Abstract
Background
In the last 15 years, there has been considerable interest in statin use as a means to reduce the likelihood of vascular events. Several clinical trials have shown that high-dose statin (HDS) treatment could reduce vascular events. In high-risk populations, lipid treatment guidelines have generally suggested prescribing statin up to the highest recommended dosage. However, there remains concern about the risk of intracerebral hemorrhage (ICH) with HDS treatment.
Methods
This was a national population-based cohort study from the National Health Insurance Research Database of Taiwan extending from July 2001 to December 2008. Patients with cerebrovascular or cardiovascular disease were enrolled. The HDS group was defined as those patients receiving more than 420 mg per year of atorvastatin or an equivalent potency statin. Moderate dose statin group (MDS) was defined as those patients receiving atorvastatin in amounts between 196-420 mg per year or an equivalent potency statin. Low dose statin (LDS) group was defined as those receiving less than 196 mg per year of atorvastatin or an equivalent statin. The primary endpoint is ICH. The secondary endpoints are myocardial infarction (MI), ischemic stroke (IS) and new-onset DM (NDM).
Results
A total of 5459 patients were enrolled in our study, with study participant ages ranging from 62.91 ± 11.85 years and a mean follow-up time of 2039 ± 6 days. After adjusting for age, gender, diabetes and hypertension, Cox regression analysis found ICH risk was lower in HDS and MDS groups compared with LDS (HR 0.49, 95% CI 0.26-0.91, p = 0.0246 and HR 0.45, 95% CI 0.24-0.86, p = 0.0157). The risk of IS is lower in patients with HDS treatment (HR 0.68, 95% CI 0.55-0.83, p < 0.01). However, the risk of MI and NDM incidence are not statistically significant between the different dose groups.
Conclusions
In the real-world data provided by Taiwan’s National Health Insurance research database, it was shown that patients who received a higher dose of statin had a reduced and not elevated risk of intracerebral hemorrhage.
Keywords: High-dose statin, Hyperlipidemia, Intracerebral hemorrhage, Ischemic stroke, New-onset DM
INTRODUCTION
Statin, the 3-hydroxy-3methyglutaryl coenzyme A inhibitor, is widely used in the treatment of cardiovascular and cerebrovascular disease. The JUPITER trial revealed that statin is effective in the primary prevention of cardiovascular disease.1 The PROVE-IT and TNT trials also showed that statin use is beneficial in secondary prevention.2-4 Moreover, several clinical trials showed that statin use could reduce the incidence of patient stroke, transient ischemic attack (TIA) and improve clinical outcomes.5,6 According to the 2011 ESC/EAS guidelines, people with high-risk characteristics should be prescribed the highest tolerable statin dose to reduce the incidence of cardiovascular disease.7 The 2013 ACC/AHA guideline for dyslipidemia also suggests using high to moderate-intensity statin for primary and secondary prevention in high-risk populations.8
However, there are some concerns about the safety of high dose statin on intracerebral hemorrhage (ICH) and new-onset diabetes mellitus (NDM). Especially, post hoc analysis of the SPARCL trial and some other studies revealed that statin therapy might increase intracerebral hemorrhage risk.9-11 However, in real world practice, there is scarce data to show the relative risk of ICH when statins are prescribed in different doses. Therefore, we analyzed the data from the Taiwan National Health Insurance database (NHIRD) and investigated the association between the incidences of intracerebral hemorrhage (ICH), myocardial infarction (MI), ischemic stroke (IS), NDM and different statin dose use.
MATERIALS AND METHODS
We enrolled patients from the Taiwan National Health Research Database (NHIRD). The Taiwan National Health Insurance (NHI) program, which has operated since 1995, enrolls virtually all the residents of Taiwan. It offered a comprehensive, unified, and universal health insurance program to all citizens. Those citizens who have established a registered domicile for at least 4 months in the Taiwan area should be enrolled in NHI program. By the end of 2004, the coverage rate in Taiwan was 99%. Therefore, the NHIRD is one of the largest and most complete nationwide population-based datasets in Taiwan and there were no statistically significant differences in age, sex, or health care costs between the sample group and all beneficiaries under the NHI program. In cooperation with the Bureau of NHI, the National Health Research Institute (NHRI) of Taiwan randomly sampled a representative database of 1,000,000 patients from the year 2005 registry of all NHI enrollees using a systematic sampling method for research purposes.
