Abstract
Deep vein thrombosis (DVT) is a potentially catastrophic condition because thrombosis, left untreated, can result in detrimental pulmonary embolism. Yet in the absence of thrombosis, anticoagulation increases the risk of bleeding. In the existing literature, knowledge about the epidemiology of DVT is primarily based on investigations among Caucasian populations. There has been little information available about the epidemiology of DVT in Taiwan, and it is generally believed that DVT is less common in Asian patients than in Caucasian patients. However, DVT is a multifactorial disease that represents the interaction between genetic and environmental factors, and the majority of patients with incident DVT have either inherited thrombophilia or acquired risk factors. Furthermore, DVT is often overlooked. Although symptomatic DVT commonly presents with lower extremity pain, swelling and tenderness, diagnosing DVT is a clinical challenge for physicians. Such a diagnosis of DVT requires a timely systematic assessment, including the use of the Wells score and a D-dimer test to exclude low-risk patients, and imaging modalities to confirm DVT. Compression ultrasound with high sensitivity and specificity is the front-line imaging modality in the diagnostic process for patients with suspected DVT in addition to conventional invasive contrast venography. Most patients require anticoagulation therapy, which typically consists of parenteral heparin bridged to a vitamin K antagonist, with variable duration. The development of non-vitamin K oral anticoagulants has revolutionized the landscape of venous thromboembolism treatment, with 4 agents available,including rivaroxaban, dabigatran, apixaban, and edoxaban. Presently, all 4 drugs have finished their large phase III clinical trial programs and come to the clinical uses in North America and Europe. It is encouraging to note that the published data to date regarding Asian patients indicates that such new therapies are safe and efficacious. Ultimately, our efforts to improve outcomes in patients with DVT rely on the awareness in the scientific and medical community regarding the importance of DVT.
Keywords: Combination therapy, Hypertension, α1-blocker
INTRODUCTION
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), represents a significant healthcare burden worldwide. For the past 150 years, DVT and PE have been categorized as two separate entities but share a common pathogenesis centered on Virchow’s triad of blood flow stasis, vessel wall damage and increased blood viscosity. Actually, both conditions are related aspects of the same dynamic disease process, VTE, that not only share risk factors and treatment but also require a coordinated approach to make the timely diagnosis.1 In particular, VTE may be lethal as PE may result in acute right ventricular failure.2-4 In Europe, it has been estimated that more than 500,000 deaths per annum are attributable to VTE or its associated complications.5 Besides the high fatality rate, VTE also has features of high recurrence rate,6,7 and intractable thrombogenesis that may cause severe chronic sequela,8,9 as well as poor patient quality of life.10 The serious and chronic nature of VTE requires considerable healthcare resources to be properly managed.11,12 Furthermore, VTE is predominantly a disease that affects the elderly.13 In fact, the incidence rates of VTE increase exponentially as the patient population increases in age.14,15 In addition, hospitalized patients undergoing major surgery and patients with acute exacerbations of a variety of medical conditions are at an increased risk for VTE.16-18 Since a greater proportion of frail elderly patients have either complex medical or surgical conditions, it is expected that an ever-increasing number of patients would be diagnosed with VTE in their coming years.19,20
Clinically, it has been long postulated that PE is a sequel of DVT as clots, forming in the lower extremity or pelvic veins, break off and travel into the pulmonary circulation.21 Indeed, more than 40% of patients with DVT have asymptomatic PE identified by imaging stduies,1,22 and the majority of patients with fatal PE have demonstrable DVT on autopsy, mostly asymptomatic before death.23 Even though DVT and PE are part of the same disease, VTE, early mortality rates are considerably different,14,24,25 particularly a small proportion of patients with PE have substantially worse outcomes within the first 3 months.2,26 Therefore, the clinical assessment, the diagnostic and therapeutic approaches for PE and DVT are manifestly different.4
In this document, experts have reviewed the updated information of DVT regarding epidemiology, diagnostic approaches, and pharmacological management. Ultimately, this document has made consensus recommendations for clinical practices. With these renewed efforts, we hope to remind the medical community of VTE as a mitigable disease that has been long overlooked.
EPIDEMIOLOGY
VTE is a common clinical condition in Western countries, with an incidence of 100 cases or greater in 100,000 person-years.5,14,25,27 In general, incident symptomatic DVT occurs twice as frequently as incident symptomatic PE.28,29 Among patients with lower extremity DVT, regardless of symptoms, isolated distal DVT without concomitant proximal extension represents the majority of all diagnoses with this illness.30-32 However, most of the patients with symptomatic DVT also have extensive and proximal thrombosis.33
Studies conducted in various communities have reported that the incidence of DVT ranged from 93-124 cases per 100,000 person-years in West Europe,25,34 116 cases per 100,000 person-years in North America,6 and 52-55 cases per 100,000 person-years in Australia and New Zealand.35,36 It has been generally perceived that VTE, including DVT, is less common in Asian patients than in Caucasian patients.36,37 The studies conducted in Asia, primarily in ethnic Chinese populations, consistently reported lower incidences of VTE in Asian patients with rates on average four-fold or more lower than in Caucasian patients.38 The average incidences were 17, 12, and 6 cases per 100,000 person-years for VTE, DVT, and PE, respectively.38 However, the information was comparable to data obtained in patients of Asian ethnicity who lived in Western countries.36,37 The reported incidence of VTE among the general population in Taiwan was 16-17 cases per 100,000 person-years.15,39 However, comparisons between Asian and Western data should be interpreted cautiously. Owing to the frequently stealthy nature of VTE, the diagnosis requires an elevated level of awareness both from patients and physicians, in conjunction with the comprehensive approaches and the sensitivity and specificity of diagnostic modalities. The long-time belief that VTE is less common in Asian populations may have provided false reassurance and leads to a lack of awareness.40 It remains unknown to what extent that the observed differences between Asian and Caucasian patients are genuine or reflect discrepancies between study designs, patient demographics, and physician practices. In addition, population-base epidemiology data has demonstrated yearly increases in incident VTE in Asian patients,20 particularly in high-risk and elderly patients.39,41
Risk factors
DVT, as part of the continuum of VTE, is a multifactorial disease that represents the interaction of genetic and environmental factors. The majority of patients with incident or recurrent VTE have at least one recognized risk factor.42,43 Common risk factors are listed in Table 1. Acquired risk factors are far more common than inherited thrombophilia. Additionally, advanced age, cancer, immobilization, recent trauma or surgery, and hospitalization are all important risk factors.44,45
Table 1. Risk factors for venous thromboembolism .
| Advancing age |
| Obesity |
| Recent surgery (including hip or knee replacement) |
| Major trauma (including fracture) |
| Active cancer |
| Acute medical illnesses (e.g. heart failure, respiratory failure) |
| Paralytic stroke or immobilization |
| Antiphospholipid syndrome |
| Inherited thrombophilia |
| Previous venous thromboembolism |
| Congenital venous malformation |
| Varicose veins |
| Central venous catheter or vena cava filter |
| Long-distance travel |
| Pregnancy/antepartum |
| Oral contraceptives or hormone replacement therapy |
Orthopedic surgery
Major orthopedic surgeries such as hip fracture repair and total hip/knee arthroplasty, including replacement surgery, represent a substantially high risk for VTE.19 Prior to the 1980s, while no thromboprophylaxis was conducted, the rates of symptomatic VTE in patients after hip fracture surgery without prophylaxis ranged from 15 to 30%.46 In the modern era, with thromboprophylaxis in Western populations, symptomatic VTE in patients undergoing major orthopedic surgery was 2.7%, of which 1.5% had DVT.47 However, it should be noted that the asymptomatic DVT confirmed by venograms was 3 times higher than symptomatic DVT.48 Recent meta-analysis also suggested that asymptomatic DVT accounted for most of the events in patients after knee arthroplasty.49
Customarily, it has been perceived that Asian patients are at a lower risk for VTE following orthopedic procedures.50 However, evidence supporting an underestimated incidence of VTE in Asia stems from the review of postoperative VTE reported in Asian countries.40,51,52 Overall, the adjusted incidences of total DVT were 13%, 16%, 50%, and 18% for general surgery, total hip replacement, total knee replacement, and hip fracture surgery, respectively.40 Regardless of the methodologies used for diagnosing DVT, the reported incidences of post-operative DVT were notably high across Asian countries.51 The SMART study was a prospective investigation of the incidence of symptomatic VTE up to 1 month postoperative in Asia.53 Patients undergoing major orthopedic surgery without thromboprophylaxis were included, and patients from Taiwan were jointly enrolled in the study.54 Among 2,420 Asian patients, postoperative symptomatic DVT occurred in 0.9% of patients; whereas in 326 evaluable venograms, 35.6% of patients had asymptomatic DVT.54 The AIDA program also showed a similar result to the SMART venography study. Asian patients undergoing total knee replacement had the highest risk for incident DVT.55 Findings of the SMART and AIDA studies were consistent with Western data.40 Some studies based upon claims data inferred that the incidence of symptomatic VTE after knee or hip arthroplasty was low in Taiwan.56,57 However, a single hospital report based on routine postoperative venograms indicated that 63.6% of patients with total knee arthroplasty had DVT, of which the majority were symptomatic distal DVT.32 The risk of late DVT and thrombosis propagation was similar in patients in Taiwan with isolated distal DVT, or a more complicated DVT.58 Apparently, further research is needed to elucidate those discrepancies.
Medical illness
VTE is also a common complication in patients who suffer critical illness.59 In Europe and North America, VTE occurred in 14.9% of patients hospitalized for acute illness without thromboprophylaxis, of which 4.9% had proximal DVT on venograms.60 Symptomatic DVT represented only 10% of the total number of asymptomatic events in patients with acute medical illness.61 The risk of asymptomatic DVT, including both proximal and distal DVT, proven by venograms was as high as 9.0%.62 Nevertheless, prospective observations in Asian countries indicated that the rate of symptomatic VTE without thromboprophylaxis in hospitalized non-surgical patients was 1.1%,63 which was comparable to rates in Western populations.64
In addition, most of Asian patients were not assigned with thromboprophylaxis measures in the setting of acute illness or surgical conditions. The low reported incidence of symptomatic VTE may only reflect a reduced awareness by patients and physicians. Finally, autopsy data suggested a similar incidence of PE in Asia and in the West.65-67 Some of the apparent differences in the incidence of symptomatic VTE between Western and Asian populations probably arose from variations in access to healthcare.68
Inherited thrombophilia
The common inheritable factors for thrombophilia include factor V Leiden, prothrombin G20210A, and deficiencies of natural inhibitors.21,44,69 Factor V Leiden and prothrombin G20210A are more prevalent in Caucasians,70,71 whereas protein S deficiency is the most common defect, followed by protein C and antithrombin deficiency in Asians.40 In Taiwan, factor V Leiden and prothrombin genetic mutations are rare conditions; the most common inherited thrombophilia is protein S deficiency regardless of the presence of VTE.72-75 The ethnic differences in the distribution of genetic predisposition to VTE are shown in Table 2.
Table 2. Ethnic differences in the distribution of inherited thrombophilias .
| Factor V Leiden | Prothrombin G20210A mutation | Protein S deficiency | Protein C deficiency | Antithrombin deficiency | |
| General populations, % | |||||
| Caucasians | 4.871 | 2.0, 71 2.740 | 0.03-0.1340 | 0.2-0.471 | 0.02-0.1671 |
| Asians | 0-0.240 | 0-0.240 | 0.06-3.740 | 0.3-1.140 | 0-2.340 |
| Taiwanese | NA | NA | 6.474 | 4.074 | 6.474 |
| Patients with VTE, % | |||||
| Caucasians | 18.871 | 7.171 | 2.371 | 3.771 | 1.971 |
| Asians | 040 | 040 | 10.7-17.840 | 8.9-10.740 | 4.7-8.140 |
| Taiwanese | 01,73,75 | 073 | 3.6,75 8.0,73 32.9,72 33.674 | 4.0,73 10.7,75 17.2,74 18.872 | 3.5,72 4.0,73 5.2,74 7.175 |
NA, not applicable; VTE, venous thromboembolism.
It is important to realize the limitations of thrombophilia testing. Diagnosing a heritable thrombophilia has rarely changed the course of patient management. The presence of a heritable thrombophilia increases the risk of first thrombosis in varying degrees,21 and it does not strongly predict the risk of recurrence after discontinuation of anticoagulation in unselected VTE patients.19,76,77 It is generally recommended not to offer heritable thrombophilia testing to patients with provoked VTE.78 In NICE recommendations, the only clear indications to perform heritable thrombophilia testing are when consideration is given to discontinuing anticoagulation following an unprovoked VTE, and for patients with a history of a first-degree relative with VTE.79
Recommendations
• Asian patients might have a lower risk for symptomatic VTE compared with Caucasian patients.
• Thromboprophylaxis should be incorporated into the daily care regimen for Asian patients complicated with high-risk medical or surgical conditions.
• Factor V Leiden and prothrombin G20210A mutation are rare in Asian patients with VTE, and therefore should not be routinely tested.
