Abstract
Background
Given the favorable impact of α1-blockers on lipid and glucose metabolism, this study was designed to compare the efficacy of two extended-release α1-blockers (bunazosin and doxazosin) as an add-on treatment in subjects with stage 1 or 2 essential hypertension which was inadequately controlled by valsartan 80 mg/day.
Methods
After a 5-week treatment of valsartan monotherapy, subjects with inadequately controlled hypertension were randomized to receive either extended-release bunazosin (n = 47) or doxazosin (n = 46) after breakfast for 8 weeks. Office sitting blood pressure (BP), 24-hour ambulatory BP, and metabolic profiles were measured at baseline, start of study drug, and study end.
Results
In the intention-to-treat population (n = 93), the average daily doses of bunazosin and doxazosinwere 2.8 mg and 3.6 mg, respectively. The two add-on treatments achieved significant and similar BP reductions from monotherapy (bunazosin, 13.2/9.3 mmHg; doxazosin, 9.2/8.5 mmHg, all p < 0.001). However, in patients with stage 2 hypertension, patients randomized to the bunazosin group, compared to those in the doxazosin group, achieved a significantly greater reduction in sitting systolic BP (14.4 ± 8.1 vs. 6.6 ± 13.8 mmHg, p = 0.015). In addition, patients who received bunazosin had significant changes in night-day systolic and diastolic BP ratios compared with those who received doxazosin (-0.02 vs. 0.02, p = 0.04 and 0 vs. 0.04, p = 0.04). No significant changes in metabolic profiles were observed in both add-on groups. Both drugs were well-tolerated, but adverse events related to the study drugs were marginally more frequent in the doxazosin group than in the bunazosin group (20% vs. 6%, p = 0.058).
Conclusions
Both extended-release bunazosin and doxazosinwerewell-tolerated and similarly effective as add-on therapy in hypertensive patients uncontrolled by valsartan monotherapy. However, add-on treatment with bunazosin seemed to be associated with favorable night-day BP ratio and greater sitting systolic BP reductions in stage 2 hypertensive patients.
Keywords: Combination therapy, Hypertension, α1-blocker
INTRODUCTION
Most clinical studies and large-scale trials have shown the importance of reducing blood pressure (BP) to help prevent cardiovascular events in hypertensive patients.1,2 However, only a limited number of hypertensive patients achieve their target BP by monotherapy.3 To obtain optimal control of BP, it has been reported that the combined use of multiple anti-hypertensive drugs might be necessary, particularly in diabetic, renal and high-risk patients.4,5
Based on the findings of the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), α1-blockers are not considered as first-line anti-hypertensive drugs in most hypertension treatment guidelines.3,6,7 Nevertheless, it has been shown that α1-blockers are effective in reducing BP as second- or third-line anti-hypertensive agents.8-11 In addition to their anti-hypertensive property, α1-blockers also exert a favorable impact on both glucose and lipid metabolism.12-15 Even more, α1-blockers can alleviate urinary symptoms associated with benign prostatic hypertrophy. They are therefore indicated, when co-administered with other anti-hypertensive agents, in hypertensive patients with clinical features indicating metabolic syndrome and/or benign prostatic hypertrophy.16
Drugs in the class of α1-blockers include bunazosin, doxazosin, terazosin and prazosin. Among these, do-xazosin has been the most studied. Bunazosin, a qu-inazoline derivative α1-blocker structurally analogous to prazosin, also exerts a good BP-lowering effect as well as having positive impacts on lipid and glucose profiles.13,17,18 In this study, we aimed to evaluate the ef-ficacy of these two extended-release formulations of α1-blockers, bunazosin and doxazosin, as second-line anti-hypertensive agents, in stages 1 and 2 Taiwanese hypertensive patients who were inadequately controlled by an angiotensin II antagonist, valsartan. This study was registered with ClinicalTrials.gov, number NCT 00130156.
METHODS
Patients
We enrolled patients with stage 1 or 2 essential hypertension [systolic BP (SBP) ≥ 140 mmHg but < 180 mmHg and/or diastolic BP (DBP) ≥ 90 mmHg but < 110 mmHg] who were 20 to 80 years of age. Patients were excluded if they had heart failure, atrial fibrillation, a history of myocardial infarction, prior cerebrovascular accident, poorly controlled diabetes mellitus (HbA1c > 10%), severe obesity (body mass index > 30 kg/m2), malignancy, and significant hepatic or renal disease. During the study period, patients were not allowed to take non-study anti-hypertensive and lipid-lowering drugs.