Study sample
This study population enrolled all patients having inpatient claims with a diagnosis of cerebrovascular or cardiovascular disease, including intracerebral hemorrhage (ICD-9-CM: 430.xx, 431.xx, 767.0, 772.2), acute or chronic ischemic cerebrovascular disease (434.xx, 436. xx, V17.1), and coronary heart disease (CHD, ICD-9-CM: 410.xx, 411.xx, 412.xx, 414.xx) between July 1, 2001, and December 31, 2008. During that period, all patients should have received statin treatment if they were otherwise eligible. The data of a patient’s first claim with cardiovascular or cerebrovascular disease diagnosis was considered the index date. In accordance with the Anatomical, Therapeutic and Chemical Classification System with Defined Daily Dose (ATC/DDD), we selected simvastatin, lovastatin, atorvastatin, fluvastatin, pravastatin, and rosuvastatin as the major study drugs of interest. The equivalent potency of statin other than atorvastatin was that simvastatin was 0.5, lovastatin and pravastatin were 0.25, fluvastatin was 0.125 and rosuvastatin was 2 by ATC/DDD definition. Claims for treatment were identified in the 365 days after each patient’s index date. In order to reveal the different effects of statin dose, we calculated accumulated dose per year of atorvastatin or equivalent potency statin dosage of patients and equally divided dosages into high-dose statin (HDS), moderate-dose statin (MDS) and low-dose statin (LDS) groups. The HDS group was defined as those patients taking more than atorvastatin 420 mg equivalent potency statin per year. The MDS group was defined as those taking atorvastatin 196-420 mg per year or equivalent potency statin. The LDS group was defined as those taking equal to or less than atorvastatin 196 mg equivalent potency statin per year from the NHIRD records.
We analyzed the end-points of this study in two ways. First, we classified all enrolled patients into HDS, MDS and LDS groups (Figure 1), and then prospectively followed the incidence of intracerebral hemorrhage, myocardial infarction, ischemic stroke and new-onset diabetes events after statin treatment as end-points. Second, because hyperlipidemia was a significant risk factor for cardiovascular and cerebrovascular disease, we initially separated two sub-groups of patients with or without hyperlipidemia diagnosis (ICD-9-CM code: 272.2, 272.4, 272.9) and re-calculated the incidence of end-points to exclude baseline confounding factor.
Figure 1.
Flow chart of study method.
Statistical analysis
The Chi-square test and one-way ANOVA were used to compare the distribution of categorical and continuous characteristics between different dose statin treatments. Time-to-event analysis involved estimating the probability that an event will occur at different time points. The follow-up time for events began at the date of diagnosis and ended at the date of different events as they developed, or upon last observation up to December 31, 2008. The Kaplan-Meier estimates were computed for the risk among different categories compared by Log-rank tests. The primary endpoint was intracerebral hemorrhage, and the secondary end-points were myocardial infarction, ischemic stroke and new-onset DM.
Two sets of hazard rate ratios (HR) were computed for analysis factors by Cox regression analyses. The univariate HR was estimated from separate Cox regressions focusing on one analysis factor at a time. The multivariable-adjusted HR is computed from Cox regression with additional variable adjustments including gender, age, diabetes and hypertension (ICD-9: 401-405). All data processing and statistical analysis were performed with SAS 9.2 software. The p value less than 0.05 is considered statistically significant.
RESULTS
Clinical characteristics
A total of 5459 patients were enrolled, with a mean age of 62.91 ± 11.85 years; the mean follow-up time was 2039 ± 6 days. Patients in the HDS group were younger and had a higher incidence of CHD. There were 4256 patients with and 1203 patients without any history of hyperlipidemia. There were 2177 patients taking more than 420 mg atorvastatin or equivalent potency statin per year which comprised the HDS group. Also, 1617 patients taking 196-420 mg atorvastatin or equivalent potency statin per year were in the MDS group. Lastly, 1665 patients taking less than 196 mg atorvastatin or equivalent potency statin per year were deemed the low statin (LDS) treatment group. The baseline characteristics of patients are shown in Table 1. The total of the followed patient cardiovascular, cerebrovascular and new-onset DM events of enrolled patients are listed in Table 2.