Diagnosis
Venous thrombosis in the lower extremity can involve the superficial leg veins, the deep veins of the calf, and proximal veins, that include popliteal, superficial femoral, common femoral, and iliac veins. DVT at the calf is generally asymptomatic as complete dissolution of small thrombi occurs quite frequently.30 These thrombi can extend proximally and become dangerous, whereas more proximal DVT results in symptoms associated with venous outflow obstruction, venous or perivascular inflammation, or PE.80,81
Diagnosing DVT is an ongoing clinical challenge for physicians. The accurate and timely diagnosis of this disease is mandatory in patients with suspected DVT because thrombi left untreated can result in detrimental PE, whereas anticoagulation in the absence of thrombosis is medically inappropriate. Common symptoms such as pain, swelling and tenderness make it sometimes difficult to distinguish DVT from other medical conditions such as heart failure, local infection, or the popliteal cyst.82,83 As a result, the clinical diagnosis of DVT has been proven to be surprisingly unreliable.84
Contrast venography
Contrast venography is the benchmark for diagnosing DVT.80 The diagnosis is established based on the presence of a constant intraluminal filling defect on at least two projections. Treatment with anticoagulants could be safely withheld in patients with a technically adequate normal venogram, and only 1.3% of patients developed symptomatic DVT within 3 months.80 However, venography is invasive, not uniformly available, and has limitations for patients with renal insufficiency and allergic reactions to contrast medium. To illustrate the entire venous drainage of the lower extremity, a dorsal foot vein cannulation is necessary. The technical difficulties frequently encountered have been cannulation failure,85 and inadequate visualization of a venous segment.86
A certain and reliable diagnosis of DVT can only be established by means of imaging. On the other hand, ruling out acute DVT can be safely achieved by using a standardized clinical probability assessment and a D-dimer blood test. The main advantage of such an approach is the reduction in the required number of imaging tests, which are in general time consuming, costly and associated with radiation exposure and other complications. Therefore, venography is currently seldom used, but still serves as a reference standard and should be used if other tests are unable to definitely confirm or exclude the diagnosis of DVT.87
Clinical assessment
An important first step is to assess the patient’s pretest probability of DVT based on medical history and physical examination. Although several structured clinical probability scoring systems have been developed to stratify patients,88-91 the system that has been most extensively studied and widely used is the Wells score.84 This scoring system,which incorporates medical history and physical examination, is listed in Table 3. The rule that originally categorizes patients as having a low, moderate, or high probability of DVT has been extensively validated by more than 8,000 patients.92,93 A simplified version of the Wells score dichotomizes patients as being unlikely (prevalence of DVT: 6%), or likely (prevalence of DVT: 28%) to have DVT for practical purposes.94 It has been shown that the Wells scoring system has excellent interobserver reliability, regardless of the extent of physician experience.84,95 However, except for Japanese population, the Wells score has not yet been extensively validated in Asian populations.96
Table 3. MIB-PEDVT-HO2 acronym for Wells prediction rules for diagnosing deep vein thrombosis .
| Clinical characteristics | Points |
| Malignancy, active | +1 |
| Immobilization of the lower extremities or paralysis | +1 |
| Bed rest > 3 days or major surgery < 12 weeks | +1 |
| Pitting edema confined to the symptomatic leg | +1 |
| Engorgement of entire leg | +1 |
| Diameter difference on affected calf > 3 cm | +1 |
| Veins (superficial, nonvaricose) dilation of the affected leg | +1 |
| Tenderness along the distribution of the deep veins | +1 |
| History of deep vein thrombosis | +1 |
| Other diagnoses at least as likely as deep vein thrombosis | -2 |
When an extensive clinical history of the patient has been obtained, the clinician can get confirmation of whether any instances of active cancer (Malignancy), paraplysis that leads to immobilization (Immobilization of the lower extremities or paralysis), and prolonged bed rest or a recent major surgery (Bed rest > 3 days or major surgery < 12 weeks) are major risk factors for DVT and thus related to the patient’s course of disease. The presentation indicative of DVT includes leg swelling (Pitting edema confined to the symptomatic leg and/or Engorgement of the entire leg, and a Diameter difference on the affected calf > 3 cm), visible collateral circulation (Veins, nonvaricose superficial dilation of the affected leg), and leg pain (Tenderness along the distribution of the deep veins). A prior history of DVT (History of deep vein thrombosis) is also an independent risk factor. Finally, if there is an alternative tentative diagnosis other than DVT (Other diagnoses at least as likely as deep vein thrombosis), the likelihood of DVT is decreased. In this instance, the acronym MIB-PEDVT-HO2 is a convenient tool.
D-dimer
The major criticism in using the Wells score to assess DVT probability is the subjective element of considering an alternative diagnosis. This clinical probability can be subsequently supplemented by the combination with a determination of the level of D-dimer for better risk stratification.94
Fibrin D-dimer is the degradation product of cross-linked fibrin. D-dimer levels are typically elevated in patients with acute thrombosis because of simultaneous activation of coagulation and fibrinolysis. The sensitivity of an elevated level of D-dimer is high, but the specificity is rather moderate.97 A high D-dimer level is also observed in conditions such as malignancy, infection, pregnancy, post surgery or trauma, and increasing age. Consequently, a negative result can facilitate the exclusion of VTE.98
A number of D-dimer assays are available. The meta-analysis of 217 D-dimer tests evaluated for DVT indicated that the sensitivities of the D-dimer enzyme-linked immunofluorescence assay, microplate enzyme-linked immunosorbent assay and latex quantitative assay are 96%, 94%, and 93%, respectively; they are superior to those of the whole-blood D-dimer assay, latex semiquantitative assay and latex qualitative assay as the sensitivities are 83%, 85%, and 69%, respectively.99 The higher sensitivity yields a higher negative predictive value and introduces fewer concerns regarding false-negative results. In addition, the age-dependent decrease in specificity of D-dimer with suspected PE was observed.100 Recent investigations suggested that age-adjusted cut-offs (age × 10 ug/L above 50 years) improved specificity of D-dimer testing in the elderly with suspected PE.101-103 The European guidelines have adopted the age-adjusted D-dimer cut-offs for the diagnosis of acute PE.4 The clinical application of the aforementioned aspect regarding D-dimer testing has not been prospectively validated in patients with suspected DVT and not yet endorsed by American guidelines for the diagnosis of DVT.
Compression ultrasound
Compression ultrasound (CUS) is the front-line imaging modality in the diagnostic process for patients with suspected DVT. The sensitivity and specificity of the CUS in diagnosing proximal DVT is 94% and 98%, respectively.104 However, the sensitivity decreases in detecting asymptomatic proximal DVT with similar specificity.105 The sensitivity of CUS in diagnosing distal DVT is relatively low, at 57%,104 and is only 48% for detecting asymptomatic calf vein thrombosis.105 For proximal DVT, ultrasound studies have a positive predictive value of 100% and 71% for symptomatic and asymptomatic events, respectively, whereas the negative predictive value for symptomatic and asymptomatic events are 100% and 94%, respectively.106
Conventionally, 3 protocols are presently available. The proximal CUS (so-called 2-point CUS) assesses compressibility of the femoral and popliteal veins. Loss of compressibility of either segment is diagnostic for incident proximal DVT. The second approach is to conduct a complete CUS along the proximal till distal deep veins of the leg. The prior study suggested that only 0.5% of patients withholding anticoagulation after a single, negative, complete CUS had distal DVT within 3 months and no proximal DVT occurred.82 Although it has good accuracy, complete CUS is time consuming and a modest proportion of imaging results are inadequate, particularly in the calf veins.107 As an alternative to the aforementioned measures, repeated proximal CUS after 5-7 days is recommended in patients with an initial negative proximal CUS finding. This approach detects if any distal DVT propagates into the proximal vein that may increase the risk of PE. The repeated proximal CUS strategy in the intermediate to high-risk patients is safe with regard to withholding anticoagulation.108,109
Computed tomography and magnetic resonance imaging
Both computed tomography venography (CTV) and magnetic resonance venography (MRV) are alternatives to CUS as a means to diagnose DVT.110 In particular, CTV provides direct imaging of the inferior vena cava, pelvic and lower extremity veins immediately after computed tomography pulmonary angiography. Since DVT is the most important risk for PE, it seems ideal that a single examination simplifies and shortens VTE work-up. However, this single comprehensive imaging modality for VTE requires a larger amount of iodinated contrast medium to produce adequate opacification of the pulmonary arteries, and pelvic and lower extremity veins,111,112 and exposure to ionizing radiation may be greater than for either test alone.113,114 The pooled analysis showed that for the diagnosis of DVT, in particular proximal DVT, CTV had a sensitivity and specificity of 96% and 95%, respectively.115 In addition, to facilitate the diagnosis of DVT, MRV has a sensitivity and specificity of 92% and 95%, respectively, and the sensitivity for the diagnosis of proximal DVT increases to 94%.116
The sensitivity and specificity of both CTV and MRV for the diagnosis of DVT are comparable to CUS. However, the safety of withholding anticoagulation on the basis of a normal CTV or MTV result has yet to be evaluated prospectively. Furthermore, CTV has generally been tested along with computed tomography pulmonary angiogram in patients with suspected PE. CTV, however, has yet to be more extensively assessed as a standalone diagnostic modality in patients with suspected DVT. Therefore, both imaging modalities should not be routinely used as front-line approaches.87 It is reasonable to utilize CTV or MRV in conditions that CUS cannot be performed or is less reliable, such as for patients with morbid obesity or casts, and patients with suspected DVT in the iliac or inferior cava vein or suspected venous anomaly.87,98,117
Recommendations
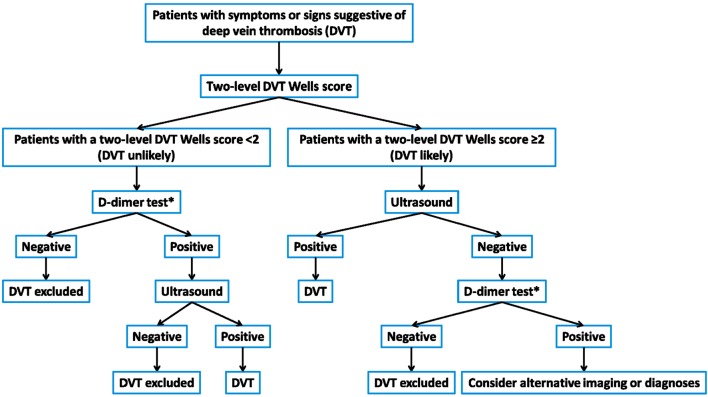
• To diagnose DVT requires systematic assessment (Figure 1).
Figure 1.
Diagnosis flowchart. * Moderately or highly sensitive D-dimer test.
• An acronym of MIB-PEDVT-HO2 for the Wells score is the comprehensive initial to stratify patients.
• The combination of the Wells score and a D-dimer test is validated in deciding whether to initially withhold anticoagulation.
• CUS, either proximal, whole leg or repeated measurements, should constitute the first-line imaging for most patients with the increased likelihood of DVT.
• CTV and MRV have similar sensitivities and specificities to CUS in diagnosing DVT. However, those measures should be reserved for conditions where patients cannot be evaluated properly by CUS or thrombosis in pelvic veins, or inferior vena cava is suspected.
• Contrast venography is the reference modality for diagnosing DVT and is reserved for occasions when other tests are unable to definitely establish or exclude DVT.
Treatment
DVT can become complicated with PE, and has a high recurrence at the early stage without treatment.118 The risk of recurrence and post-thrombotic syndrome (PTS) develop steadily after the first episode of DVT.7,119 The most effective manner in which to prevent thrombus extension, recurrence and late complications primarily depends on effective pharmacological and/or mechanical treatment.120
Thrombolysis
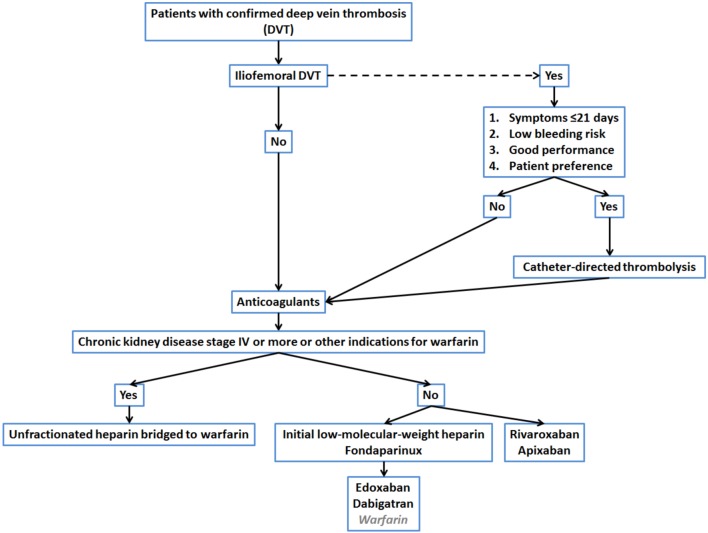
Despite effective anticoagulation, only 20%, 39% and 58% of the patients had normal CUS at 3 months, 6 months and 1 year, respectively.121 Other studies reported a similar rate of the presence of residual thrombus at 3 months and beyond.122,123 The presence of residual thrombus has been associated with the risk of recurrence and the occurrence of PTS.121,124,125 The possibility to reduce thrombus burden and to restore venous drainage in the early stage of DVT in patients with extreme high risk for thromboembolism complications is an appealing one. The evidence suggested that systemic thrombolysis had the potential to reduce PTS at the expense of an increase in major bleeding. As a result, systemic thrombolysis is not generally recommended by the current guidelines.120,126 A variety of endovascular interventions have been tested in patients with iliofemoral DVT, including catheter-directed thrombolysis (CDT) and/or thrombectomy. CDT reduces PTS at 24 months with a number needed to treat of 7 in a randomized trial that involved 209 patients with iliofemoral DVT.127 There was no significant reduction in recurrence by CDT in this small-sized trial. However, bleeding is a primary concern of a broad application of thrombolysis.128 Currently, CDT is preferred for patients with iliofemoral DVT and/or limb-threatening circulatory compromise, acute or subacute symptoms, and a low risk of bleeding.120,126
Endovascular thrombectomy and stenting have reduced recurrence and PTS at 30 months compared with anticoagulation only in a randomized trial involving 169 patients with proximal DVT.129 Whether pharmacomechanical CDT truly reduces recurrence and PTS in patients with proximal DVT is being evaluated currently in a larger trial with a target of more than 600 patients and a primary endpoint of PTS occurrence.130
Conventional anticoagulation
The initial and standard pharmacological approach in patients with DVT started with parenteral anticoagulants, either unfractionated or low-molecular-weight heparin, followed by long-term vitamin K antagonists (VKAs). The importance of effective initial parenteral anticoagulants has been emphasized by the results in early trials.131,132 It has been suggested to start parenteral anticoagulation within a short period of time while waiting for the results of diagnostic tests in patients with intermediate to high clinical suspicion of acute DVT.120 In general, twice-daily low-molecular-weight heparin (LMWH) administration is preferred over once-daily LMWH administration or unfractionated heparin for a lower risk of inducing major bleeding and heparin-induced thrombocytopenia.133,134
An oral VKA is preferably initiated on the same day as the start of parenteral anticoagulants, which should be continued for at least 5 days until the international normalized ratio (INR) is 2.0 or above for 2 consecutive days.120,135 The targeted INR for VKA treatment is 2.0-3.0. The subtherapeutic dose is associated with higher recurrence,136 and the occurrence of PTS.137 To maintain adequate anticoagulation without an excessive risk of bleeding in the use of VKAs, primarily warfarin, is a lesson that every clinician has learned for the past 50 years due to the limitations of new effective treatment.138
Non-vitamin K oral anticoagulants (NOACs)
NOACs have been developed to optimize VTE management and overcome the limitations of traditional treatments. Five NOACs have been extensively tested in patients with VTE in clinical trials. Except for ximelagatran, which has been withdrawn, rivaroxaban, dabigatran, apixaban, and edoxaban have been approved in North America and Europe for VTE treatment; edoxaban has completed the largest clinical trial ever in VTE treatment. Except for acute treatment, rivaroxaban, dabigatran, and apixaban have been compared with placebo or warfarin in the setting of extended treatment for VTE.
