acute effects: anticoagulation, antiaggregation and vasodilatation. In our study population, adequate antithrombotic effect was obtained by pre-procedural aspirin and high dose clopidogrel; cilostazol did not provide any additional benefit. Despite the different mechanism of action of cilostazol, adding cilostazol to conventional dual antiplatelet therapy before PCI did not prevent periprocedural injury, which emphasized that antiplatelet efficacy with the addition of cilostazol might not cause a reduction in cardiac enzyme level increases after elective PCI.19
There are many studies that exhibit the prevention effect of statin therapy for PPMIJ. However, study groups generally consisted of patients with acute coronary syndrome.20 There are few studies that have included patients with stable coronary artery disease who were generally on statin therapy longer than 7 days.21 In the ARMYDA trial it was stated that atorvastatin therapy during the 7 days preceding PCI prevented PPMIJ.21 In the Naples-II trial, the researchers reported that statin therapy reduces the incidence of periprocedural myocardial infarction in elective PCI.17
In subgroup analysis, we determined that cilostazol and statin therapy combination prevented PPMIJ in patients who were not on statin therapy prior to PCI. A single high dose statins’ “pleiotropic” effects that would prevent PPMIJ were unlikely to act in such a short time interval. This perspective suggests that acute single high dose cilostazol provided PPMIJ prevention. In addition, we already know that cilostazol improves statins’ acute positive effects on inflammation and endothelial functions.
The SYNTAX score emphasizes angiographic coronary anatomy and lesion characteristics,22 and has been shown to predict PCI success and MACE in long-term follow-up.23 There is an increased risk of periprocedural injury during complex and long lesion interventions. In our study, high SYNTAX score and long lesion length were found to be predictive for PPMIJ. By calculating SYNTAX score before the procedure and determining the lesion length, preventive therapies may reduce the risk of PPMIJ in subjects with a high SYNTAX score.
After one-month follow-up, similar rates of MACE and bleeding complications were recorded in both groups. Single high dose cilostazol therapy did not cause an increase in bleeding, unlike triple antiplatelet therapy.15 On the other hand, single high-dose cilostazol was found ineffective in preventing MACE.
Our study had some limitations. First, this was a single center study with a relatively small number of patients. Second, aspirin and/or clopidogrel resistance was not evaluated in study subjects. However our randomized study may serve as a guide for larger-scale studies.
CONCLUSIONS
This prospective randomized trial supported the proposition that, as an addition to statin therapy, cilostazol pre-treatment had no effect on PPMIJ in patients undergoing elective PCI. However, in patients who were not on statin therapy before elective PCI, cilostazol and statin treatment before the procedure yields some element of prevention against PPMIJ. Additionally, cilostazol and statin combination may also decrease procedure-related complications in this specific patient group.
Acknowledgments
This study was presented in the 2013 European Society of Cardiology Congress. The authors would like to thank to Assistant Professor Giuseppe Biondi-Zoccai and Dr. Dirk Belmans for their contribution in statistical assessment.
CONFLICT OF INTEREST
None declared.
Statement regarding conflicts of interest
This study was not financially supported, and the authors have no relevant conflicts of interest to disclose.
REFERENCES
- 1.Selvanayagam JB, Porto I, Channon K, et al. Troponin elevation after percutaneous coronary intervention directly represents the extent of irreversible myocardial injury:insights from cardiovascular magnetic resonance imaging. Circulation. 2005;111:1027–1032. doi: 10.1161/01.CIR.0000156328.28485.AD. [DOI] [PubMed] [Google Scholar]
- 2.Nienhuis MB, Ottervanger JP, Bilo HJ, et al. Prognostic value of troponin after elective percutaneous coronary intervention:a meta-analysis. Catheter Cardiovasc Interv. 2008;71:318–324. doi: 10.1002/ccd.21345. [DOI] [PubMed] [Google Scholar]
- 3.Herrmann J, Von Birgelen C, Haude M, et al. Prognostic implication of cardiac troponin T increase following stent implantation. Heart. 2002;87:549–553. doi: 10.1136/heart.87.6.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mason RP, Walter MF, Day CA, Jacob RF. Active metabolite of atorvastatin inhibits membrane cholesterol domain formation by an antioxidant mechanism. J Biol Chem. 2006;281:9337–9345. doi: 10.1074/jbc.M513000200. [DOI] [PubMed] [Google Scholar]
- 5.Laufs U, La Fata V, Plutzky J, Liao JK. Upregulation of endothelial nitric oxide synthase by HMG-CoA reductase inhibitors. Circulation. 1998;97:1129–1135. doi: 10.1161/01.cir.97.12.1129. [DOI] [PubMed] [Google Scholar]
- 6.Kültürsay H. Results of the rosuvastatin studies in Turkey. Arch Turk So. 2007;35(Suppl 1):24–30. [Google Scholar]