Table 1. Baseline characteristics of study patients .
| Variable, n (%) | Total patients (n = 5459) | LDS group (n = 1665) | MDS group (n = 1617) | HDS group (n = 2177) | p-value | |
| Gender | Female | 2309 | 730 (43.8) | 719 (44.5) | 860 (39.5) | 0.003 |
| Male | 3150 | 935 (56.2) | 898 (55.5) | 1317 (60.5) | ||
| Age | 62.91 ± 11.85 | 63.62 ± 11.92 | 62.87 ± 11.80 | 62.40 ± 11.82 | 0.007 | |
| Hypertension | 4041 | 1240 (74.5) | 1167 (72.2) | 1634 (75.1) | 0.12 | |
| Hyperlipidemia | 3893 | 1123 (67.4) | 1132 (70.0) | 1638 (75.2) | < 0.001 | |
| Diabetes mellitus | 2458 | 764 (45.9) | 733 (45.3) | 961 (44.1) | 0.54 |
HDS, high-dose statin; LDS, low-dose statin; MDS, moderate-dose statin.
Table 2. Cardiovascular and cerebrovascular events during follow-up .
| Variable, n (%) | Total patients event (n = 5459) | LDS group (n = 1665) | MDS group (n = 1617) | HDS group (n = 2177) | p-value |
| ICH | 59 | 31 (1.9) | 13 (0.8) | 15 (0.7) | < 0.001 |
| Myocardial Infarction | 1664 | 552 (33.2) | 532(32.9) | 580 (26.6) | < 0.001 |
| Ischemic stroke | 584 | 233 (14.0) | 191 (11.8) | 160 (7.3) | < 0.001 |
| New-onset DM | 436 | 143 (17.8) | 141 (18.1) | 152 (14.3) | 0.05 |
HDS, high-dose statin; ICH, intracerebral hemorrhage; LDS, low-dose statin; MDS, moderate-dose statin.
Intracerebral hemorrhage
In the entire studied population, Kaplan-Meier survival analysis showed that patients in the LDS had a higher risk of ICH (p = 0.0251) (Figure 2). Cox regression found that both HDS and MDS use was associated with a lower risk of ICH compared with LDS (HR 0.49, 95% CI 0.26-0.91, p = 0.02, and HR 0.45, 95% CI 0.24-0.86, p = 0.02) (Table 3) after adjustment for gender, age, hypertension, diabetes and hyperlipidemia. The subgroup analysis found that reduction of the ICH incidence is significant in patients with hyperlipidemia history and MDS treatment (HR 0.47, 95% CI 0.23-1.95, p = 0.03). In the HDS group, there was a trend towards a lower risk of ICH in patients with hyperlipidemia compared with the LDS group (HR 0.54, 95% CI 0.28-1.04, p = 0.06). However, for patients without a history of hyperlipidemia, there was no reduction effect of ICH incidence in both the HDS and MDS groups compared with the LDS group (HR 0.24, 95% CI 0.03-1.99, p = 0.18 and HR 0.39, 95% CI 0.08-1.94, p = 0.25).
Figure 2.
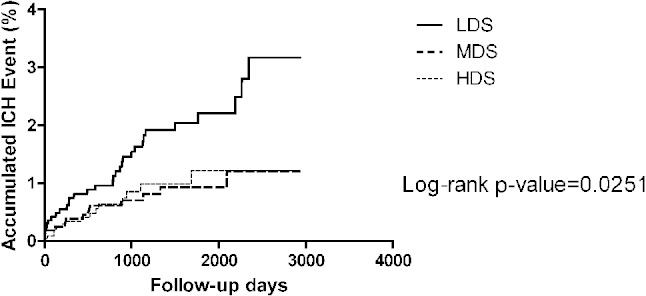
The Kaplan-Meier figure of the intracerebral hemorrhage (ICH) among different dose groups. HDS, high-dose statin; LDS, low-dose statin; MDS, moderate-dose statin.