Rivaroxaban
Patients with acute DVT or PE were enrolled in the EINSTEIN program. In the EINSTEIN DVT, 3,449 patients with acute symptomatic DVT were randomized to receive oral rivaroxaban alone (15 mg twice daily for 3 weeks, followed by 20 mg once daily) or subcutaneous enoxaparin followed by warfarin for 3, 6, or 12 months.139 In the EINSTEIN PE, 4,832 patients who had acute symptomatic PE with or without DVT, were randomized according to the same protocol.140 In both the EINSTEIN DVT and EINSTEIN PE, a single-drug approach by rivaroxaban was non-inferior to the standard treatment with regard to VTE recurrence and had no excessive major or clinically relevant nonmajor bleeding.139,140 In particular, there was no signal of excessive VTE recurrence by day 21, at the end of the twice-daily treatment. The pool analysis of 8,282 patients showed very similar results of separate trials but there was significantly less major bleeding in patients with rivaroxaban compared to the standard therapy (hazard ratio: 0.54; 95% confidence interval: 0.37-0.79, p = 0.002).141
Dabigatran
Dabigatran has been tested in 2 trials for acute VTE treatment (RE-COVER and RE-COVER II).142,143 The RE-COVER series consisted of 2 trials because of low rate of recurrent VTE observed in the first trial with a small population. Dabigatran 150 mg twice daily was compared with warfarin for 6 months after the initial parenteral heparin. There were 2,564 and 2,589 patients with acute VTE enrolled in the RE-COVER and the RE-COVER II, respectively. In both trials, dabigatran was non-inferior to warfarin with regard to recurrent VTE but had significantly less major or clinically relevant nonmajor bleeding (hazard ratio: 0.63; 95% confidence interval: 0.47-0.84, in the RECOVER; hazard ratio: 0.62; 95% confidence interval: 0.45-0.84, in the RECOVER II). The extent of major bleeding was similar in patients with dabigatran or warfarin. The conclusions were consistent in the pooled analysis.142
Apixaban
In the AMPLIFY study, 5,400 patients with acute VTE were randomized to receive apixaban (10 mg twice daily for 7 days, followed by 5 mg twice daily) or subcutaneous enoxaparin followed by warfarin for 6 months.144 Apixaban, as a single-drug approach, was non-inferior to the standard treatment with respect to recurrent VTE. Both major bleeding or clinically relevant nonmajor bleeding were significantly less in patients with apixaban (relative risk: 0.44, 95% confidence interval: 0.36-0.55, p < 0.001; relative risk: 0.31, 95% confidence interval: 0.17-0.55, p < 0.001, respectively).
Edoxaban
Edoxaban has been compared with warfarin in the largest phase III clinical trial in VTE treatment. In the HOKUSAI VTE, 8,240 patients with acute VTE were randomly assigned to edoxaban (60 mg once daily or 30 mg once daily in patients with renal impairment or low body weight) or warfarin for 3 to 12 months after the initial parenteral heparin.145 Edoxaban was non-inferior to warfarin with respect to recurrent VTE. Furthermore, edoxaban had significantly less major or clinically relevant nonmajor bleeding (hazard ratio: 0.81; 95% confidence interval: 0.71-0.94, p = 0.004), and had no excessive risk for major bleeding.
The risk of recurrent VTE and bleeding are fundamental considerations for recommendation of VTE management. Although clinical trials of NOACs in VTE treatment have differences with regard to the study designs, the conclusions are rather similar. The design and results of pivotal NOAC trials for VTE treatment are listed in Table 4 and Table 5. The availability of NOACs that do not require monitoring will create a treatment shift for patients with VTE as an effective, safer, and more convenient treatment for patients, physicians, and healthcare systems.146 The additional advantage of NOACs against VKAs is that Asians are prone to bleeding when treated with VKAs, and the optimal INR use has yet to be determined in Asian patients.147,148 In trials evaluating NOACs for stroke prevention in atrial fibrillation, NOACs have less critical bleeding than VKAs in Asian patients than in patients with other ethnicities.147,149,150 In trials evaluating NOACs for VTE treatment, both rivaroxaban and edoxaban have published data of Asian patients.150,151 Both analyses reconfirmed the similar efficacy and the probable safety advantage of rivaroxaban and edoxaban against dose-adjusted VKAs.
Table 4. Patient characteristics in clinical trials of non-vitamin K oral anticoagulants for venous thromboembolism treatment .
| EINSTEIN-DVT | EINSTEIN-PE | RE-COVER | RE-COVER II | AMPLIFY | HOKUSAI-VTE | |||||||
| Rivaroxaban | VKA | Rivaroxaban | VKA | Dabigatran | VKA | Dabigatran | VKA | Apixaban | VKA | Edoxaban | VKA | |
| Number of patients | 1,731 | 1,718 | 2,419 | 2,413 | 1,273 | 1,266 | 1,280 | 1,288 | 2,691 | 2,704 | 4,118 | 4,122 |
| Design | Open-label | Open-label | Double-blinded | Double-blinded | Double-blinded | Double-blinded | ||||||
| Treatment duration | 3, 6 or 12 months* | 3, 6 or 12 months* | 6 months | 6 months | 6 months | 3, 6 or 12 months* | ||||||
| Initial treatment | Rivaroxaban 15 mg twice daily for 3 weeks | LMWH ≥ 5 days | Rivaroxaban 15 mg twice daily for 3 weeks | LMWH ≥ 5 days | LMWH or UFH ≥ 5 days | LMWH or UFH ≥ 5 days | Apixaban 10 mg twice daily for 7 days | LMWH or UFH ≥ 5 days | LMWH or UFH ≥ 5 days | |||
| Long-term treatment | Rivaroxaban 20 mg once daily | INR: 2-3 | Rivaroxaban 20 mg once daily | INR: 2-3 | Dabigatran 150 mg twice daily | INR: 2-3 | Dabigatran 150mg twice daily | INR: 2-3 | Apixaban 5mg twice daily | INR: 2-3 | Edoxaban 60 mg once daily# | INR: 2-3 |
| Age, years | 56 | 56 | 58 | 58 | 55 | 54 | 55 | 55 | 57 | 57 | 56 | 56 |
| Female, % | 43 | 44 | 46 | 48 | 42 | 41 | 39 | 40 | 42 | 41 | 43 | 43 |
| Index event, % | ||||||||||||
| DVT | 99 | 99 | 0 | 0 | 69 | 69 | 69 | 68 | 65 | 66 | 60 | 60 |
| PE ± DVT | 1 | 1 | 100 | 100 | 31 | 31 | 31 | 32 | 35 | 34 | 40 | 40 |
| Risk factor, % | ||||||||||||
| Unprovoked | 61 | 63 | 65 | 64 | NR | NR | NR | NR | 90 | 90 | 66 | 65 |
| Prior VTE | 19 | 19 | 19 | 20 | 26 | 25 | 19 | 16 | 17 | 15 | 19 | 18 |
| Cancer | 7 | 5 | 5 | 5 | 5 | 5 | 4 | 4 | 2 | 3 | 9 | 10 |
| TTR, % | NA | 58 | NA | 63 | NA | 60 | NA | 57 | NA | 60 | NA | 64 |
* Treatment duration was left to the discretion of the treating physician. # An edoxaban 30 mg once daily was administered in certain conditions.
DVT, deep vein thrombosis; INR, international normalized ratio; LMWH, low-molecular-weight heparin; NA, not applicable; NR, not reported; PE, pulmonary embolism; TTR, time in therapeutic range; UFH, unfractionated heparin; VKA, vitamin K antagonist; VTE, venous thromboembolism.
Table 5. Hazard ratio (95% confident interval) of selected outcomes for non-vitamin K oral anticoagulants in venous thromboembolism treatment .
| Rivaroxaban vs. VKAs EINSTEIN pooled analysis | Dabigatran vs. VKAs RE-COVER pooled analysis | Apixaban vs. VKAs AMPLIFY* | Edoxaban vs. VKAs HOKUSAI-VTE | |
| Recurrent VTE | 0.89 (0.66-1.19) | 1.09 (0.76-1.57) | 0.84 (0.60-1.18) | 0.82 (0.60-1.14) # |
| Major bleeding | 0.54 (0.37-0.79) | 0.73 (0.48-1.11) | 0.31 (0.17-0.55) | 0.84 (0.59-1.21) |
| Major or CRNM bleeding | 0.93 (0.81-1.06) | 0.62 (0.50-0.76) | 0.44 (0.36-0.55) | 0.81 (0.71-0.94) |
| Death | 0.89 (0.67-1.18) | 1.00 (0.67-1.51) | 0.79 (0.53-1.19) | 1.05 (0.82-1.35) |
* Relative risks are reported for the AMPLIFY study. # Recurrent VTE during on-treatment period.
CRNM, clinically relevant nonmajor; VKA, vitamin K antagonist; VTE, venous thromboembolism.
Recommendations
• Timely administration of anticoagulation is essential in the treatment of DVT (Figure 2).
Figure 2.
Treatment flowchart.
• Conventional treatment involves parenteral heparin bridging to VKAs with a maintenance target INR of 2.0-3.0.
• Two strategies have been tested in 6 pivotal trials of NOACs for VTE treatment; the single-drug approach using rivaroxban or apixaban, and the 2-phase approach using parenteral heparin bridged to dabigatran or edoxaban are as effective as the conventional treatment with regard to the recurrence of VTE.
• With respect to major bleeding, NOACs seem to be safer than the conventional treatment.
Duration
A 3-phase anticoagulation treatment is recommended by the current guidelines.120 The initial phase of intensified anticoagulants is followed by minimal 3-month long-term oral anticoagulants for the majority of patients.152-154 After the first 2 phases, whether patients require an extended phase of anticoagulants depends on balances between risk for VTE recurrence without and bleeding with anticoagulants,155 and the preference of patients. DVT, as part of VTE, is a chronic disease with a high rate of recurrence. Previous epidemiological data indicated that more than 30% of patients developed recurrence within 10 years.7,119,156,157 In general, the long-term recurrence rate is not extremely different between DVT and PE. Rather, recurrence is more likely to occur in the same clinical manifestation as the index event.24,118
According to the most recent data from randomized trials, the risk of early recurrence (on anticoagulant treatment) is about 1-2%.158 Factors associated with recurrence are listed in Table 6. Risk factors for recurrence during anticoagulation include immobilization, cancer, and chronic pulmonary condition,76 in which immobilization > 3 days is the strongest risk factor.159 The risk of recurrence after terminating anticoagulant therapy markedly differs depending on whether the initial event is associated with one or more risk factors.152,160,161 The long-term recurrence rate has been reported to reach 13% at 1 year, 23% at 5 years, and 30% at 10 years.162 Risk factors for recurrence after discontinuing anticoagulation include male sex,163 elevated body mass index,164 low levels of high-density lipoprotein cholesterol,165 idiopathic presentation, thrombophilia, increasing age, shorter duration of anticoagulation, cancer, and residual thrombus.7,121,166
Table 6. Risk factors for recurrent venous thromboembolism .
| While taking anticoagulant drugs |
| Immobilization |
| Cancer |
| Chronic obstructive pulmonary disease |
| After anticoagulant drugs are discontinued |
| Unprovoked event or associated with cancer |
| Male sex |
| Obesity |
| Lack of recanalization of DVT on venous ultrasound examination |
| Presence of antiphospholipid antibody |
In fact, the provoked nature of an index event is a major determinant for the risk of VTE recurrence.15,167 In patients with proximal DVT and PE, the estimated cumulative risk of recurrence after stopping anticoagulants is as follows: VTE provoked by surgery, 1% after 1 year and 3% after 5 years; VTE provoked by a nonsurgical reversible risk factor, 5% after 1 year and 15% after 5 years; and unprovoked VTE, 10% recurrence after 1 year and 30% after 5 years, and 15% annually for patients with cancer.120
Previous trials have compared different antithrombotic strategies in the reduction of VTE recurrence in patients with an unprovoked event.152,160,168 These trials indicated that extending anticoagulation for 1 year has yet to eliminate long-term recurrence in patients with unprovoked VTE. Two trials have intended to extend the anticoagulation treatment beyond 1 year.169,170 In the ELATE study, low-intensity warfarin (target INR of 1.5-1.9) has been compared with standard-intensity warfarin (target INR of 2.0-3.0), and patients have been followed for an average of 2.4 years. The recurrence rate was 1.9 per 100 patient-years in the low-intensity group compared with 0.7 per 100 patient-years in the standard-intensity group (p = 0.03).170 In the PREVENT study, 508 patients with unprovoked VTE were randomized to low-intensity warfarin (target INR of 1.5-2.0) or placebo. The trial was terminated after 2 years of therapy due to extreme beneficial effect with warfarin. The recurrent VTE was 2.6 per 100 patient-years with warfarin compared with 7.2 per 100 patient-years with placebo (p < 0.001).169 Incidentially, the major bleeding was remarkable low in both trials (around 1 per 100 patient-years in all treatment groups).