- 7.Bai Y, Muqier, Murakami H, et al. Cilostazol protects the heart against ischaemia reperfusion injury in a rabbit model of myocardial infarction:focus on adenosine,nitric oxide and mitochondrial ATP-sensitive potassium channels. Clin Exp Pharmacol Physiol. 2011;38:658–665. doi: 10.1111/j.1440-1681.2011.05550.x. [DOI] [PubMed] [Google Scholar]
- 8.Manickavasagam S, Ye Y, Lin Y, et al. The cardioprotective effect of a statin and cilostazol combination:relationship to Akt and endothelial nitric oxide synthase activation. Cardiovasc Drugs Ther. 2007;21:321–330. doi: 10.1007/s10557-007-6036-0. [DOI] [PubMed] [Google Scholar]
- 9.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition ofmyocardial infarction. European Heart Journal. 2012;33:2551–2567. doi: 10.1093/eurheartj/ehs184. [DOI] [PubMed] [Google Scholar]
- 10.Rao AK, Pratt C, Berke A, et al. Thrombolysis In Myocardial Infarction (TIMI) trial—phase 1:hemorrhagic manifestations and changes in plasma fibrinogen and the fibrinolytic system in patients treated with recombinant tissue plasminogen activator and streptokinase. J Am Coll Cardiol. 1988;11:1–11. doi: 10.1016/0735-1097(88)90158-1. [DOI] [PubMed] [Google Scholar]
- 11.Babu GG, Walker JM, Yellon DM, Hausenloy DJ. Peri-procedural myocardial injury during percutaneous coronary intervention:an important target for cardioprotection. European Heart Journal. 2011;32:23–32. doi: 10.1093/eurheartj/ehq393. [DOI] [PubMed] [Google Scholar]
- 12.Goto S. Cilostazol:potential mechanism of action for antithrombotic effects accompanied by a low rate of bleeding. Atheros. 2005;6:3–11. doi: 10.1016/j.atherosclerosissup.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Shakur Y, Yoshitake M, Kambayashi Ji J. Cilostazol (pletal):a dual inhibitor of cyclic nucleotide phosphodiesterase type 3 and adenosine uptake. Cardiovasc Drug Rev. 2001;19:369–386. doi: 10.1111/j.1527-3466.2001.tb00076.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee SW, Park SW, Hong MK, et al. Triple versus dual antiplatelet therapy after coronary stenting:impact on stent thrombosis. J Am Coll Cardiol. 2005;46:1833–1837. doi: 10.1016/j.jacc.2005.07.048. [DOI] [PubMed] [Google Scholar]
- 15.Lee SW, Park SW, Kim YH, et al. Drug-eluting stenting followed by cilostazol treatment reduces late restenosis in patients with diabetes mellitus:the DECLARE-DIABETES trial. J Am Coll Cardiol. 2008;51:1181–1187. doi: 10.1016/j.jacc.2007.11.049. [DOI] [PubMed] [Google Scholar]
- 16.Lee SW, Park SW, Kim YH, et al. Comparison of triple versus dual antiplatelet therapy after drug-eluting stent implantation (from the DECLARE-Long trial) Am J Cardiol. 2007;100:1103–1108. doi: 10.1016/j.amjcard.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 17.Briguori C, Visconti G, Focaccio A, et al. Novel approaches for preventing or limiting events (Naples) II trial:impact of a single high loading dose of atorvastatin on periprocedural myocardial infarction. J Am Coll Cardiol. 2009;54:2157–2163. doi: 10.1016/j.jacc.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Kim BK, Oh SJ, Yoon SJ, et al. A randomized study assessing the effects of pretreatment with cilostazol on periprocedural myonecrosis after percutaneous coronary intervention. Yonsei Med J. 2011;52:717–726. doi: 10.3349/ymj.2011.52.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Claeys MJ, Van der Planken MG, Bosmans JM, et al. Does pre-treatment with aspirin and loading dose clopidogrel obviate the need for glycoprotein IIb/IIIa antagonists during elective coronary stenting? A focus on peri-procedural myonecrosis. Eur Heart J. 2005;26:567–575. doi: 10.1093/eurheartj/ehi071. [DOI] [PubMed] [Google Scholar]
- 20.Briel M, Schwartz GG, Thompson PL, et al. Effects of early treatment with statins on short-term clinical outcomes in acute coronary syndromes:a meta-analysis of randomized controlled trials. JAMA. 2006;295:2046–2056. doi: 10.1001/jama.295.17.2046. [DOI] [PubMed] [Google Scholar]
- 21.Pasceri V, Patti G, Nusca A, et al. ARMYDA Investigators. Randomized trial of atorvastatin for reduction of myocardial damage during coronary intervention:results from the ARMYDA (Atorvastatin for Reduction of MYocardial Damage during Angioplasty) study. Circulation. 2004;110:674–678. doi: 10.1161/01.CIR.0000137828.06205.87. [DOI] [PubMed] [Google Scholar]
- 22.Park DW, Seung KB, Kim YH, et al. Long-term safety and efficacy of stenting versus coronary artery bypass grafting for unprotected left main coronary artery disease:5-year results from the MAIN-COMPARE (Revascularization for Unprotected Left Main Coronary Artery Stenosis:Comparison of Percutaneous Coronary Angioplasty Versus Surgical Revascularization) registry. J Am Coll Cardiol. 2010;56:117–124. doi: 10.1016/j.jacc.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 23.van Gaal WJ, Ponnuthurai FA, Selvanayagam J, et al. The Syntax score predicts peri-procedural myocardial necrosis during percutaneous coronary intervention. Int J Cardiol. 2009;135:60–65. doi: 10.1016/j.ijcard.2008.03.033. [DOI] [PubMed] [Google Scholar]