Table 3. Risk of intracerebral hemorrhage, ischemic stoke, myocardial infarction and new-onset DM of study patients .
| Class | 1Intracerebral hemorrhage | 1Myocardial infarction | 1Ischemic stroke | 2New-onset DM | |||||||||||||
| Crude HR | Adjust HR | Adjusted 95% CI | p value | Crude HR | Adjust HR | Adjusted 95% CI | p value | Crude HR | Adjust HR | Adjusted 95% CI | p value | Crude HR | Adjust HR | Adjusted 95% CI | p value | ||
| Total population | LDS (n = 1665) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| MDS (n = 1617) | 0.45 | 0.45 | 0.24-0.86 | 0.02 | 1.01 | 1.02 | 0.90-1.15 | 0.78 | 0.85 | 0.87 | 0.72-1.05 | 0.12 | 1.05 | 1.04 | 0.83-1.32 | 0.72 | |
| HDS (n = 2177) | 0.49 | 0.49 | 0.26-0.91 | 0.02 | 0.96 | 0.97 | 0.86-1.09 | 0.55 | 0.66 | 0.68 | 0.55-0.83 | < 0.001 | 1.13 | 1.14 | 0.90-1.43 | 0.28 |
1 Adjusted variables including gender, age, diabetes and hypertension; 2 Adjusted variables including gender, age, and hypertension.
CI, confidence interval; HDS, high-dose statin; HR, hazard ratio; LDS, low-dose statin; MDS, moderate-dose statin.
Myocardial infarction
Kaplan-Meier survival analysis showed that there was no significant difference in risk of MI between the different statin dose groups (p = 0.09) (Figure 3). There was no significant reduction of MI in both HDS and MDS groups compared with the LDS group in the Cox regression analysis (HR 0.97, 95% CI 0.86-1.09, p = 0.55 and HR 1.02, 95% CI 0.90-1.15, p = 0.78) (Table 3). Furthermore, the subgroup analysis found there was a trend towards a lower risk of MI in patients without history of hyperlipidemia and HDS treatment compared with the LDS group (HR 0.72, 95% CI 0.52-1.00, p = 0.051).
Figure 3.
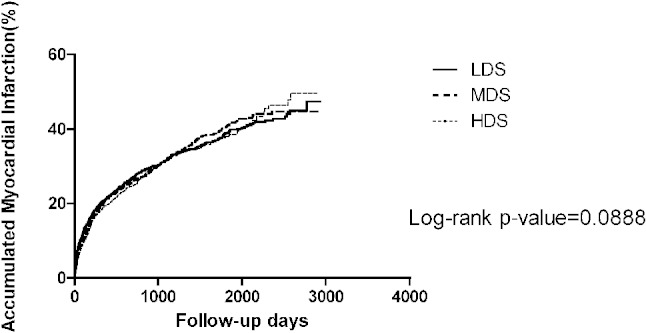
The Kaplan-Meier figure of the myocardial infarction among different dose groups. HDS, high-dose statin; LDS, low-dose statin; MDS, moderate-dose statin.
Ischemic stroke
Additionally, Kaplan-Meier survival analysis showed that higher dose of statin use was associated with lower risk of IS (p = 0.009) (Figure 4). Cox regression analysis showed that HDS use was associated with a lower risk of ischemic stroke occurrence compared with LDS group (HR 0.68, 95% CI 0.55-0.83, p < 0.01) (Table 3). The beneficial effect of HDS use in reduction of IS risk was found in patients with hyperlipidemia (HR 0.63, 95% CI 0.51-0.79, p < 0.01). For patients without a history of hyperlipidemia, there was no significantly lower IS risk whether HDS and MDS were prescribed compared with LDS use (HR 0.93, 95% CI 0.53-1.64, p = 0.79 and HR 1.03, 95% CI 0.59-1.8, p = 0.92).
Figure 4.
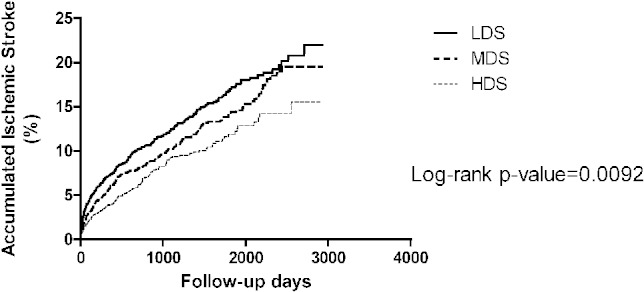
The Kaplan-Meier figure of the ischemic stroke among different dose groups. HDS, high-dose statin; LDS, low-dose statin; MDS, moderate-dose statin.