Study design
This was a single-center, randomized, open-label, parallel group, active-controlled study. Every subject started with a 2-week placebo run-in phase. Eligible subjects were treated with 80 mg valsartan once daily after breakfast for 5 weeks. Patients who showed adequate responses, i.e. SBP < 140 mmHg and DBP < 90 mmHg or BP reduction > 10%, were excluded from this study. The remaining patients then received an extended-release formulation of α1-blocker, either buna-zosin or doxazosin once daily after breakfast for 8 weeks. The initial dose of bunazosin and doxazosin was 3 mg and 4 mg respectively; 4 weeks later, there was a titration accompanied by a double dose of bunazosin or doxazosin if patients had inadequate BP control (SBP ≥ 140 mmHg or DBP ≥ 90 mmHg).
The primary efficacy outcome was to compare the reduction of sitting SBP and DBP as well as 24-hour ambulatory BP. Blood pressure was measured by mercurial sphygmomanometer after a 15-minute rest. Measurements were repeated 3 times, and taken at 2-minute intervals. The 24-hour ambulatory BP measurements were performed after a 5-week valsartan monotherapy (start of add-on therapy) and after an 8-week α1-blocker add-on therapy. The secondary outcome included changes of waist circumference, body weight, lipid profiles and fasting glucose level. Safety was evaluated by assessing adverse events and routine physical and labo-ratory examinations in each visit.
This study was approved by the Ethics Committee of National Taiwan University Hospital. The study protocol was conducted in accordance with the Declaration of Helsinki and regulations of the Taiwan Department of Health. All study patients provided written informed consent.
Statistical analysis
Our target sample size was approximately 70 patients. This assumption was made based on previous studies on α1-blockers, to detect a difference in SBP change of 4 mmHg with a standard deviation of 6 mmHg between both groups. Because an exclusion rate of 40% (ineligible to reach the add-on therapy stage) was expected, more than 117 patients should be enrolled. Statistical comparisons for baseline demographic variables were carried out (two-tailed), with an associated 5% level of significance. The Wilcoxon rank sum test was used for continuous variables and the Fisher’s exact test was used for categorical variables.
The efficacy analyses were performed in the intention-to-treat population, which included all patients who were randomized to an add-on treatment and received at least one dose of study medication. The last-observation-carried-forward method was used for the last visit analyses. The primary statistical analysis was comparison of BP changes within and between two treatment groups, which were analyzed by Wilcoxon signed-rank test and Wilcoxon rank sum test, respectively. Pre-specified subgroup analyses in patients stratified by the stages of baseline BP and the presence or absence of the metabolic syndrome were also performed.
RESULTS
Patient disposition
One hundred and thirty-three patients were enrolled in our study, and the disposition of study patients is shown in Figure 1. Forty (30%) of them had adequate responses to the 5-week valsartan monotherapy and were ineligible for the subsequent add-on α1-blocker treatment. Thus, 93 patients (51 men, 42 women) were randomized to receive bunazosin (n = 47) or doxazosin (n = 46). Thereafter, all of them formed the intention-to-treat population. The baseline characteristics of the two add-on treatment groups were similar (Table 1). Based on data obtained after the 2-week run-in period, forty-six patients had stage 1 hypertension (bunazosin, n = 25; doxazosin, n = 21), and the rest 47 patients had stage 2 hypertension (bunazosin, n = 22; doxazosin, n = 25).
Figure 1.
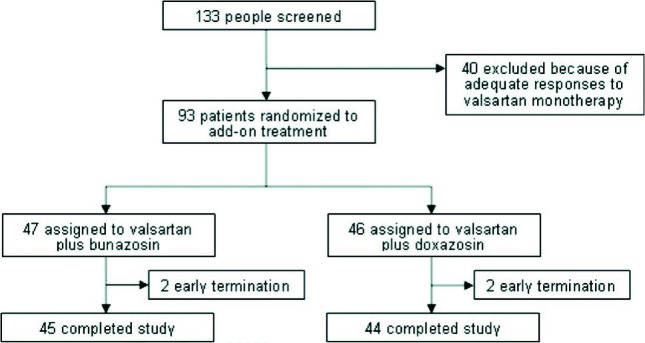
Disposition of study patients.