Besides warfarin, extended treatment trials for VTE involving rivaroxaban, dabigatran and apixaban have been completed, with variable treatment duration and comparators.139,171,172 All 3 NOACs have largely reduced recurrent or fatal VTE and had no excessive risk for major bleeding compared with placebo. The recurrent or fatal VTE and major bleeding was similar with dabigatran and standard-intensity warfarin, but the risk of major or clinically relevant bleeding event was lower with dabigatran compared with standard-intensity warfarin.171 The information associated with the NOACs trials involving extended treatment for VTE is listed in Table 7.
Table 7. Clinical trials of non-vitamin K oral anticoagulants in extended treatment for venous thromboembolism .
| Dabigatran | Rivaroxaban | Apixaban | |||
| RE-MEDY | RE-SONATE | EINSTEIN-EXT | AMPLIFY-EXT | AMPLIFY-EXT | |
| Comparator | Warfarin | Placebo | Placebo | Placebo | Placebo |
| Treatment | Dabigatran 150 twice daily | Dabigatran 150 twice daily | Rivaroxaban 20 mg once daily | Apixaban 2.5 mg twice daily | Apixaban 5 mg twice daily |
| Treatment duration | 18 months | 6 months | 6 or 12 months | 12 months | 12 months |
| Recurrent VTE* | 1.44 (0.78-2.64) | 0.08 (0.02-0.25) | 0.18 (0.09-0.39) | 0.19 (0.11-0.33) | 0.20 (0.11-0.34) |
| Major bleeding* | 0.52 (0.27-1.02) | NA | NA | 0.49 (0.09-2.64) | 0.25 (0.03-2.24) |
* Hazard ratio (95% confident interval).
NA, not applicable; VKA, vitamin K antagonist; VTE, venous thromboembolism.
In addition to anticoagulants, aspirin has been used for VTE prevention in high-risk patients.173 Recently, 2 extended treatment trials compared low-dose aspirin (100 mg once daily) with placebo in patients with unprovoked VTE.174,175 The pool analysis of 2 trials indicated that the recurrence rate was 5.1% per year with low-dose aspirin compared with 7.5% per year with placebo (p = 0.008), whereas there was no excessive risk for major bleeding.176
Despite the probability that extended treatment is effective and generally safe in reducing VTE recurrence in patients with higher risk for recurrence, anticoagulation treatment of indefinite duration, or even probably for a lifetime, is a major undertaking both for patients and healthcare professionals.177 The decision of the treatment duration for DVT requires a complex evaluation that balances the risks of recurrence in the absence of anticoagulation against the risks of bleeding complications with continued pharmacological therapy.152,178 There is not yet a universally accepted method for predicting the risk of VTE recurrence in the absence of anticoagulation. The DASH scoring system and Vienna prediction model are newly developed assessment for patients with unprovoked VTE.179,180 To address the risk of major bleeding with anticoagulation in the first 3 months of treatment, the practice guidelines recommend a complex matrix whereas the RIETE scoring is a simple assessment.181
Recommendations
• The long-term treatment comprises minimally a 3-month duration of oral anticoagulants.
• Patients without a transient reversible risk factor for VTE are more likely to develop recurrences after cessation of anticoagulant treatment.
• For extended treatment, normal or low intensity warfarin, dabigatran, rivaroxaban, apixaban, and aspirin are effective on the reduction of VTE recurrence in patients with unprovoked VTE.
• Whether patients require an extended phase of anticoagulants depends on balances between the risk of VTE recurrence without anticoagulants and bleeding with anticoagulants, and the preference of patients.
• There is not yet a widely accepted scoring system for prediction of VTE recurrence without anticoagulation and bleeding with anticoagulation.
Pregnancy-related VTE
Pregnancy is associated with a hypercoagulable state characterized by increased concentrations of procoagulant factors and by a simultaneous decrease of anticoagulant factors.182 It is important to note that the likelihood of VTE is increased at least 4-fold during pregnancy compared with a non-pregnant status, and is a major cause of maternal mortality in the developed world.183,184 The overall incidence of VTE in pregnancy is about 1-2 cases per 1,000 deliveries in the West, and around 1 case per 10,000 individuals in Asia.20,185 The risk of VTE remains high until the first 6 weeks after delivery have passed.186 Furthermore, VTE occurs at a rate of 5.4, 7.2, and 4.3 cases per 10,000 pregnancies for antepartum, peripartum, and postpartum, respectively.185 Isolated DVT, particularly left iliac and/or femoral vein, is more common in pregnant patients due to the enlarged uterus’ compressive effects on veins.187
The use of diagnostic algorithms for DVT, the combination of structured clinical prediction rules and the use of D-dimer testing, in non-pregnant patients is neither adequate nor validated when applied in pregnancy. Leg swelling, the cardinal sign and symptom of DVT, may be associated with a normal pregnancy and levels of D-dimer increase with the progression of a normal pregnancy.188 The cornerstone of diagnosing DVT in pregnancy is the demonstration of presence of a clot by CUS. Compression maneuvers should be performed along the entire venous system from the femoral to the popliteal vein. The sensitivity and negative predictive value are 90.9% and 98.9%, respectively,189 with a reduced accuracy for isolated calf- and iliac-vein thrombosis.190 CUS could be repeated within 7 days if the initial study is negative,191,192 or MRV could be used if the initial CUS study is negative or for detecting iliac-vein thrombosis.193
It is safe to withhold anticoagulation in pregnant patients with suspected DVT following negative serial CUS and iliac vein imaging.194 Maternal complications from anticoagulant therapy are similar to those seen in non-pregnant patients.195 However, additional considerations regarding the health and life of the fetus will affect patients’ choices on anticoagulation therapy. For the treatment of acute DVT, particularly proximal DVT with or without PE, inpatient treatment with parenteral heparin is recommended and VKAs should only be considered in exceptional circumstances.192,196 Pregnant women were excluded from participating in clinical trials evaluating NOACs, and NOACs are not approved for such conditions and should not be used in breast-feeding patients. In general, LMWH is recommended over unfractionated heparin for use in pregnant patients. It is reasonable to use the twice-daily regimen of LMWH in pregnancy, especially for the first month when the risk of recurrence is the greatest. This practice also stems from the altered renal elimination of LMWH and the impact of weight gain during pregnancy.
No study has assessed the optimal duration of anticoagulant therapy for treatment of pregnancy-related VTE. In non-pregnant patients with VTE, evidence supports a minimum duration of 3 months of treatment. Major guidelines recommend anticoagulants, which should be continued for at least 6 weeks postpartum (for a minimum total therapy duration of 3 months).196,197 And for pregnant women with a prior history of VTE associated with transient risk factors, withholding antepartum anticoagulation therapy is generally safe.198,199
Cancer-related VTE
Patients with cancer are at the increased risk for VTE,200 and carry a substantial risk for VTE recurrence.15,201 Meanwhile, VTE can be attributed to major morbidity and mortality in patients with cancer,202 and has a significant negative impact on quality of life in patients with cancer.203 Although long-term VKAs are warranted and prevent recurrence in the majority of patients, VKA treatment therapy is problematic in patients with cancer because drug interactions, malnutrition, vomiting, and liver dysfunction can lead to unpredictable levels of anticoagulation in addition to the potential need for invasive procedures requiring interruption of VKAs.204 These limitations may contribute to the higher risk of recurrent thromboembolism and bleeding in patients with cancer than in patients without cancer.
Weight-adjusted LMWH as a parenteral agent is effective and appealing for treatment in patients with cancer. The remote and recent clinical trials have demonstrated the advantage of treatment with LMWH over VKAs for 6 months.205,206 NOACs had at least a similar clinical benefit as VKAs in the subgroup analyses,141,207,208 and might be even comparable to LMWH in the pooled analyses.209,210 However, additional studies are needed to confirm this concept and to compare NOACs with LMWH directly in patients with cancer.211 Currently, 3 protocols that will randomize cancer patients with VTE to either NOACs or LMWH with a variable treatment duration are approved (NCT02583191, NCT02585713, NCT02073682). Despite practicing guidelines recommending LMWH for at least 3-6 months and possibly indefinitely for patients with active cancer, the optimal duration of LMWH treatment beyond 6 months is yet to be determined by the evidence from the randomized controlled trials.212,213 In the Hokusai VTE-cancer study, patients will be treated for an extended period up to 12 months on the basis of the risk-benefit assessment by the treating physician and/or on patient preference.214
Areas with uncertainty
Although a large number of cohort studies have provided convincing data, and the clinical assessment pathway has been long constructed, these studies describe and have been validated mainly in Western populations. The therapeutic trials had a very low proportion of both young and elderly patients, and patients with minimal comorbidity. In addition, a small proportion of Asian patients participated in these trials. The NOACs have been tested in extended treatment with limited follow-up periods. It will be necessary to elaborate on (i) whether Asian patients indeed have a low risk for the development of DVT and PE, (ii) whether the diagnostic algorithm is feasible if Asian patients truly have a lower prevalence of DVT and PE, (iii) long-term recurrence without and bleeding risk with anticoagulation treatment in Asian patients, and (iv) whether treatment for VTE in patients with pregnancy or cancer is practical in Asian patients who might have different thrombosis and bleeding profiles. Nevertheless, the accumulation of clinical experience with new therapeutics in the real-world will have to proceed at a prudent pace. For those trials that enrolled but have yet to publish data regarding Asian patients, we welcome public dissemination of the research results, so that physicians, patients, and other health care professionals can benefit from the results.215,216
SUMMARY
The heavy burden of DVT, as part of VTE continuum, is seriously overlooked in Taiwan. Routine risk assessment and thromboprophylaxis for high-risk patient have not been established, and the guidelines are not yet developed. On the other hand, the diagnosis of DVT requires a sophisticated evaluation from pre-test risk assessment until imaging confirmation. The implantation of the comprehensive diagnostic algorithm facilitates the timely administration of therapeutics for the appropriate patients. For patients with DVT, anticoagulation is essential to prevent thrombus extension and recurrence. The conventional strategy of initial parenteral heparin followed by warfarin is effective, but somehow inconvenient and has its limitations. Quickly-adopted NOACs as an alternative strategy for acute VTE treatment have their unique features with respect to each other. Some of the NOACs are taken once daily, others twice daily, some require the use of a parenteral heparin lead in, and others use the single-drug approach. The single-drug approach without preceding parenteral treatments is appealing to low risk outpatients whereas the conventional parenteral lead in strategy is feasible for intermediate/high risk inpatients. Nevertheless, NOACs have been proven effectively safe in the large-scale clinical trials, particularly with respect to the important outcome of bleeding. Finally, DVT is a chronic disease and unprovoked DVT carries a higher risk for recurrence. Extended treatment should be considered in such patients in part based upon the weight of prevention of recurrence and risk of bleeding. Growing studies have paid attention to the idea of constructing a more user-friendly scoring system to predict VTE recurrence without and bleeding events with anticoagulation. Finally, this consensus document hopefully can upsurge the awareness of DVT in the medical community, and improve patient outcomes during the acute phase and thereafter in the long run.
DISCLOSURES
Dr. Kang-Ling Wang received some benefit from Bayer, Boehringer Ingelheim, and Daiichi Sankyo. Dr. Kou-Gi Shyu has been on the speaker’s bureau for AstraZeneca, Bayer, Daiichi Sankyo, MSD, Pfizer, Sanofi, and Takeda. Dr. Chern-En Chiang has been on the speaker’s bureau for Bayer, Boehringer Ingelheim, Daiichi Sankyo, and Pfizer.
FUNDING SOURCES
This work was supported, in part, by grants from the Ministry of Health and Welfare (MOHW104-TDU-B-211-113-003), the Ministry of Science and Technology (102-2314-B-182A-060-MY2 and 104-2314-B-182A-131), and Chang Gung Memorial Hospital (CIRPG3E0011).