New-onset diabetes
We didn’t find a significant association between statin treatment and risk of NDM both in the Kaplan-Meier survival and Cox regression analysis (Figure 5 and Table 3). In the subgroups analysis, even with HDS treatment, there was no statistical significance of the increase of NDM risk in patients with hyperlipidemia or non-hyperlipidemia compared with LDS use (HR 1.12, 95% CI 0.87-1.42, p = 0.38 and HR 1.16, 95% CI 0.58-2.32, p = 0.68).
Figure 5.
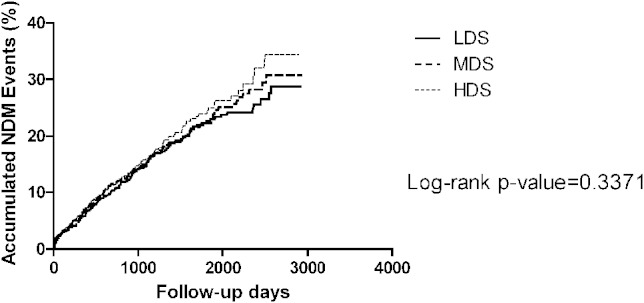
The Kaplan-Meier figure of the new-onset diabetes among different dose groups. HDS, high-dose statin; LDS, low-dose statin; MDS, moderate-dose statin.
DISCUSSION
There are three major findings in our perspective study. First, we found that a higher dose of statin use is associated with lower but not higher risk of ICH. Second, patients who received a higher dose of statin had a lower risk of ischemic stroke. Third, we didn’t find statin dose was associated with occurrence of new onset of diabetes.
In recent studies, a moderate to high intensity of statin use showed cardiovascular benefit in high-risk patients for secondary prevention.3,12 Therefore, the 2011 ESC/EAS and 2013 ACC/AHA guidelines suggested high to moderate statin intensity for preventing recurrent cardiovascular or cerebrovascular disease. In this real world study, patients who received a higher dose of statin treatment had a lower risk of ischemic stroke compared with the low statin group. Surprisingly, for patients without hyperlipidemia at baseline, there was no similarly beneficial effect of statin treatment in reducing the incidence of ischemic stroke, even under high-dose statin treatment. This implies that the protective effect of statin in ischemic stroke is significant for patients with hyperlipidemia, especially for those receiving high-dose statin. A possible explanation is that people without hyperlipidemia are at a relatively low risk. Thus, for patients without hyperlipidemia, the potential benefit of higher dose of statin treatment may require further evaluation for secondary prevention.
Although many large clinical trials had shown that statin use provided benefits in reduction of cardiovascular and cerebrovascular events both in the primary and secondary prevention,5,13,14 questions regarding the incidence of ICH after statin prescription remain unresolved.3,15,16 Previous studies indicated that statin use increases the incidence of ICH, especially in patients with a history of hemorrhagic stroke, old age or high blood pressure.17,18 Even for patients with ischemic stroke, the incidence of ICH has been found to be associated with statin use but not with basic cholesterol level.19 Low LDL level and statin treatment were considered to play a role in increasing fibrinolytic activity, and an independent risk factor for intracerebral bleeding in some clinical outcome studies.20,21 However, in our study, we analyzed real world data from Taiwan’s NHIRD and found that there was a significant reduction in the incidence of ICH in those patients with HDS and MDS treatment compared with LDS treatment. This represents a different result as compared with previous investigations. In some earlier studies, higher dose of statin provided a proven benefit for cerebrovascular disease, including acting to reduce vasospasm, anti-inflammatory, angiogenesis, neurogenesis and synaptogenesis.22,23 It also decreased the peri-hematomal cell death after ICH.24 The outcome of one clinical study was also revealed that inpatient statin cessation would be associated with worse outcomes and after ICH.25 The use of a high dose of statin may not only decrease the occurrence of ICH, but also create a better prognosis after ICH.