Table 1. Baseline demographic and clinical characteristics of patients .
| Bunazosin group (N = 47) | Doxazosin group (N = 46) | p value | |
| Age, years | 54.0 ± 10.8 | 55.0 ± 9.6 | 0.546 |
| Male, n (%) | 26 (55.3) | 25 (54.4) | 0.999 |
| Body weight, kg | 70.8 ± 11.2 | 66.8 ± 10.3 | 0.060 |
| Waist circumference, cm | 89.5 ± 8.5 | 87.5 ± 6.5 | 0.459 |
| Current smoker, n (%) | 4 (8.5) | 1 (2.2) | 0.361 |
| Treated hypertension | 30 (63.8) | 26 (56.5) | 0.528 |
| Systolic BP, mmHg | 143.1 ± 8.40 | 139.3 ± 11.7 | 0.211 |
| Diastolic BP, mmHg | 93.0 ± 7.0 | 91.5 ± 7.1 | 0.412 |
| Total cholesterol (mg/dl) | 206.4 ± 34.9 | 203.2 ± 45.9 | 0.403 |
| Triglycerides (mg/dl) | 138.7 ± 77.9 | 152.9 ± 97.6 | 0.634 |
| HDL-C (mg/dl) | 45.9 ± 11.8 | 43.9 ± 11.8 | 0.365 |
| LDL-C (mg/dl) | 133.5 ± 30.2 | 128.4 ± 39.3 | 0.308 |
| Fasting glucose (mg/dl) | 96.2 ± 15.9 | 100.2 ± 26.1 | 0.845 |
Values are number (%) or mean ± SD. BP, blood pressure; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Four patients did not complete the study. Two patients in the doxazosin group discontinued due to adverse events, and two patients in the bunazosin group withdrew their consent.
Efficacy
Both add-on treatment groups achieved significant reductions in sitting SBP and DBP after 8-week α1-blocker add-on therapy (p < 0.001), but the difference between the two groups did not reach statistical significance (bunazosin, 13.2/9.3 mmHg; doxazosin, 9.2/8.5 mmHg) (Table 2). However, there was a trend (p = 0.052) towards a greater reduction in sitting SBP in the bunazosin group. The response rate was similar between both groups (bunazosin, 80.9% vs. doxazosin, 78.3%, p = 0.802). The average daily doses of bunazosin and doxazosin were 2.8 mg and 3.6 mg (p = 0.087), respectively, in the intention-to-treat population.
Table 2. Changes in sitting systolic and diastolic BP after add-on treatment with α1-blockers .
| Valsartan + Bunazosin (N = 47) | Valsartan + Doxazosin (N = 46) | p value (between groups) | |
| Systolic BP (mmHg) | |||
| Start of add-on therapy | 143.1 ± 8.4 | 139.3 ± 11.7 | 0.211 |
| Study end | 129.9 ± 10.2 | 130.1 ± 16.6 | 0.533 |
| Change | -13.2 ± 8.7 | -9.2 ± 15.0 | 0.052 |
| Percentage change | -9.2 ± 6.0% | -6.5 ± 9.6% | 0.077 |
| p value (within group) | < 0.001 | < 0.001 | |
| Diastolic BP (mmHg) | |||
| Start of add-on therapy | 93.0 ± 7.0 | 91.5 ± 7.1 | 0.412 |
| Study end | 83.8 ± 7.0 | 82.9 ± 10.2 | 0.460 |
| Change | -9.3 ± 5.0 | -8.5 ± 6.6 | 0.746 |
| Percentage change | -9.9 ± 5.2% | -9.5 ± 7.4% | 0.842 |
| p value (within group) | < 0.001 | < 0.001 |
BP, blood pressure.
In patients with stage 2 hypertension, treatment with bunazosin caused greater SBP reduction than with doxazosin (14.4 ± 8.1 vs. 6.6 ± 13.8 mmHg, p = 0.015, Table 3). However, in patients with stage 1 hypertension, the two add-on treatments achieved a similar drop in SBP and DBP. Patients with or without the metabolic syndrome did not result in significant differences in treatment-related BP changes between them (data not shown).