REFERENCES
- 1. Moser KM, Fedullo PF, LitteJohn JK, Crawford R. Frequent asymptomatic pulmonary embolism in patients with deep venous thrombosis. JAMA. 1994;271(3):223–225. [PubMed] [Google Scholar]
- 2. Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER) Lancet. 1999;353(9162):1386–1389. doi: 10.1016/s0140-6736(98)07534-5. [DOI] [PubMed] [Google Scholar]
- 3. Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Prognostic role of echocardiography among patients with acute pulmonary embolism and a systolic arterial pressure of 90 mm Hg or higher. Arch Intern Med. 2005;165(15):1777–1781. doi: 10.1001/archinte.165.15.1777. [DOI] [PubMed] [Google Scholar]
- 4. Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(43):3033–3069. doi: 10.1093/eurheartj/ehu283. [DOI] [PubMed] [Google Scholar]
- 5. Cohen AT, Agnelli G, Anderson FA, et al. Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost. 2007;98(4):756–764. doi: 10.1160/TH07-03-0212. [DOI] [PubMed] [Google Scholar]
- 6. Spencer FA, Gore JM, Lessard D, et al. Patient outcomes after deep vein thrombosis and pulmonary embolism: the Worcester Venous Thromboembolism Study. Arch Intern Med. 2008;168(4):425–430. doi: 10.1001/archinternmed.2007.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica. 2007;92(2):199–205. doi: 10.3324/haematol.10516. [DOI] [PubMed] [Google Scholar]
- 8. Pengo V, Lensing AW, Prins MH, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350(22):2257–2264. doi: 10.1056/NEJMoa032274. [DOI] [PubMed] [Google Scholar]
- 9. Kahn SR. The post-thrombotic syndrome: the forgotten morbidity of deep venous thrombosis. J Thromb Thrombolysis. 2006;21(1):41–48. doi: 10.1007/s11239-006-5574-9. [DOI] [PubMed] [Google Scholar]
- 10. Roberts LN, Patel RK, Donaldson N, et al. Post-thrombotic syndrome is an independent determinant of health-related quality of life following both first proximal and distal deep vein thrombosis . Haematologica. 2014;99(3):e41–e43. doi: 10.3324/haematol.2013.089870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lefebvre P, Laliberte F, Nutescu EA, et al. All-cause and disease-related health care costs associated with recurrent venous thromboembolism. Thromb Haemost. 2013;110(6):1288–1297. doi: 10.1160/TH13-05-0425. [DOI] [PubMed] [Google Scholar]
- 12. Sorensen HT, Horvath-Puho E, Pedersen L, et al. Venous thromboembolism and subsequent hospitalisation due to acute arterial cardiovascular events: a 20-year cohort study. Lancet. 2007;370(9601):1773–1779. doi: 10.1016/S0140-6736(07)61745-0. [DOI] [PubMed] [Google Scholar]
- 13. Silverstein MD, Heit JA, Mohr DN, et al. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998;158(6):585–593. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 14. White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107(23 Suppl 1):I4–I8. doi: 10.1161/01.CIR.0000078468.11849.66. [DOI] [PubMed] [Google Scholar]
- 15. Lee CH, Lin LJ, Cheng CL, et al. Incidence and cumulative recurrence rates of venous thromboembolism in the Taiwanese population. J Thromb Haemost. 2010;8(7):1515–1523. doi: 10.1111/j.1538-7836.2010.03873.x. [DOI] [PubMed] [Google Scholar]
- 16. Alikhan R, Cohen AT, Combe S, et al. Risk factors for venous thromboembolism in hospitalized patients with acute medical illness: analysis of the MEDENOX Study. Arch Intern Med. 2004;164(9):963–968. doi: 10.1001/archinte.164.9.963. [DOI] [PubMed] [Google Scholar]
- 17. Samama MM. An epidemiologic study of risk factors for deep vein thrombosis in medical outpatients: the Sirius study. Arch Intern Med. 2000;160(22):3415–3420. doi: 10.1001/archinte.160.22.3415. [DOI] [PubMed] [Google Scholar]
- 18. Cohen AT, Alikhan R, Arcelus JI, et al. Assessment of venous thromboembolism risk and the benefits of thromboprophylaxis in medical patients. Thromb Haemost. 2005;94(4):750–759. [PubMed] [Google Scholar]
- 19. Anderson FA, Jr., Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107(23 Suppl 1):I9–I16. doi: 10.1161/01.CIR.0000078469.07362.E6. [DOI] [PubMed] [Google Scholar]
- 20. Jang MJ, Bang SM, Oh D. Incidence of venous thromboembolism in Korea: from the Health Insurance Review and Assessment Service database. J Thromb Haemost. 2011;9(1):85–91. doi: 10.1111/j.1538-7836.2010.04108.x. [DOI] [PubMed] [Google Scholar]
- 21. Kyrle PA, Eichinger S. Deep vein thrombosis. Lancet. 2005;365(9465):1163–1174. doi: 10.1016/S0140-6736(05)71880-8. [DOI] [PubMed] [Google Scholar]
- 22. Meignan M, Rosso J, Gauthier H, et al. Systematic lung scans reveal a high frequency of silent pulmonary embolism in patients with proximal deep venous thrombosis. Arch Intern Med. 2000;160(2):159–164. doi: 10.1001/archinte.160.2.159. [DOI] [PubMed] [Google Scholar]
- 23. Sandler DA, Martin JF. Autopsy proven pulmonary embolism in hospital patients: are we detecting enough deep vein thrombosis? J R Soc Med. 1989;82(4):203–205. doi: 10.1177/014107688908200407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Murin S, Romano PS, White RH. Comparison of outcomes after hospitalization for deep venous thrombosis or pulmonary embolism. Thromb Haemost. 2002;88(3):407–414. [PubMed] [Google Scholar]
- 25. Naess IA, Christiansen SC, Romundstad P, et al. Incidence and mortality of venous thrombosis: a population-based study. J Thromb Haemost. 2007;5(4):692–699. doi: 10.1111/j.1538-7836.2007.02450.x. [DOI] [PubMed] [Google Scholar]
- 26. Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Massive pulmonary embolism. Circulation. 2006;113(4):577–582. doi: 10.1161/CIRCULATIONAHA.105.592592. [DOI] [PubMed] [Google Scholar]
- 27. Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol. 2008;28(3)::370–372. doi: 10.1161/ATVBAHA.108.162545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guijarro R, Montes J, Sanroman C, et al. Venous thromboembolism in Spain. Comparison between an administrative database and the RIETE registry. Eur J Intern Med. 2008;19(6):443–446. doi: 10.1016/j.ejim.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 29. Spencer FA, Emery C, Joffe SW, et al. Incidence rates, clinical profile, and outcomes of patients with venous thromboembolism. The Worcester VTE study. J Thromb Thrombolysis. 2009;28(4):401–409. doi: 10.1007/s11239-009-0378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kakkar VV, Howe CT, Flanc C, Clarke MB. Natural history of postoperative deep-vein thrombosis. Lancet. 1969;2(7614):230–232. doi: 10.1016/s0140-6736(69)90002-6. [DOI] [PubMed] [Google Scholar]
- 31. Galanaud JP, Sevestre MA, Genty C, et al. Incidence and predictors of venous thromboembolism recurrence after a first isolated distal deep vein thrombosis. J Thromb Haemost. 2014;12(4):436–443. doi: 10.1111/jth.12512. [DOI] [PubMed] [Google Scholar]
- 32. Wang CJ, Wang JW, Chen LM, et al. Deep vein thrombosis after total knee arthroplasty. J Formos Med Assoc. 2000;99(11):848–853. [PubMed] [Google Scholar]
- 33. Cogo A, Lensing AW, Prandoni P, Hirsh J. Distribution of thrombosis in patients with symptomatic deep vein thrombosis. Implications for simplifying the diagnostic process with compression ultrasound. Arch Intern Med. 1993;153(24):2777–2780. [PubMed] [Google Scholar]
- 34. Oger E EPI-GETBP Study Group. Groupe d’Etude de la Thrombose de Bretagne Occidentale. Incidence of venous thromboembolism: a community-based study in Western France. Thromb Haemost . 2000;83(5):657–660. [PubMed] [Google Scholar]
- 35. Ho WK, Hankey GJ, Eikelboom JW. The incidence of venous thromboembolism: a prospective, community-based study in Perth, Western Australia. Med J Aust. 2008;189(3):144–147. doi: 10.5694/j.1326-5377.2008.tb01947.x. [DOI] [PubMed] [Google Scholar]
- 36. Liao S, Woulfe T, Hyder S, et al. Incidence of venous thromboembolism in different ethnic groups: a regional direct comparison study. J Thromb Haemost. 2014;12(2):214–219. doi: 10.1111/jth.12464. [DOI] [PubMed] [Google Scholar]
- 37. White RH, Zhou H, Murin S, Harvey D. Effect of ethnicity and gender on the incidence of venous thromboembolism in a diverse population in California in 1996. Thromb Haemost. 2005;93(2):298–305. doi: 10.1160/TH04-08-0506. [DOI] [PubMed] [Google Scholar]
- 38. Cohen A, Chiu KM, Park K, et al. Managing venous thromboembolism in Asia: winds of change in the era of new oral anticoagulants. Thromb Res. 2012;130(3):291–301. doi: 10.1016/j.thromres.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 39. Lee CH, Cheng CL, Lin LJ, et al. Epidemiology and predictors of short-term mortality in symptomatic venous thromboembolism. Circ J. 2011;75(8):1998–2004. doi: 10.1253/circj.cj-10-0992. [DOI] [PubMed] [Google Scholar]
- 40. Angchaisuksiri P. Venous thromboembolism in Asia--an unrecognised and under-treated problem? Thromb Haemost. 2011;106(4):585–590. doi: 10.1160/TH11-03-0184. [DOI] [PubMed] [Google Scholar]
- 41. Yu YB, Gau JP, Liu CY, et al. A nation-wide analysis of venous thromboembolism in 497,180 cancer patients with the development and validation of a risk-stratification scoring system. Thromb Haemost. 2012;108(2):225–235. doi: 10.1160/TH12-01-0010. [DOI] [PubMed] [Google Scholar]
- 42.Anderson FA, Jr., Wheeler HB. Physician practices in the management of venous thromboembolism: a community-wide survey. J Vasc Surg. 1992;16(5):707–714. doi: 10.1067/mva.1992.41080. [DOI] [PubMed] [Google Scholar]
- 43. Kyrle PA, Rosendaal FR, Eichinger S. Risk assessment for recurrent venous thrombosis. Lancet. 2010;376(9757):2032–2039. doi: 10.1016/S0140-6736(10)60962-2. [DOI] [PubMed] [Google Scholar]
- 44. Goldhaber SZ. Venous thromboembolism: epidemiology and magnitude of the problem. Best Pract Res Clin Haematol. 2012;25(3):235–242. doi: 10.1016/j.beha.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 45. Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet. 2012;379(9828):1835–1846. doi: 10.1016/S0140-6736(11)61904-1. [DOI] [PubMed] [Google Scholar]
- 46. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e278S–e325S. doi: 10.1378/chest.11-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bjornara BT, Gudmundsen TE, Dahl OE. Frequency and timing of clinical venous thromboembolism after major joint surgery. J Bone Joint Surg Br. 2006;88(3):386–391. doi: 10.1302/0301-620X.88B3.17207. [DOI] [PubMed] [Google Scholar]
- 48. Bergqvist D, Benoni G, Bjorgell O, et al. Low-molecular-weight heparin (enoxaparin) as prophylaxis against venous thromboembolism after total hip replacement. N Engl J Med. 1996;335(10):696–700. doi: 10.1056/NEJM199609053351002. [DOI] [PubMed] [Google Scholar]
- 49. Lee WS, Kim KI, Lee HJ, et al. The incidence of pulmonary embolism and deep vein thrombosis after knee arthroplasty in Asians remains low: a meta-analysis. Clin Orthop Relat Res. 2013;471(5):1523–1532. doi: 10.1007/s11999-012-2758-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chung LH, Chen WM, Chen CF, et al. Deep vein thrombosis after total knee arthroplasty in asian patients without prophylactic anticoagulation. Orthopedics. 2011;34(1):15. doi: 10.3928/01477447-20101123-05. [DOI] [PubMed] [Google Scholar]
- 51. Cohen AT Asia-Pacific Thrombosis Advisory B. Asia-Pacific Thrombosis Advisory Board consensus paper on prevention of venous thromboembolism after major orthopaedic surgery. Thromb Haemost. 2010;104(5):919–930. doi: 10.1160/TH10-03-0190. [DOI] [PubMed] [Google Scholar]
- 52. Liew NC, Chang YH, Choi G, et al. Asian venous thromboembolism guidelines: prevention of venous thromboembolism. Int Angiol. 2012;31(6):501–516. [PubMed] [Google Scholar]
- 53. Leizorovicz A, Turpie AG, Cohen AT, et al. Epidemiology of venous thromboembolism in Asian patients undergoing major orthopedic surgery without thromboprophylaxis. The SMART study. J Thromb Haemost. 2005;3(1):28–34. doi: 10.1111/j.1538-7836.2004.01094.x. [DOI] [PubMed] [Google Scholar]
- 54. Leizorovicz A Committee SVSS. Epidemiology of post-operative venous thromboembolism in Asian patients. Results of the SMART venography study. Haematologica. 2007;92(9):1194–1200. doi: 10.3324/haematol.10819. [DOI] [PubMed] [Google Scholar]
- 55. Piovella F, Wang CJ, Lu H, et al. Deep-vein thrombosis rates after major orthopedic surgery in Asia. An epidemiological study based on postoperative screening with centrally adjudicated bilateral venography. J Thromb Haemost. 2005;3(12):2664–2670. doi: 10.1111/j.1538-7836.2005.01621.x. [DOI] [PubMed] [Google Scholar]
- 56. Lee CH, Cheng CL, Chang CH, et al. Universal pharmacological thromboprophylaxis for total knee arthroplasty may not be necessary in low-risk populations: a nationwide study in Taiwan. J Thromb Haemost. 2012;10(1):56–63. doi: 10.1111/j.1538-7836.2011.04555.x. [DOI] [PubMed] [Google Scholar]