The beneficial effect of higher dose of statin treatment for MI was not statistical demonstrated in this study. One possible explanation is that most people in Taiwan received a relatively low dose of statin. Alternatively, some physicians may have discontinued statin prescription in 3-6 months pursuant to limitations caused by National Health Insurance reimbursement. Although recent guidelines and studies have shown that high-potency statin use could effectively reduce cardiovascular events,8,26 some reports revealed that the lower dose of statin use is actually beneficial for the Asian population.27,28 Statin response for different races, especially for Asian population, may be a possible explanation for our results. However, this proposition needs more investigation for further confirmation.
In recent years, there have been many studies showing the increase of risk of NDM after statin use.29,30 Although it may be related to attenuated adipocyte maturation and glucose transporter-4 expression, the mechanisms are still unclear.31,32 We found there is no significant difference of NDM in MDS or HDS use compared with LDS treatment, no matter whether patients were with or without a history of hyperlipidemia in this study. A possible explanation is that there are confounding factors between statin therapy and NDM such as body weight, lipid profile and glucose levels which were not obtained in the NHIRD. Besides, patients may have limited lifestyle modification or weight loss after cerebrovascular and cardiovascular events in Taiwan. These modifications may also reduce the risk of NDM. In the WOSCOPS trial, it was shown that lifestyle modification and statin prescription reduces progression to DM by 30%.33
LIMITATIONS
There were some limitations in our study. First, all of the encoded data in the national health insurance database is provided for administrative purpose, not for scientific use. However, the accuracy of prescription data about statin use and dosage has been proved in previous NHIRD research. Second, the national health insurance database could not reveal certain personal profile information such as smoking habit, body mass index or baseline lipid profiles. Therefore, we were not in the position to know with specificity the exact change of lipid profile after different statin dosage. Third, the dosage of statin provided to patients may be restricted by the insurance system itself. Therefore, this may result in the underestimation of the benefit of high dose statin prescription. Fourth, this study was designed as a secondary prevention clinical trial. For patients without cardiovascular or cerebrovascular disease, the benefits of statin, especially for ICH, still require further investigation. Fifth, many factors were associated with clinical endpoints such as the presence of atrial fibrillation, antiplatelet and anticoagulant agents. We only adjusted for gender, age, diabetes, and hypertension. However, our study did provide valuable preliminary real-world data about the use of statins in Taiwan. Further large-scale study may be needed to confirm our findings.
CONCLUSION
In our review of the data provided through the NHIRD, we have provided an analysis of real-world usage of statin drug in Taiwan. The results we derived from such study suggest that higher statin dose treatment was associated with a lower, and not a higher, risk of ICH.
Acknowledgments
This study is based in part on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health, and managed by the National Health Research Institutes (Registered number 98178). The interpretation and conclusions contained herein do not represent those of the Bureau of National Health Insurance, Department of Health or National Health Research Institutes.
At the same time, the authors wish to give thanks for assistance provided by the Statistical Analysis Laboratory, Department of Internal Medicine, Kaohsiung Medical University Hospital.