Table 3. Changes in sitting systolic and diastolic BP after add-on treatment with α1-blockers in stage 1 and stage 2 hypertensive patients .
| Stage 1 hypertension at baseline | Valsartan + Bunazosin (N = 25) | Valsartan + Doxazosin (N = 21) | p value (between groups) |
| Systolic BP (mmHg) | |||
| Start of add-on therapy | 140.6 ± 5.3 | 138.6 ± 11.1 | 0.894 |
| Study end | 128.4 ± 12.0 | 126.3 ± 13.8 | 0.433 |
| Change | -12.2 ± 9.2 | -12.3 ± 13.8 | 0.825 |
| p value (within group) | < 0.001 | < 0.001 | |
| Diastolic BP (mmHg) | |||
| Start of add-on therapy | 91.7 ± 4.8 | 89.2 ± 6.8 | 0.204 |
| Study end | 82.3 ± 7.0 | 79.5 ± 8.6 | 0.255 |
| Change | -9.4 ± 5.2 | -9.7 ± 5.6 | 0.947 |
| Percentage change | -10.1 ± 5.6% | -10.1 ± 5.9% | 0.802 |
| p value (within group) | < 0.001 | < 0.001 | |
| Stage 2 hypertension at baseline | Valsartan + Bunazosin (N = 22) | Valsartan + Doxazosin (N = 25) | p value (between groups) |
| Systolic BP (mmHg) | |||
| Start of add-on therapy | 145.9 ± 10.4 | 139.9 ± 12.3 | 0.098 |
| Study end | 131.6 ± 9.3 | 133.3 ± 18.2 | 0.693 |
| Change | -14.4 ± 8.1 | -6.6 ± 13.8 | 0.015 |
| Percentage change | -9.7± 5.3% | -4.7 ± 9.5% | 0.020 |
| p value (within group) | < 0.001 | < 0.001 | |
| Diastolic BP (mmHg) | |||
| Start of add-on therapy | 94.6 ± 8.8 | 93.4 ± 6.9 | 0.536 |
| Study end | 85.5 ± 7.8 | 85.8 ± 10.7 | 0.796 |
| Change | -9.9 ± 5.0 | -7.6 ± 7.4 | 0.798 |
| Percentage change | -9.5 ± 4.9% | -8.2 ± 8.0% | 0.840 |
| p value (within group) | < 0.001 | < 0.001 |
BP, blood pressure.
The changes of day-time, night-time, and mean 24-hour ambulatory SBP and DBP were similar between both groups. Nevertheless, treatment with bunazosin was associated with significant changes of night-day ratios of SBP and DBP compared with doxazosin (-0.02 vs. 0.02, p = 0.04; 0 vs. 0.04, p = 0.04) (Table 4). Both add-on treatments did not result in significant changes in waist circumference, serum levels of triglycerides, high-density lipoprotein, total cholesterol or fasting glucose (Table 5).
Table 4. Changes of night-day ratio of 24-hour ambulatory pressure after add-on treatment with α1-blockers .
| Valsartan + Bunazosin (N = 47) | Valsartan + Doxazosin (N = 46) | p value (between groups) | |
| Night-Day SBP ratio | |||
| Start of add-on therapy | 0.93 ± 0.06 | 0.91 ± 0.07 | 0.208 |
| Study end | 0.91 ± 0.08 | 0.93 ± 0.09 | 0.262 |
| Change | -0.02 ± 0.08 | 0.02 ± 0.08 | 0.040 |
| p value (within group) | 0.115 | 0.375 | |
| Night-Day DBP ratio | |||
| Start of add-on therapy | 0.93 ± 0.08 | 0.91 ± 0.08 | 0.260 |
| Study end | 0.93 ± 0.09 | 0.95 ± 0.11 | 0.242 |
| Change | 0.00 ± 0.09 | 0.04 ± 0.09 | 0.038 |
| p value (within group) | 0.859 | 0.020 | |
| Night-Day mean BP ratio | |||
| Start of add-on therapy | 0.93 ± 0.07 | 0.91 ± 0.08 | 0.215 |
| Study end | 0.92 ± 0.08 | 0.94 ± 0.19 | 0.237 |
| Change | -0.01 ± 0.08 | 0.03 ± 0.08 | 0.032 |
| p value (within group) | 0.494 | 0.070 |
DBP, diastolic blood pressure; SBP, systolic blood pressure.