- 57. Wu PK, Chen CF, Chung LH, et al. Population-based epidemiology of postoperative venous thromboembolism in Taiwanese patients receiving hip or knee arthroplasty without pharmacological thromboprophylaxis. Thromb Res. 2014;133(5):719–724. doi: 10.1016/j.thromres.2014.01.039. [DOI] [PubMed] [Google Scholar]
- 58. Wang CJ, Wang JW, Weng LH, et al. Clinical significance of muscular deep-vein thrombosis after total knee arthroplasty. Chang Gung Med J. 2007;30(1):41–46. [PubMed] [Google Scholar]
- 59. Attia J, Ray JG, Cook DJ, et al. Deep vein thrombosis and its prevention in critically ill adults. Thromb Res. 2001;161(10):1268–1279. doi: 10.1001/archinte.161.10.1268. [DOI] [PubMed] [Google Scholar]
- 60. Turpie AG. Thrombosis prophylaxis in the acutely ill medical patient: insights from the prophylaxis in MEDical patients with ENOXaparin (MEDENOX) trial. Am J Cardiol. 2000;86(12B):48M–52M. doi: 10.1016/s0002-9149(00)01481-8. [DOI] [PubMed] [Google Scholar]
- 61. Leizorovicz A, Cohen AT, Turpie AG, et al. Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110(7):874–879. doi: 10.1161/01.CIR.0000138928.83266.24. [DOI] [PubMed] [Google Scholar]
- 62. Cohen AT, Davidson BL, Gallus AS, et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006;332(7537):325–329. doi: 10.1136/bmj.38733.466748.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Leung V, Leung V, Lui W, et al. Incidence of deep vein thrombosis in hospitalized Chinese medical patients is similar to that in western populations. Thromb Res. 2006;118(6):763–764. doi: 10.1016/j.thromres.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 64. Dunn AS, Brenner A, Halm EA. The magnitude of an iatrogenic disorder: a systematic review of the incidence of venous thromboembolism for general medical inpatients. Thromb Haemost. 2006;95(5):758–762. [PubMed] [Google Scholar]
- 65. Nakamura M, Fujioka H, Yamada N, et al. Clinical characteristics of acute pulmonary thromboembolism in Japan: results of a multicenter registry in the Japanese Society of Pulmonary Embolism Research. Clin Cardiol. 2001;24(2):132–138. doi: 10.1002/clc.4960240207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chau KY, Yuen ST, Wong MP. Clinicopathological pattern of pulmonary thromboembolism in Chinese autopsy patients: comparison with Caucasian series. Pathology . 1997;29(3):263–266. doi: 10.1080/00313029700169035. [DOI] [PubMed] [Google Scholar]
- 67. Dickens P, Knight BH, Ip P, Fung WS. Fatal pulmonary embolism: a comparative study of autopsy incidence in Hong Kong and Cardiff, Wales. Forensic Sci Int. 1997;90(3):171–174. doi: 10.1016/s0379-0738(97)00157-6. [DOI] [PubMed] [Google Scholar]
- 68. Zakai NA, McClure LA. Racial differences in venous thromboembolism. J Thromb Haemost. 2011;9(10):1877–1882. doi: 10.1111/j.1538-7836.2011.04443.x. [DOI] [PubMed] [Google Scholar]
- 69. Seligsohn U, Lubetsky A. Genetic susceptibility to venous thrombosis. N Engl J Med. 2001;344(16):1222–1231. doi: 10.1056/NEJM200104193441607. [DOI] [PubMed] [Google Scholar]
- 70. Margaglione M, Grandone E. Population genetics of venous thromboembolism. A narrative review. Thromb Haemost. 2011;105(2):221–231. doi: 10.1160/TH10-08-0510. [DOI] [PubMed] [Google Scholar]
- 71. De Stefano V, Rossi E, Paciaroni K, Leone G. Screening for inherited thrombophilia: indications and therapeutic implications. Haematologica. 2002;87(10):1095–1108. [PubMed] [Google Scholar]
- 72. Shen MC, Lin JS, Tsay W. High prevalence of antithrombin III, protein C and protein S deficiency, but no factor V Leiden mutation in venous thrombophilic Chinese patients in Taiwan. Thromb Res. 1997;87(4):377–385. doi: 10.1016/s0049-3848(97)00141-2. [DOI] [PubMed] [Google Scholar]
- 73. Ho CH, Chau WK, Hsu HC, et al. Causes of venous thrombosis in fifty Chinese patients. Am J Hematol. 2000;63(2):74–78. doi: 10.1002/(sici)1096-8652(200002)63:2<74::aid-ajh3>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 74. Shen MC, Lin JS, Tsay W. Protein C and protein S deficiencies are the most important risk factors associated with thrombosis in Chinese venous thrombophilic patients in Taiwan. Thromb Res. 2000;99(5):447–452. doi: 10.1016/s0049-3848(00)00265-6. [DOI] [PubMed] [Google Scholar]
- 75. Chen TY, Su WC, Tsao CJ. Incidence of thrombophilia detected in southern Taiwanese patients with venous thrombosis. Ann Hematol. 2003;82(2):114–117. doi: 10.1007/s00277-002-0603-z. [DOI] [PubMed] [Google Scholar]
- 76. Goldhaber SZ. Risk factors for venous thromboembolism. J Am Coll Cardiol. 2010;56(1):1–7. doi: 10.1016/j.jacc.2010.01.057. [DOI] [PubMed] [Google Scholar]
- 77. De Stefano V, Rossi E. Testing for inherited thrombophilia and consequences for antithrombotic prophylaxis in patients with venous thromboembolism and their relatives. A review of the Guidelines from Scientific Societies and Working Groups. Thromb Haemost. 2013;110(4):697–705. doi: 10.1160/TH13-01-0011. [DOI] [PubMed] [Google Scholar]
- 78. Chong LY, Fenu E, Stansby G, Hodgkinson S Guideline Development G. Management of venous thromboembolic diseases and the role of thrombophilia testing: summary of NICE guidance. BMJ. 2012;344:e3979. doi: 10.1136/bmj.e3979. [DOI] [PubMed] [Google Scholar]
- 79. Howard LS, Hughes RJ. NICE guideline: management of venous thromboembolic diseases and role of thrombophilia testing. Thorax. 2013;68(4):391–393. doi: 10.1136/thoraxjnl-2012-202376. [DOI] [PubMed] [Google Scholar]
- 80. Hull R, Hirsh J, Sackett DL, et al. Clinical validity of a negative venogram in patients with clinically suspected venous thrombosis. Circulation. 1981;64(3):622–625. doi: 10.1161/01.cir.64.3.622. [DOI] [PubMed] [Google Scholar]
- 81. Hirsh J, Hoak J Council on Thrombosis (in consultation with the Council on Cardiovascular Radiology), American Heart Association. Management of deep vein thrombosis and pulmonary embolism. A statement for healthcare professionals. Circulation. 1996;93(12):2212–2245. doi: 10.1161/01.cir.93.12.2212. [DOI] [PubMed] [Google Scholar]
- 82. Elias A, Mallard L, Elias M, et al. A single complete ultrasound investigation of the venous network for the diagnostic management of patients with a clinically suspected first episode of deep venous thrombosis of the lower limbs. Thromb Haemost. 2003;89(2):221–227. [PubMed] [Google Scholar]
- 83. Grune S, Orlik J, Von Korn H, et al. Clinical signs in the diagnosis of deep vein thrombosis. Int Angiol. 2011;30(1):64–70. [PubMed] [Google Scholar]
- 84. Wells PS, Hirsh J, Anderson DR, et al. Accuracy of clinical assessment of deep-vein thrombosis. Lancet. 1995;345(8961):1326–1330. doi: 10.1016/s0140-6736(95)92535-x. [DOI] [PubMed] [Google Scholar]
- 85. Heijboer H, Buller HR, Lensing AW, et al. A comparison of real-time compression ultrasonography with impedance plethysmography for the diagnosis of deep-vein thrombosis in symptomatic outpatients. N Engl J Med. 1993;329(19):1365–1369. doi: 10.1056/NEJM199311043291901. [DOI] [PubMed] [Google Scholar]
- 86. Lensing AW, Buller HR, Prandoni P, et al. Contrast venography, the gold standard for the diagnosis of deep-vein thrombosis: improvement in observer agreement. Thromb Haemost. 1992;67(1):8–12. [PubMed] [Google Scholar]
- 87. Bates SM, Jaeschke R, Stevens SM, et al. Diagnosis of DVT: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl): e351S– e418S. doi: 10.1378/chest.11-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Constans J, Nelzy ML, Salmi LR, et al. Clinical prediction of lower limb deep vein thrombosis in symptomatic hospitalized patients. Thromb Haemost. 2001;86(4):985–990. [PubMed] [Google Scholar]
- 89. Kahn SR, Joseph L, Abenhaim L, Leclerc JR. Clinical prediction of deep vein thrombosis in patients with leg symptoms. Thromb Haemost. 1999;81(3):353–357. [PubMed] [Google Scholar]
- 90. Buller HR, Ten Cate-Hoek AJ, Hoes AW, et al. Safely ruling out deep venous thrombosis in primary care. Ann Intern Med. 2009;150(4):229–235. [PubMed] [Google Scholar]
- 91. Goodacre S, Sutton AJ, Sampson FC. Meta-analysis: the value of clinical assessment in the diagnosis of deep venous thrombosis. Ann Intern Med. 2005;143(2):129–139. doi: 10.7326/0003-4819-143-2-200507190-00012. [DOI] [PubMed] [Google Scholar]
- 92. Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet. 1997;350(9094):1795–1798. doi: 10.1016/S0140-6736(97)08140-3. [DOI] [PubMed] [Google Scholar]
- 93. Wells PS, Owen C, Doucette S, et al. Does this patient have deep vein thrombosis? JAMA. 2006;295(2):199–207. doi: 10.1001/jama.295.2.199. [DOI] [PubMed] [Google Scholar]
- 94. Wells PS, Anderson DR, Rodger M, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med. 2003;349(13):1227–1235. doi: 10.1056/NEJMoa023153. [DOI] [PubMed] [Google Scholar]
- 95. Cornuz J, Ghali WA, Hayoz D, et al. Clinical prediction of deep venous thrombosis using two risk assessment methods in combination with rapid quantitative D-dimer testing. Am J Med. 2002;112(3):198–203. doi: 10.1016/s0002-9343(01)01110-x. [DOI] [PubMed] [Google Scholar]
- 96. Yamaki T, Nozaki M, Sakurai H, et al. Combined use of pretest clinical probability score and latex agglutination D-dimer testing for excluding acute deep vein thrombosis. J Vasc Surg. 2009;50(5):1099–1105. doi: 10.1016/j.jvs.2009.06.059. [DOI] [PubMed] [Google Scholar]
- 97. Righini M, Perrier A, De Moerloose P, Bounameaux H. D-Dimer for venous thromboembolism diagnosis: 20 years later. J Thromb Haemost. 2008;6(7):1059–1071. doi: 10.1111/j.1538-7836.2008.02981.x. [DOI] [PubMed] [Google Scholar]
- 98. Huisman MV, Klok FA. Diagnostic management of acute deep vein thrombosis and pulmonary embolism. J Thromb Haemost. 2013;11(3):412–422. doi: 10.1111/jth.12124. [DOI] [PubMed] [Google Scholar]
- 99. Di Nisio M, Squizzato A, Rutjes AW, et al. Diagnostic accuracy of D-dimer test for exclusion of venous thromboembolism: a systematic review. J Thromb Haemost. 2007;5(2):296–304. doi: 10.1111/j.1538-7836.2007.02328.x. [DOI] [PubMed] [Google Scholar]
- 100. Righini M, Goehring C, Bounameaux H, Perrier A. Effects of age on the performance of common diagnostic tests for pulmonary embolism. Am J Med. 2000;109(5):357–361. doi: 10.1016/s0002-9343(00)00493-9. [DOI] [PubMed] [Google Scholar]
- 101. Douma RA, le Gal G, Sohne M, et al. Potential of an age adjusted D-dimer cut-off value to improve the exclusion of pulmonary embolism in older patients: a retrospective analysis of three large cohorts. BMJ. 2010;340:c1475. doi: 10.1136/bmj.c1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Schouten HJ, Geersing GJ, Koek HL, et al. Diagnostic accuracy of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and meta-analysis. BMJ. 2013;346:f2492. doi: 10.1136/bmj.f2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA. 2014;311(11):1117–1124. doi: 10.1001/jama.2014.2135. [DOI] [PubMed] [Google Scholar]
- 104. Goodacre S, Sampson F, Thomas S, et al. Systematic review and meta-analysis of the diagnostic accuracy of ultrasonography for deep vein thrombosis. BMC Med Imaging. 2005;5:6. doi: 10.1186/1471-2342-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wells PS, Lensing AW, Davidson BL, et al. Accuracy of ultrasound for the diagnosis of deep venous thrombosis in asymptomatic patients after orthopedic surgery. A meta-analysis. Ann Intern Med. 1995;122(1):47–53. doi: 10.7326/0003-4819-122-1-199501010-00008. [DOI] [PubMed] [Google Scholar]
- 106. Kearon C, Julian JA, Newman TE, Ginsberg JS. Noninvasive diagnosis of deep venous thrombosis. McMaster Diagnostic Imaging Practice Guidelines Initiative. Ann Intern Med. 1998;128(8):663–677. doi: 10.7326/0003-4819-128-8-199804150-00011. [DOI] [PubMed] [Google Scholar]
- 107. Gottlieb RH, Widjaja J, Tian L, et al. Calf sonography for detecting deep venous thrombosis in symptomatic patients: experience and review of the literature. J Clin Ultrasound. 1999;27(8):415–420. doi: 10.1002/(sici)1097-0096(199910)27:8<415::aid-jcu1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 108. Cogo A, Lensing AW, Koopman MM, et al. Compression ultrasonography for diagnostic management of patients with clinically suspected deep vein thrombosis: prospective cohort study. BMJ. 1998;316(7124):17–20. doi: 10.1136/bmj.316.7124.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ten Wolde M, Hagen PJ, Macgillavry MR, et al. Non-invasive diagnostic work-up of patients with clinically suspected pulmonary embolism; results of a management study. J Thromb Haemost. 2004;2(7):1110–1117. doi: 10.1111/j.1538-7836.2004.00769.x. [DOI] [PubMed] [Google Scholar]