REFERENCES
- 1. Albert MA, Glynn RJ, Fonseca FA, et al. Race, ethnicity, and the efficacy of rosuvastatin in primary prevention: the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial. Am Heart J. 2011;162:106–114. doi: 10.1016/j.ahj.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 2. Murphy SA, Cannon CP, Wiviott SD, et al. Reduction in recurrent cardiovascular events with intensive lipid-lowering statin therapy compared with moderate lipid-lowering statin therapy after acute coronary syndromes from the PROVE IT-TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis In Myocardial Infarction 22) trial. J Am Coll Cardiol. 2009;54:2358–2362. doi: 10.1016/j.jacc.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 3. Mora S, Wenger NK, Demicco DA, et al. Determinants of residual risk in secondary prevention patients treated with high-versus low-dose statin therapy: the Treating to New Targets (TNT) study. Circulation. 2012;125:1979–1987. doi: 10.1161/CIRCULATIONAHA.111.088591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goldstein LB, Amarenco P, Zivin J, et al. Statin treatment and stroke outcome in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Stroke. 2009;40:3526–3531. doi: 10.1161/STROKEAHA.109.557330. [DOI] [PubMed] [Google Scholar]
- 5. Fuentes B, Martinez-Sanchez P, Diez-Tejedor E. Lipid-lowering drugs in ischemic stroke prevention and their influence on acute stroke outcome. Cerebrovasc Dis. 2009;27(Suppl 1):126–133. doi: 10.1159/000200450. [DOI] [PubMed] [Google Scholar]
- 6. Yu AY, Keezer MR, Zhu B, et al. Pre-stroke use of antihypertensives, antiplatelets, or statins and early ischemic stroke outcomes. Cerebrovasc Dis. 2009;27:398–402. doi: 10.1159/000207444. [DOI] [PubMed] [Google Scholar]
- 7. Reiner Z, Catapano AL, De Backer G, et al. ESC/EAS Guidelines for the management of dyslipidaemias. The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Atherosclerosis. 2011;217:3–46. doi: 10.1016/j.atherosclerosis.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 8. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 9. Goldstein LB, Amarenco P, Szarek M, et al. Hemorrhagic stroke in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels study. Neurology. 2008;70:2364–2370. doi: 10.1212/01.wnl.0000296277.63350.77. [DOI] [PubMed] [Google Scholar]
- 10. Manktelow BN, Potter JF. Interventions in the management of serum lipids for preventing stroke recurrence. Cochrane Database Syst Rev. 2009:Cd002091. doi: 10.1002/14651858.CD002091.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vergouwen MD, de Haan RJ, Vermeulen M, Roos YB. Effect of statin treatment on vasospasm, delayed cerebral ischemia, and functional outcome in patients with aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis update. Stroke. 2010;41:e47–e52. doi: 10.1161/STROKEAHA.109.556332. [DOI] [PubMed] [Google Scholar]
- 12. Puri R, Nissen SE, Shao M, et al. Antiatherosclerotic effects of long-term maximally intensive statin therapy after acute coronary syndrome: insights from Study of Coronary Atheroma by Intravascular Ultrasound: Effect of Rosuvastatin Versus Atorvastatin. Arterioscler Thromb Vasc Biol. 2014;34:2465–2472. doi: 10.1161/ATVBAHA.114.303932. [DOI] [PubMed] [Google Scholar]
- 13. Mora S, Glynn RJ, Boekholdt SM, et al. On-treatment non-high-density lipoprotein cholesterol, apolipoprotein B, triglycerides, and lipid ratios in relation to residual vascular risk after treatment with potent statin therapy: JUPITER (justification for the use of statins in prevention: an intervention trial evaluating rosuvastatin) J Am Coll Cardiol. 2012;59:1521–1528. doi: 10.1016/j.jacc.2011.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feher A, Pusch G, Koltai K, et al. Statin therapy in the primary and the secondary prevention of ischaemic cerebrovascular diseases. Int J Cardiol. 2011;148:131–138. doi: 10.1016/j.ijcard.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 15. Schwartz GG, Chaitman BR, Goldberger JJ, Messig M. High-dose atorvastatin and risk of atrial fibrillation in patients with prior stroke or transient ischemic attack: analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Am Heart J. 2011;161:993–999. doi: 10.1016/j.ahj.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 16. Rocco A, Sykora M, Ringleb P, Diedler J. Impact of statin use and lipid profile on symptomatic intracerebral haemorrhage, outcome and mortality after intravenous thrombolysis in acute stroke. Cerebrovasc Dis. 2012;33:362–368. doi: 10.1159/000335840. [DOI] [PubMed] [Google Scholar]