Table 5. Changes in metabolic profiles after add-on treatment with α 1-blockers .
| Valsartan + Bunazosin (N = 47) | Valsartan + Doxazosin (N = 46) | p value (between groups) | |
| Waist circumference (cm) | |||
| Start of add-on therapy | 89.5 ± 8.5 | 87.5 ± 6.5 | 0.459 |
| Study end | 89.5 ± 8.0 | 87.9 ± 7.0 | 0.381 |
| p value (within group) | 0.779 | 0.832 | |
| Triglycerides (mg/dl) | |||
| Start of add-on therapy | 138.7 ± 77.9 | 152.9 ± 97.6 | 0.634 |
| Study end | 162.4 ± 117.9 | 143.3 ± 87.7 | 0.529 |
| p value (within group) | 0.210 | 0.632 | |
| High-density lipoprotein cholesterol (mg/dl) | |||
| Start of add-on therapy | 45.9 ± 11.8 | 43.9 ± 11.8 | 0.365 |
| Study end | 44.1 ± 10.6 | 43.4 ± 10.4 | 0.902 |
| p value (within group) | 0.082 | 0.685 | |
| Fasting glucose (mg/dl) | |||
| Start of add-on therapy | 96.2 ± 15.9 | 100.2 ± 26.1 | 0.845 |
| Study end | 96.9 ± 15.0 | 98.0 ± 18.0 | 0.767 |
| p value (within group) | 0.377 | 0.636 |
Safety
The two combination treatments were well-tolerated, and only two patients discontinued the study drug due to adverse events. A total of 91 adverse events occurred in fifty-three (53%) patients and most of them (96%) were mild in intensity. The incidence of adverse events was similar in the two groups (59.5% in buna-zosin vs. 54.4% in doxazosin, p = 0.677). The most commonly reported adverse events were dizziness (10 events), headache (4 events) and palpitation (4 events). No event of heart failure was reported during the entire study period. As judged by the investigators, 3 (6%) patients in the bunazosin group and 9 (20%) in the do-xazosin group experienced adverse events related to the study drugs (p = 0.058).
DISCUSSION
The current study was the first head-to-head comparison to assess the clinical efficacy of two different long-acting α1-blockers as second-line anti-hypertensive agents in patients with stage 1 or 2 hypertension whose BP could not be controlled by valsartan monotherapy. Although the two study drugs produced similar extents of BP reductions in the whole intention-to-treat popu-lation, bunazosin achieved a greater reduction in sitting SBP in patients with stage 2 hypertension at baseline. Moreover, patients treated with bunazosin had a lower night-day BP ratio, which might imply bunazosin had a relatively steadier BP-lowering effect than doxazosin. In contrast to previous studies, both α1-blockers seemed to be metabolically neutral in this study. Both combination treatments were generally well-tolerated, despite a marginally higher treatment-related adverse event rate that was reported in patients receiving doxazosin.
The observation that bunazosin exerted a stronger BP-lowering potency, compared to doxazosin, in patients with stage 2 hypertension was of clinical significance. The differences observed in the BP-lowering effect between agents of variable BP-lowering potency would be more apparent in patients with higher baseline BP than in patients with lower BP. It might explain why buna-zosin only showed its relative superior potency in patients with higher baseline BP. Criticism could be forthcoming that the relatively weak potency of doxazosin observed in our study might be due to inadequate dosage. However, based on previous reports, the doses we chose were just the lowest effective dose of bunazosin and doxazosin.18,19 Besides, the extent of BP reduction in our study was similar to that observed in previous studies.9,20
Twenty-four hour BP control is of equal importance in assessing the efficacy of antihypertensive agents as well as sitting BP. It has been speculated that the higher rates of stroke and congestive heart failure in the doxazosin limb of the ALLHAT trial might be partially due to the use of a short-acting formulation of doxazosin. In another study, the Hypertension and Lipid Trial (HALT), it has been shown that the mean night-day SBP ratio increased significantly from 0.89 to 0.91 after treatment with doxazosin.21 Despite an improved pharmacokinetic profile, an extended-release formulation of doxazosin, compared with extended-release bunazosin, was still less effective in reducing night-time BP in our study. Therefore, doxazosin treatment resulted in a higher night-day BP ratio. There is a growing body of evidence showing that night-day BP ratio not only predicts hypertension-related end-organ damage but also cardiovascular mortality.22,23 Although we are not certain whether the relatively lower night-day BP ratio in the bunazosin group will result in better cardiovascular outcomes, it is evident that extended-release bunazosin as an add-on treatment did provide a more sustained and steady BP-lowering effect than extended-release doxazosin.