- 110. Kanne JP, Lalani TA. Role of computed tomography and magnetic resonance imaging for deep venous thrombosis and pulmonary embolism. Circulation. 2004;109(12 Suppl 1):I15–I21. doi: 10.1161/01.CIR.0000122871.86662.72. [DOI] [PubMed] [Google Scholar]
- 111. Bruce D, Loud PA, Klippenstein DL, et al. Combined CT venography and pulmonary angiography: how much venous enhancement is routinely obtained? AJR Am J Roentgenol. 2001;176(5):1281–1285. doi: 10.2214/ajr.176.5.1761281. [DOI] [PubMed] [Google Scholar]
- 112. Garg K, Kemp JL, Wojcik D, et al. Thromboembolic disease: comparison of combined CT pulmonary angiography and venography with bilateral leg sonography in 70 patients. AJR Am J Roentgenol. 2000;175(4):997–1001. doi: 10.2214/ajr.175.4.1750997. [DOI] [PubMed] [Google Scholar]
- 113. Rademaker J, Griesshaber V, Hidajat N, et al. Combined CT pulmonary angiography and venography for diagnosis of pulmonary embolism and deep vein thrombosis: radiation dose. J Thorac Imaging. 2001;16(4):297–299. doi: 10.1097/00005382-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 114. Reichert M, Henzler T, Krissak R, et al. Venous thromboembolism: additional diagnostic value and radiation dose of pelvic CT venography in patients with suspected pulmonary embolism. Eur J Radiol. 2011;80(1):50–53. doi: 10.1016/j.ejrad.2010.12.101. [DOI] [PubMed] [Google Scholar]
- 115. Thomas SM, Goodacre SW, Sampson FC, van Beek EJ. Diagnostic value of CT for deep vein thrombosis: results of a systematic review and meta-analysis. Clin Radiol. 2008;63(3):299–304. doi: 10.1016/j.crad.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 116. Sampson FC, Goodacre SW, Thomas SM, van Beek EJ. The accuracy of MRI in diagnosis of suspected deep vein thrombosis: systematic review and meta-analysis. Eur Radiol. 2007;17(1):175–181. doi: 10.1007/s00330-006-0178-5. [DOI] [PubMed] [Google Scholar]
- 117. Dupas B, el Kouri D, Curtet C, et al. Angiomagnetic resonance imaging of iliofemorocaval venous thrombosis. Lancet. 1995;346(8966):17–19. doi: 10.1016/s0140-6736(95)92650-x. [DOI] [PubMed] [Google Scholar]
- 118. Kearon C. Natural history of venous thromboembolism. Circulation. 2003;107(23 Suppl 1):I22–I30. doi: 10.1161/01.CIR.0000078464.82671.78. [DOI] [PubMed] [Google Scholar]
- 119. Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125(1):1–7. doi: 10.7326/0003-4819-125-1-199607010-00001. [DOI] [PubMed] [Google Scholar]
- 120. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl): e419S– e494S. doi: 10.1378/chest.11-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Prandoni P, Lensing AW, Prins MH, et al. Residual venous thrombosis as a predictive factor of recurrent venous thromboembolism. Ann Intern Med. 2002;137(12):955–960. doi: 10.7326/0003-4819-137-12-200212170-00008. [DOI] [PubMed] [Google Scholar]
- 122. Prandoni P, Prins MH, Lensing AW, et al. Residual thrombosis on ultrasonography to guide the duration of anticoagulation in patients with deep venous thrombosis: a randomized trial. Ann Intern Med. 2009;150(9):577–585. doi: 10.7326/0003-4819-150-9-200905050-00003. [DOI] [PubMed] [Google Scholar]
- 123. Enden T, Klow NE, Sandvik L, et al. Catheter-directed thrombolysis vs. anticoagulant therapy alone in deep vein thrombosis: results of an open randomized, controlled trial reporting on short-term patency. J Thromb Haemost. 2009;7(8):1268–1275. doi: 10.1111/j.1538-7836.2009.03464.x. [DOI] [PubMed] [Google Scholar]
- 124. Hull RD, Marder VJ, Mah AF, et al. Quantitative assessment of thrombus burden predicts the outcome of treatment for venous thrombosis: a systematic review. Am J Med. 2005;118(5):456–464. doi: 10.1016/j.amjmed.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 125. de Wolf MA, Wittens CH, Kahn SR. Incidence and risk factors of the post-thrombotic syndrome. Phlebology. 2012;27(Suppl 1):85–94. doi: 10.1258/phleb.2011.012s06. [DOI] [PubMed] [Google Scholar]
- 126. Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123(16):1788–1830. doi: 10.1161/CIR.0b013e318214914f. [DOI] [PubMed] [Google Scholar]
- 127. Enden T, Haig Y, Klow NE, et al. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet. 2012;379(9810):31–38. doi: 10.1016/S0140-6736(11)61753-4. [DOI] [PubMed] [Google Scholar]
- 128. Watson L, Broderick C, Armon MP. Thrombolysis for acute deep vein thrombosis. Cochrane Database Syst Rev. 2014;1:CD002783. doi: 10.1002/14651858.CD002783.pub3. [DOI] [PubMed] [Google Scholar]
- 129. Sharifi M, Bay C, Mehdipour M, et al. Thrombus Obliteration by Rapid Percutaneous Endovenous Intervention in Deep Venous Occlusion (TORPEDO) trial: midterm results. J Endovasc Ther. 2012;19(2):273–280. doi: 10.1583/11-3674MR.1. [DOI] [PubMed] [Google Scholar]
- 130. Vedantham S, Goldhaber SZ, Kahn SR, et al. Rationale and design of the ATTRACT Study: a multicenter randomized trial to evaluate pharmacomechanical catheter-directed thrombolysis for the prevention of postthrombotic syndrome in patients with proximal deep vein thrombosis. Am Heart J. 2013;165(4):523–530.e3. doi: 10.1016/j.ahj.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Brandjes DP, Heijboer H, Buller HR, et al. Acenocoumarol and heparin compared with acenocoumarol alone in the initial treatment of proximal-vein thrombosis. N Engl J Med. 1992;327(21):1485–1489. doi: 10.1056/NEJM199211193272103. [DOI] [PubMed] [Google Scholar]
- 132. Raschke RA, Reilly BM, Guidry JR, et al. The weight-based heparin dosing nomogram compared with a “standard care” nomogram. A randomized controlled trial. Ann Intern Med. 1993;119(9):874–881. doi: 10.7326/0003-4819-119-9-199311010-00002. [DOI] [PubMed] [Google Scholar]
- 133. Cossette B, Pelletier ME, Carrier N, et al. Evaluation of bleeding risk in patients exposed to therapeutic unfractionated or low-molecular-weight heparin: a cohort study in the context of a quality improvement initiative. Ann Pharmacother. 2010;44(6):994–1002. doi: 10.1345/aph.1M615. [DOI] [PubMed] [Google Scholar]
- 134. Prandoni P, Siragusa S, Girolami B, et al. The incidence of heparin-induced thrombocytopenia in medical patients treated with low-molecular-weight heparin: a prospective cohort study. Blood. 2005;106(9):3049–3054. doi: 10.1182/blood-2005-03-0912. [DOI] [PubMed] [Google Scholar]
- 135. Research Committee of the British Thoracic Society. Optimum duration of anticoagulation for deep-vein thrombosis and pulmonary embolism. Lancet. 1992;340(8824):873–876. [PubMed] [Google Scholar]
- 136. Palareti G, Legnani C, Cosmi B, et al. Poor anticoagulation quality in the first 3 months after unprovoked venous thromboembolism is a risk factor for long-term recurrence. J Thromb Haemost. 2005;3(5):955–961. doi: 10.1111/j.1538-7836.2005.01330.x. [DOI] [PubMed] [Google Scholar]
- 137. van Dongen CJ, Prandoni P, Frulla M, et al. Relation between quality of anticoagulant treatment and the development of the postthrombotic syndrome. J Thromb Haemost. 2005;3(5):939–942. doi: 10.1111/j.1538-7836.2005.01333.x. [DOI] [PubMed] [Google Scholar]
- 138. Haas S. New oral Xa and IIa inhibitors: updates on clinical trial results. J Thromb Thrombolysis. 2008;25(1):52–60. doi: 10.1007/s11239-007-0108-7. [DOI] [PubMed] [Google Scholar]
- 139. Investigators E. Bauersachs R, Berkowitz SD, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363(26):2499–2510. doi: 10.1056/NEJMoa1007903. [DOI] [PubMed] [Google Scholar]
- 140. Investigators E-P. Buller HR, Prins MH, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366(14):1287–1297. doi: 10.1056/NEJMoa1113572. [DOI] [PubMed] [Google Scholar]
- 141. Prins MH, Lensing AW, Bauersachs R, et al. Oral rivaroxaban versus standard therapy for the treatment of symptomatic venous thromboembolism: a pooled analysis of the EINSTEIN-DVT and PE randomized studies. Thromb J. 2013;11(1):21. doi: 10.1186/1477-9560-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Schulman S, Kakkar AK, Goldhaber SZ, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation. 2014;129(7):764–772. doi: 10.1161/CIRCULATIONAHA.113.004450. [DOI] [PubMed] [Google Scholar]
- 143. Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361(24):2342–2352. doi: 10.1056/NEJMoa0906598. [DOI] [PubMed] [Google Scholar]
- 144. Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799–808. doi: 10.1056/NEJMoa1302507. [DOI] [PubMed] [Google Scholar]
- 145. Hokusai VTEI, Buller HR, Decousus H, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369(15):1406–1415. doi: 10.1056/NEJMoa1306638. [DOI] [PubMed] [Google Scholar]
- 146. Verhamme P, Bounameaux H. Direct oral anticoagulants for acute venous thromboembolism: closing the circle? Circulation. 2014;129(7):725–727. doi: 10.1161/CIRCULATIONAHA.113.007478. [DOI] [PubMed] [Google Scholar]
- 147. Chiang CE, Wang KL, Lip GY. Stroke prevention in atrial fibrillation: an Asian perspective. Thromb Haemost. 2014;111(5):789–797. doi: 10.1160/TH13-11-0948. [DOI] [PubMed] [Google Scholar]
- 148. Wang KL, Chiang CE. Optimal international normalized ratio for atrial fibrillation in Asians and Japanese: do we really know? Circ J. 2013;77(9):2242–2243. doi: 10.1253/circj.cj-13-0885. [DOI] [PubMed] [Google Scholar]
- 149. Lip GY, Wang KL, Chiang CE. Non-vitamin K antagonist oral anticoagulants (NOACs) for stroke prevention in Asian patients with atrial fibrillation: time for a reappraisal. Int J Cardiol. 2015;180:246–254. doi: 10.1016/j.ijcard.2014.11.182. [DOI] [PubMed] [Google Scholar]
- 150. Nakamura M, Wang YQ, Wang C, et al. Efficacy and safety of edoxaban for treatment of venous thromboembolism: a subanalysis of East Asian patients in the Hokusai-VTE trial. J Thromb Haemost. 2015;13(9):1606–1614. doi: 10.1111/jth.13055. [DOI] [PubMed] [Google Scholar]
- 151. Wang Y, Wang C, Chen Z, et al. Rivaroxaban for the treatment of symptomatic deep-vein thrombosis and pulmonary embolism in Chinese patients: a subgroup analysis of the EINSTEIN DVT and PE studies. Thromb J. 2013;11(1):25. doi: 10.1186/1477-9560-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med. 1999;340(12):901–907. doi: 10.1056/NEJM199903253401201. [DOI] [PubMed] [Google Scholar]
- 153. Hull R, Delmore T, Genton E, et al. Warfarin sodium versus low-dose heparin in the long-term treatment of venous thrombosis. N Engl J Med. 1979;301(16):855–858. doi: 10.1056/NEJM197910183011602. [DOI] [PubMed] [Google Scholar]
- 154. Schulman S, Rhedin AS, Lindmarker P, et al. Duration of Anticoagulation Trial Study Group. A comparison of six weeks with six months of oral anticoagulant therapy after a first episode of venous thromboembolism. N Engl J Med. 1995;332(25):1661–1665. doi: 10.1056/NEJM199506223322501. [DOI] [PubMed] [Google Scholar]
- 155. Kaatz S, Fu AC, AbuDagga A, et al. Association between anticoagulant treatment duration and risk of venous thromboembolism recurrence and bleeding in clinical practice. Thromb Res. 2014;134(4):807–813. doi: 10.1016/j.thromres.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 156. Heit JA, Mohr DN, Silverstein MD, et al. Predictors of recurrence after deep vein thrombosis and pulmonary embolism: a population-based cohort study. Arch Intern Med. 2000;160(6):761–768. doi: 10.1001/archinte.160.6.761. [DOI] [PubMed] [Google Scholar]
- 157. Schulman S, Lindmarker P, Holmstrom M, et al. Post-thrombotic syndrome, recurrence, and death 10 years after the first episode of venous thromboembolism treated with warfarin for 6 weeks or 6 months. J Thromb Haemost. 2006;4(4):734–742. doi: 10.1111/j.1538-7836.2006.01795.x. [DOI] [PubMed] [Google Scholar]
- 158. Agnelli G, Becattini C. Treatment of DVT: how long is enough and how do you predict recurrence. J Thromb Thrombolysis. 2008;25(1):37–44. doi: 10.1007/s11239-007-0103-z. [DOI] [PubMed] [Google Scholar]
- 159. Nijkeuter M, Sohne M, Tick LW, et al. The natural course of hemodynamically stable pulmonary embolism: Clinical outcome and risk factors in a large prospective cohort study. Chest. 2007;131(2):517–523. doi: 10.1378/chest.05-2799. [DOI] [PubMed] [Google Scholar]
- 160. Agnelli G, Prandoni P, Santamaria MG, et al. Warfarin Optimal Duration Italian Trial Investigators. Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. N Engl J Med. 2001;345(3):165–169. doi: 10.1056/NEJM200107193450302. [DOI] [PubMed] [Google Scholar]
- 161. Pinede L, Ninet J, Duhaut P, et al. Comparison of 3 and 6 months of oral anticoagulant therapy after a first episode of proximal deep vein thrombosis or pulmonary embolism and comparison of 6 and 12 weeks of therapy after isolated calf deep vein thrombosis. Circulation. 2001;103(20):2453–2460. doi: 10.1161/01.cir.103.20.2453. [DOI] [PubMed] [Google Scholar]