- 17. Westover MB, Bianchi MT, Eckman MH, Greenberg SM. Statin use following intracerebral hemorrhage: a decision analysis. Arch Neurol. 2011;68:573–579. doi: 10.1001/archneurol.2010.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eckman MH, Rosand J, Knudsen KA, et al. Can patients be anticoagulated after intracerebral hemorrhage? A decision analysis. Stroke. 2003;34:1710–1716. doi: 10.1161/01.STR.0000078311.18928.16. [DOI] [PubMed] [Google Scholar]
- 19. Meier N, Nedeltchev K, Brekenfeld C, et al. Prior statin use, intracranial hemorrhage, and outcome after intra-arterial thrombolysis for acute ischemic stroke. Stroke. 2009;40:1729–1737. doi: 10.1161/STROKEAHA.108.532473. [DOI] [PubMed] [Google Scholar]
- 20. Bang OY, Saver JL, Liebeskind DS, et al. Cholesterol level and symptomatic hemorrhagic transformation after ischemic stroke thrombolysis. Neurology. 2007;68:737–742. doi: 10.1212/01.wnl.0000252799.64165.d5. [DOI] [PubMed] [Google Scholar]
- 21. Haslinger B, Goedde MF, Toet KH, Kooistra T. Simvastatin increases fibrinolytic activity in human peritoneal mesothelial cells independent of cholesterol lowering. Kidney Int. 2002;62:1611–1619. doi: 10.1046/j.1523-1755.2002.00601.x. [DOI] [PubMed] [Google Scholar]
- 22. Chen J, Zhang ZG, Li Y, et al. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- 23. Amin-Hanjani S, Stagliano NE, Yamada M, et al. Mevastatin, an HMG-CoA reductase inhibitor, reduces stroke damage and upregulates endothelial nitric oxide synthase in mice. Stroke. 2001;32:980–986. doi: 10.1161/01.str.32.4.980. [DOI] [PubMed] [Google Scholar]
- 24. Jung KH, Chu K, Jeong SW, et al. HMG-CoA reductase inhibitor, atorvastatin, promotes sensorimotor recovery, suppressing acute inflammatory reaction after experimental intracerebral hemorrhage. Stroke. 2004;35:1744–1749. doi: 10.1161/01.STR.0000131270.45822.85. [DOI] [PubMed] [Google Scholar]
- 25. Flint AC, Conell C, Rao VA, et al. Effect of statin use during hospitalization for intracerebral hemorrhage on mortality and discharge disposition. JAMA Neurol. 2014;71:1364–1371. doi: 10.1001/jamaneurol.2014.2124. [DOI] [PubMed] [Google Scholar]
- 26. Pauriah M, Elder DH, Ogston S, et al. High-potency statin and ezetimibe use and mortality in survivors of an acute myocardial infarction: a population-based study. Heart. 2014;100:867–872. doi: 10.1136/heartjnl-2013-304678. [DOI] [PubMed] [Google Scholar]
- 27. Itakura H, Kita T, Mabuchi H, et al. Relationship between coronary events and serum cholesterol during 10 years of low-dose simvastatin therapy: long-term efficacy and safety in Japanese patients with hypercholesterolemia in the Japan Lipid Intervention Trial (J-LIT) Extension 10 Study, a prospective large-scale observational cohort study. Circ J. 2008;72:1218–1224. doi: 10.1253/circj.72.1218. [DOI] [PubMed] [Google Scholar]
- 28. Nakaya N, Mizuno K, Ohashi Y, et al. Low-dose pravastatin and age-related differences in risk factors for cardiovascular disease in hypercholesterolaemic Japanese: analysis of the management of elevated cholesterol in the primary prevention group of adult Japanese (MEGA study) Drugs and Aging. 2011;28:681–692. doi: 10.2165/11595620-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 29. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–742. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 30. Shen L, Shah BR, Reyes EM, et al. Role of diuretics, beta blockers, and statins in increasing the risk of diabetes in patients with impaired glucose tolerance: reanalysis of data from the NAVIGATOR study. BMJ. 2013;347:f6745. doi: 10.1136/bmj.f6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nakata M NS, Kusaka I, Matsuoka H, et al. Effects of statins on the adipocyte maturation and expression of glucose transporter 4 (SLC2A4): implications in glycaemic control. Diabetologia. 2006;49:1881–1892. doi: 10.1007/s00125-006-0269-5. [DOI] [PubMed] [Google Scholar]
- 32. Bang CN, Okin PM. Statin treatment, new-onset diabetes, and other adverse effects: a systematic review. Curr Cardiol Rep. 2014;16:461. doi: 10.1007/s11886-013-0461-4. [DOI] [PubMed] [Google Scholar]
- 33. Takagi T, Matsuda M, Abe M, et al. Effect of pravastatin on the development of diabetes and adiponectin production. Atherosclerosis. 2008;196:114–121. doi: 10.1016/j.atherosclerosis.2007.02.013. [DOI] [PubMed] [Google Scholar]