Previous studies have shown that both bunazosin and doxazosin can improve plasma lipid profiles, insulin sensitivity, and glucose metabolism.9,12,15,17,24 However, in this study, we could not demonstrate the metabolically beneficial effects in patients treated with either α1-blocker on top of background valsartan therapy. It is still uncertain whether background valsartan therapy can influence the metabolic effects of both α1-blockers. In addition, the study period was only 8 weeks long, which might be too short to show the changes of meta-bolic profiles.
The present study has several limitations. First, the long-term cardiovascular impact could not be assessed for both combination antihypertensive treatments due to the small sample size. Second, in our study, the drugwas administered after breakfast. However, in most daily practices, due to the potential side effect of orthostatic hypotension, α1-blocker is suggested to be taken at bedtime. Night-time dosing of doxazosin has been associated with better BP control over a 24 hour period.25 Hence, the superiority of bunazosin on the night-day BP ratio in our study becomes uncertain once both study drugs are administered at bedtime.
Both α1-blockers were generally well-tolerated and there was no apparent difference in the incidence of adverse events. Besides, no event of heart failure was reported with either agent. The satisfactory tolerance of our study drugs further supports the clinical use of α1-blockers in combination therapy.
CONCLUSION
The study results suggested that the extended-release formulation of bunazosin and doxazosin are similarly effective in reducing blood pressures in hypertensive patients uncontrolled by valsartan monotherapy. However, add-on treatment with bunazosin seemed to be associated with favorable night-day BP ratio and greater reduction in sitting SBP in patients of stage 2 hypertension. These findings need to be verified in larger-scale trials.
REFERENCES
- 1.Moser M, Hebert PR. Prevention of disease progression, left ventricular hypertrophy and congestive heart failure in hypertension treatment trials. J Am Coll Cardiol. 1996;27:1214–1218. doi: 10.1016/0735-1097(95)00606-0. [DOI] [PubMed] [Google Scholar]
- 2.Collins R, Peto R, MacMahon S, et al. Blood pressure, stroke, and coronary heart disease. Part 2, short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet. 1990;335:827–838. doi: 10.1016/0140-6736(90)90944-z. [DOI] [PubMed] [Google Scholar]
- 3.Mancia G, De Backer G, Dominiczak A, et al. 2007 guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;25:1105–1187. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 4.Katayama S, Inaba M, Morita T, et al. Blood pressure control in Japanese hypertensives with or without type 2 diabetes mellitus. Hypertens Res. 2000;23:601–605. doi: 10.1291/hypres.23.601. [DOI] [PubMed] [Google Scholar]
- 5.Messerli FH, Grossman E, Goldbourt U. Antihypertensive therapy in diabetic hypertensive patients. Am J Hypertens. 2001;14:12S–16S. doi: 10.1016/s0895-7061(01)01314-0. [DOI] [PubMed] [Google Scholar]
- 6.Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs. diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 7.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 8.Black HR. Doxazosin as combination therapy for patients with stage 1 and stage 2 hypertension. J Cardiovasc Pharmacol. 2003;41:866–869. doi: 10.1097/00005344-200306000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Chapman N, Chang CL, Dahlof B, et al. Effect of doxazosin gastrointestinal therapeutic system as third-line antihyper-tensive therapy on blood pressure and lipids in the anglo-scandinavian cardiac outcomes trial. Circulation. 2008;118:42–48. doi: 10.1161/CIRCULATIONAHA.107.737957. [DOI] [PubMed] [Google Scholar]
- 10.Black HR, Sollins JS, Garofalo JL. The addition of doxazosin to the therapeutic regimen of hypertensive patients inadequately controlled with other antihypertensive medications: a randomized, placebo-controlled study. Am J Hypertens. 2000;13:468–474. doi: 10.1016/s0895-7061(99)00225-3. [DOI] [PubMed] [Google Scholar]