- 162. Heit JA. Predicting the risk of venous thromboembolism recurrence. Am J Hematol. 2012;87(Suppl 1):S63–S67. doi: 10.1002/ajh.23128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Kyrle PA, Minar E, Bialonczyk C, et al. The risk of recurrent venous thromboembolism in men and women. N Engl J Med. 2004;350(25):2558–2563. doi: 10.1056/NEJMoa032959. [DOI] [PubMed] [Google Scholar]
- 164. Eichinger S, Hron G, Bialonczyk C, et al. Overweight, obesity, and the risk of recurrent venous thromboembolism. Arch Intern Med. 2008;168(15):1678–1683. doi: 10.1001/archinte.168.15.1678. [DOI] [PubMed] [Google Scholar]
- 165. Eichinger S, Pecheniuk NM, Hron G, et al. High-density lipoprotein and the risk of recurrent venous thromboembolism. Circulation. 2007;115(12):1609–1614. doi: 10.1161/CIRCULATIONAHA.106.649954. [DOI] [PubMed] [Google Scholar]
- 166. Hansson PO, Sorbo J, Eriksson H. Recurrent venous thromboembolism after deep vein thrombosis: incidence and risk factors. Arch Intern Med. 2000;160(6):769–774. doi: 10.1001/archinte.160.6.769. [DOI] [PubMed] [Google Scholar]
- 167. Iorio A, Kearon C, Filippucci E, et al. Risk of recurrence after a first episode of symptomatic venous thromboembolism provoked by a transient risk factor: a systematic review. Arch Intern Med. 2010;170(19):1710–1716. doi: 10.1001/archinternmed.2010.367. [DOI] [PubMed] [Google Scholar]
- 168. Agnelli G, Prandoni P, Becattini C, et al. Extended oral anticoagulant therapy after a first episode of pulmonary embolism. Ann Intern Med. 2003;139(1):19–25. doi: 10.7326/0003-4819-139-1-200307010-00008. [DOI] [PubMed] [Google Scholar]
- 169. Ridker PM, Goldhaber SZ, Danielson E, et al. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med. 2003;348(15):1425–1434. doi: 10.1056/NEJMoa035029. [DOI] [PubMed] [Google Scholar]
- 170. Kearon C, Ginsberg JS, Kovacs MJ, et al. Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med. 2003;349(7):631–639. doi: 10.1056/NEJMoa035422. [DOI] [PubMed] [Google Scholar]
- 171. Schulman S, Kearon C, Kakkar AK, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013;368(8):709–718. doi: 10.1056/NEJMoa1113697. [DOI] [PubMed] [Google Scholar]
- 172. Agnelli G, Buller HR, Cohen A, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2013;368(8):699–708. doi: 10.1056/NEJMoa1207541. [DOI] [PubMed] [Google Scholar]
- 173. Hovens MM, Snoep JD, Tamsma JT, Huisman MV. Aspirin in the prevention and treatment of venous thromboembolism. J Thromb Haemost. 2006;4(7):1470–1475. doi: 10.1111/j.1538-7836.2006.01928.x. [DOI] [PubMed] [Google Scholar]
- 174. Brighton TA, Eikelboom JW, Mann K, et al. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med. 2012;367(21):1979–1987. doi: 10.1056/NEJMoa1210384. [DOI] [PubMed] [Google Scholar]
- 175. Becattini C, Agnelli G, Schenone A, et al. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med. 2012;366(21):1959–1967. doi: 10.1056/NEJMoa1114238. [DOI] [PubMed] [Google Scholar]
- 176. Simes J, Becattini C, Agnelli G, et al. Aspirin for the Prevention of Recurrent Venous Thromboembolism: The INSPIRE Collaboration. Circulation. 2014;130(13):1062–1071. doi: 10.1161/CIRCULATIONAHA.114.008828. [DOI] [PubMed] [Google Scholar]
- 177. Goldhaber SZ. Prevention of recurrent idiopathic venous thromboembolism. Circulation. 2004;110(24 Suppl 1):IV20–IV24. doi: 10.1161/01.CIR.0000150641.65000.f2. [DOI] [PubMed] [Google Scholar]
- 178. Zhu T, Martinez I, Emmerich J. Venous thromboembolism: risk factors for recurrence. Arterioscler Thromb Vasc Biol. 2009;29(3):298–310. doi: 10.1161/ATVBAHA.108.182428. [DOI] [PubMed] [Google Scholar]
- 179. Eichinger S, Heinze G, Jandeck LM, Kyrle PA. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation. 2010;121(14):1630–1636. doi: 10.1161/CIRCULATIONAHA.109.925214. [DOI] [PubMed] [Google Scholar]
- 180. Tosetto A, Iorio A, Marcucci M, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH) J Thromb Haemost. 2012;10(6):1019–1025. doi: 10.1111/j.1538-7836.2012.04735.x. [DOI] [PubMed] [Google Scholar]
- 181. Ruiz-Gimenez N, Suarez C, Gonzalez R, et al. Findings from the RIETE Registry . Predictive variables for major bleeding events in patients presenting with documented acute venous thromboembolism. Thromb Haemost. 2008;100(1):26–31. doi: 10.1160/TH08-03-0193. [DOI] [PubMed] [Google Scholar]
- 182. Eichinger S, Weltermann A, Philipp K, et al. Prospective evaluation of hemostatic system activation and thrombin potential in healthy pregnant women with and without factor V Leiden. Thromb Haemost. 1999;82(4):1232–1236. [PubMed] [Google Scholar]
- 183. Heit JA, Kobbervig CE, James AH, et al. Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30-year population-based study. Ann Intern Med. 2005;143(10):697–706. doi: 10.7326/0003-4819-143-10-200511150-00006. [DOI] [PubMed] [Google Scholar]
- 184. Sultan AA, Tata LJ, West J, et al. Risk factors for first venous thromboembolism around pregnancy: a population-based cohort study from the United Kingdom. Blood. 2013;121(19):3953–3961. doi: 10.1182/blood-2012-11-469551. [DOI] [PubMed] [Google Scholar]
- 185. Liu S, Rouleau J, Joseph KS, et al. Epidemiology of pregnancy-associated venous thromboembolism: a population-based study in Canada. J Obstet Gynaecol Can. 2009;31(7):611–620. doi: 10.1016/S1701-2163(16)34240-2. [DOI] [PubMed] [Google Scholar]
- 186. Pomp ER, Lenselink AM, Rosendaal FR, Doggen CJ. Pregnancy, the postpartum period and prothrombotic defects: risk of venous thrombosis in the MEGA study. J Thromb Haemost. 2008;6(4):632–637. doi: 10.1111/j.1538-7836.2008.02921.x. [DOI] [PubMed] [Google Scholar]
- 187. Chan WS, Spencer FA, Ginsberg JS. Anatomic distribution of deep vein thrombosis in pregnancy. CMAJ. 2010;182(7):657–660. doi: 10.1503/cmaj.091692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Kawaguchi S, Yamada T, Takeda M, et al. Changes in d-dimer levels in pregnant women according to gestational week. Pregnancy Hypertens. 2013;3(3):172–177. doi: 10.1016/j.preghy.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 189. Le Gal G, Kercret G, Ben Yahmed K, et al. Diagnostic value of single complete compression ultrasonography in pregnant and postpartum women with suspected deep vein thrombosis: prospective study. BMJ. 2012;344:e2635. doi: 10.1136/bmj.e2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Eskandari MK, Sugimoto H, Richardson T, et al. Is color-flow duplex a good diagnostic test for detection of isolated calf vein thrombosis in high-risk patients? Angiology. 2000;51(9):705–710. doi: 10.1177/000331970005100901. [DOI] [PubMed] [Google Scholar]
- 191. Ralli E, Zezza L, Caserta D. Pregnancy and venous thromboembolism . Curr Opin Obstet Gynecol. 2014;26(6):469–475. doi: 10.1097/GCO.0000000000000115. [DOI] [PubMed] [Google Scholar]
- 192. Chan WS, Rey E, Kent NE, et al. Venous thromboembolism and antithrombotic therapy in pregnancy. J Obstet Gynaecol Can. 2014;36(6):527–553. doi: 10.1016/s1701-2163(15)30569-7. [DOI] [PubMed] [Google Scholar]
- 193. Marik PE, Plante LA. Venous thromboembolic disease and pregnancy. N Engl J Med. 2008;359(19):2025–2033. doi: 10.1056/NEJMra0707993. [DOI] [PubMed] [Google Scholar]
- 194. Chan WS, Spencer FA, Lee AY, et al. Safety of withholding anticoagulation in pregnant women with suspected deep vein thrombosis following negative serial compression ultrasound and iliac vein imaging. CMAJ. 2013;185(4):E194–E200. doi: 10.1503/cmaj.120895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Romualdi E, Dentali F, Rancan E, et al. Anticoagulant therapy for venous thromboembolism during pregnancy: a systematic review and a meta-analysis of the literature. J Thromb Haemost. 2013;11(2):270–281. doi: 10.1111/jth.12085. [DOI] [PubMed] [Google Scholar]
- 196. Bates SM, Greer IA, Middeldorp S, et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl): e691S – e736S . doi: 10.1378/chest.11-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. European Society of G; Association for European Paediatric C; German Society for Gender M. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC) Eur Heart J. 2011;32(24):3147–3197. doi: 10.1093/eurheartj/ehr218. [DOI] [PubMed] [Google Scholar]
- 198. Brill-Edwards P, Ginsberg JS, Gent M, et al. Safety of withholding heparin in pregnant women with a history of venous thromboembolism. Recurrence of Clot in This Pregnancy Study Group. N Engl J Med. 2000;343(20):1439–1444. doi: 10.1056/NEJM200011163432002. [DOI] [PubMed] [Google Scholar]
- 199. Bauersachs RM, Dudenhausen J, Faridi A, et al. Risk stratification and heparin prophylaxis to prevent venous thromboembolism in pregnant women. Thromb Haemost. 2007;98(6):1237–1245. doi: 10.1160/th07-05-0329. [DOI] [PubMed] [Google Scholar]
- 200. Lee AY, Levine MN. Venous thromboembolism and cancer: risks and outcomes. Circulation. 2003;107(23 Suppl 1):I17–I21. doi: 10.1161/01.CIR.0000078466.72504.AC. [DOI] [PubMed] [Google Scholar]
- 201. Schulman S, Zondag M, Linkins L, et al. Recurrent venous thromboembolism in anticoagulated patients with cancer: management and short-term prognosis. J Thromb Haemost. 2015;13(6):1010–1018. doi: 10.1111/jth.12955. [DOI] [PubMed] [Google Scholar]
- 202. Sorensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343(25):1846–1850. doi: 10.1056/NEJM200012213432504. [DOI] [PubMed] [Google Scholar]
- 203. Bura A, Cailleux N, Bienvenu B, et al. Incidence and prognosis of cancer associated with bilateral venous thrombosis: a prospective study of 103 patients. J Thromb Haemost. 2004;2(3):441–444. doi: 10.1111/j.1538-7933.2004.00619.x. [DOI] [PubMed] [Google Scholar]
- 204. Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100(10):3484–3488. doi: 10.1182/blood-2002-01-0108. [DOI] [PubMed] [Google Scholar]
- 205. Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146–153. doi: 10.1056/NEJMoa025313. [DOI] [PubMed] [Google Scholar]
- 206. Lee AY, Kamphuisen PW, Meyer G, et al. Tinzaparin vs Warfarin for Treatment of Acute Venous Thromboembolism in Patients With Active Cancer: A Randomized Clinical Trial. JAMA. 2015;314(7):677–686. doi: 10.1001/jama.2015.9243. [DOI] [PubMed] [Google Scholar]
- 207. Schulman S, Goldhaber SZ, Kearon C, et al. Treatment with dabigatran or warfarin in patients with venous thromboembolism and cancer. Thromb Haemost. 2015;114(1):150–157. doi: 10.1160/TH14-11-0977. [DOI] [PubMed] [Google Scholar]
- 208. Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of venous thromboembolism in cancer patients: results from the AMPLIFY trial. J Thromb Haemost. 2015;13(12):2187–2191. doi: 10.1111/jth.13153. [DOI] [PubMed] [Google Scholar]
- 209. Larsen TB, Nielsen PB, Skjoth F, et al. Non-vitamin K antagonist oral anticoagulants and the treatment of venous thromboembolism in cancer patients: a semi systematic review and meta-analysis of safety and efficacy outcomes. PLoS One. 2014;9(12):e114445. doi: 10.1371/journal.pone.0114445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Posch F, Konigsbrugge O, Zielinski C, et al. Treatment of venous thromboembolism in patients with cancer: a network meta-analysis comparing efficacy and safety of anticoagulants. Thromb Res. 2015;136(3):582–589. doi: 10.1016/j.thromres.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Carrier M, Cameron C, Delluc A, et al. Efficacy and safety of anticoagulant therapy for the treatment of acute cancer-associated thrombosis: a systematic review and meta-analysis. Thromb Res. 2014;134(6):1214–1219. doi: 10.1016/j.thromres.2014.09.039. [DOI] [PubMed] [Google Scholar]
- 212. Akl EA, Kahale L, Barba M, et al. Anticoagulation for the long-term treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev. 2014;7:CD006650. doi: 10.1002/14651858.CD006650.pub4. [DOI] [PubMed] [Google Scholar]
- 213. Francis CW, Kessler CM, Goldhaber SZ, et al. Treatment of venous thromboembolism in cancer patients with dalteparin for up to 12 months: the DALTECAN Study. J Thromb Haemost. 2015;13(6):1028–1035. doi: 10.1111/jth.12923. [DOI] [PubMed] [Google Scholar]
- 214. van Es N, Di Nisio M, Bleker SM, et al. Edoxaban for treatment of venous thromboembolism in patients with cancer. Rationale and design of the Hokusai VTE-cancer study. Thromb Haemost. 2015;114(6):1268–1276. doi: 10.1160/TH15-06-0452. [DOI] [PubMed] [Google Scholar]
- 215. Hudson KL, Collins FS. Sharing and reporting the results of clinical trials. JAMA. 2015;313(4):355–356. doi: 10.1001/jama.2014.10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Jones CW, Handler L, Crowell KE, et al. Non-publication of large randomized clinical trials: cross sectional analysis. BMJ. 2013;347:f6104. doi: 10.1136/bmj.f6104. [DOI] [PMC free article] [PubMed] [Google Scholar]