- 11.Nalbantgil S, Nalbantgil I, Onder R. Clinically additive effect between doxazosin and amlodipine in the treatment of essential hypertension. Am J Hypertens. 2000;13:921–926. doi: 10.1016/s0895-7061(99)00279-4. [DOI] [PubMed] [Google Scholar]
- 12.Schmitz G, Stumpe KO, Herrmann W, Weidinger G. Effects of bunazosin and atenolol on serum lipids and apolipoproteins in a randomised trial. Blood Press. 1996;5:354–359. doi: 10.3109/08037059609078074. [DOI] [PubMed] [Google Scholar]
- 13.Bonner G, Schmieder R, Chrosch R, Weidinger G. Effect of bunazosin and atenolol on glucose metabolism in obese, nondiabetic patients with primary hypertension. Cardiovasc Drugs Ther. 1997;11:21–26. doi: 10.1023/a:1007735420758. [DOI] [PubMed] [Google Scholar]
- 14.Grimm RH, Jr., Flack JM, Grandits GA, et al. Long-term effects on plasma lipids of diet and drugs to treat hypertension. Treatment of Mild Hypertension Study (TOMHS) Research Group. JAMA. 1996;275:1549–1556. doi: 10.1001/jama.1996.03530440029033. [DOI] [PubMed] [Google Scholar]
- 15.Hunter Hypertension Research Group. Combined effect of doxazosin and pindolol on blood pressure control and lipid concentrations in patients with essential hypertension selected from general practice. J Hum Hypertens. 1992;6:181–184. [PubMed] [Google Scholar]
- 16.Chiang CE, Wang TD, Li YH, et al. 2010 guidelines of the Taiwan Society of Cardiology for the management of hypertension. J Formos Med Assoc. 2011;109:740–773. doi: 10.1016/S0929-6646(10)60120-9. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki M, Hirose J, Asakura Y, et al. Insulin insensitivity in nonobese, nondiabetic essential hypertension and its improvement by an alpha 1-blocker (bunazosin) Am J Hypertens. 1992;5:869–874. doi: 10.1093/ajh/5.12.869. [DOI] [PubMed] [Google Scholar]
- 18.Marchegiano R, Lamenza F, Sarno D, et al. Dose-response study of bunazosin in the treatment of light-to-moderate arterial hypertension. Minerva Cardioangiol. 1993;41:451–456. [PubMed] [Google Scholar]
- 19.Heran BS, Galm BP, Wright JM. Blood pressure lowering efficacy of alpha blockers for primary hypertension. Cochrane Database Syst Rev. 2009:CD004643. doi: 10.1002/14651858.CD004643.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Ihara S, Shimamoto K, Watanabe H, et al. An alpha 1-receptor blocker reduces plasma leptin levels in hypertensive patients with obesity and hyperleptinemia. Hypertens Res. 2006;29:805–811. doi: 10.1291/hypres.29.805. [DOI] [PubMed] [Google Scholar]
- 21.Kario K, Schwartz JE, Pickering TG. Changes of nocturnal blood pressure dipping status in hypertensives by nighttime dosing of alpha-adrenergic blocker, doxazosin: results from the HALT study. Hypertension. 2000;35:787–794. doi: 10.1161/01.hyp.35.3.787. [DOI] [PubMed] [Google Scholar]
- 22.Minutolo R, Gabbai FB, Borrelli S, et al. Changing the timing of antihypertensive therapy to reduce nocturnal blood pressure in CKD: an 8-week uncontrolled trial. Am J Kidney Dis. 2007;50:908–917. doi: 10.1053/j.ajkd.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 23.Fagard RH, Celis H, Thijs L, et al. Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension. 2008;51:55–61. doi: 10.1161/HYPERTENSIONAHA.107.100727. [DOI] [PubMed] [Google Scholar]
- 24.Schmieder RE, Langenfeld MR, Gatzka CD, et al. Impact of alpha-versus beta-blockers on hypertensive target organ damage: results of a double-blind, randomized, controlled clinical trial. Am J Hypertens. 1997;10:985–991. doi: 10.1016/s0895-7061(97)00161-1. [DOI] [PubMed] [Google Scholar]
- 25.Hermida RC, Calvo C, Ayala DE, et al. Administration-time-dependent effects of doxazosin GITS on ambulatory blood pressure of hypertensive subjects. Chronobiol Int. 2004;21:277–296. doi: 10.1081/cbi-120037772. [DOI] [PubMed] [Google Scholar]


