Abstract
Hypertension is the leading cause of heart failure and cardiovascular comorbidities in developed countries. Left ventricular structural/functional alterations such as concentric remodeling or hypertrophy have been extensively studied in hypertensive heart diseases. Furthermore, it is also well-recognized that diastolic function actually deteriorates in hypertensive subjects prior to overt heart failure.
Novel imaging modality techniques such as myocardial deformation have allowed for early detection of regional/global myocardial contractile dysfunction. Myocardial deformation, which can be quantified by measuring the systolic strain and strain rate in three different directions (longitudinal, circumferential and radial), has facilitated new insights into the understanding of cardiac systolic mechanics in subjects with early stage myocardial damage.
Previous studies had shown that longitudinal function remains the most sensitive parameter in identifying hypertension-related myocardial dysfunction, particularly for those patients who had developed LV hypertrophy. Instead, preserved or enhanced short-axis function, when presented as circumferential or radial strains, may remain relatively preserved or enhanced in order to compensate for longitudinal functional decline. In this manner, global cardiac pumping in terms of ejection fraction may remain relatively unchanged.
The early recognition of subclinical systolic dysfunction and associated mechanical compensation in the context of hypertension is crucial, which potentially helps to identify a disease stage that is still responsive to therapeutic intervention.
Keywords: Concentric remodeling, Hypertension, Myocardial mechanics, Strain, Subclinical systolic dysfunction
INTRODUCTION
Hypertension is one of the most commonly encountered patient problems in daily clinical practice. As a leading cause of adverse cardiovascular events in developed societies, the underlying mechanisms, pathophysiologies and clinical consequences of hypertension are increasingly becoming more widely recognized.1-3 Hypertensive heart disease, especially when accompanied by aging, is associated with numerous adverse cardiac structural/functional remodeling, repercussions that extend to the cellular level, including abnormal myocyte growth and collagen fiber deposition, leading to myocardial fibrosis, cardiomyopathy and eventually the development of heart failure (HF).4,5
Diastolic dysfunction characterized by ventricular filling abnormalities, including decreased diastolic distensibility and impaired relaxation, is thought to represent an early and important pathophysiological intermediate step between hypertension and HF.6 Nearly half of all patients with hypertension encounter evidence of diastolic dysfunction,7 which actually represents an attractive target for disease prevention because the high prevalence of hypertension may translate to a greater population-attributable risk.8 However, the exact mechanism of the transition from asymptomatic diastolic dysfunction to diastolic heart failure remains unclear.
The deleterious factors for impaired cardiac function in subjects with hypertension are multi-factorial, such as apoptosis of cardiomyocytes, adaptive ventricular remodeling, increased mechanical stress with subsequent ventricular hypertrophy, phenotype alterations of cardiomyocytes, interstitial and perivascular fibrosis, endothelial dysfunction, microvascular insufficiency, disturbances in intracellular calcium turnover, and neurohormonal factors.3,9-12 Identifying subclinical systolic dysfunction among hypertensive subjects might be helpful in targeting these patients for preventive treatment delivery.
Currently suggested phenotypic classification of HF is based on normal or reduced left ventricular (LV) ejection fraction (EF). However, it has long been debated whether systolic function is really “normal” in HF with preserved EF (HFpEF),13 owing to the possibility that deteriorated myocardial function may happen earlier; thus, it may be of substantial clinical benefit to look further into more complex and sophisticated mechanics than a conventional estimate such as EF.14 Furthermore, preserved global LV systolic function in a resting state does not necessarily guarantee the normal response of LV to patient exercise.15
ETIOLOGY
The presence of objective evidence indicating obvious diastolic dysfunction (e.g., elevated diastolic filling pressures or decreased mitral annulus diastolic relaxation velocities) or diminished cardiac output had been used to support the clinical diagnosis of abnormal myocardial function.16 Patients with HF and reduced EF (HFrEF) typically exhibit progressive LV chamber dilation, eccentric remodeling, and systolic impairment in terms of reduced EF. Specific therapies focusing on the reversal of these structural and functional alterations may reduce morbidity and mortality in these patients.17 By contrast, the pathophysiology underlying the development of HFpEF among hypertensives remains only partially understood.18 Recent large clinical trials have indicated that the presence of concentric remodeling/LV hypertrophy, left atrial (LA) enlargement, and diastolic dysfunction are the most common features of HFpEF.19
Concentric ventricular remodeling or overt hypertrophy occurs most commonly in hypertension and is more likely to be associated with normal or even reduced LV end-diastolic volume (LVEDV) accompanied by increased ventricular stiffness and limited distensibility.20 On the other hand, eccentric hypertrophy was defined by similarly increased LV mass though larger LV diameter and LVEDV.21 The development of LV hypertrophy is actually a combined consequence of chronic pressure or volume overload. To compensate for chronic pressure overload in hypertensive subjects, LV wall thickness gradually increases in order to normalize wall stress, leading to concentric LV remodeling and hypertrophy.20 Activation of several biological processes including various hormones, growth factors and cytokines also contribute to protein genesis by promoting muscle cell growth, leading to structural alterations and remodeling.22 It is thus believed that in untreated hypertension, progression from LV hypertrophy to HF is accompanied by serial events/processes such as ischemia, myocytes apoptosis or fibrosis, and eventually systolic dysfunction.23
Strain and strain rate for assessment of cardiac function
Advances in ultrasound technology such as tissue Doppler imaging (TDI) and strain or strain rate imaging, either by Doppler tissue method24 or two-dimensional (2D) speckle-tracking, has appreciably broadened our understanding of cardiac mechanics and has been shown to identify early stage myocardial dysfunction secondary to hypertension.25,26 The use of TDI to measure regional myocardial velocity as part of diastolic functional assessment integration is less prone to load changes, and is mainly limited by its angle dependency, tethering effects and translational artifacts.27-29 Instead, strain imaging may provide less angle-dependent evaluation of regional and global LV function and further help to identify altered myocardial systolic mechanics at an early stage.30
Strain as a linear measure of tissue deformation can be expressed as a percentage of the length changes during systole between two separate points in the myocardium from its original status.31 Strain rate is the derivative of strain, which represents the rate of deformation changes over a period of time. As mentioned before, speckle-tracking echocardiography has been successfully applied to the measurement of myocardial strain and strain rate.
The infrastructure of myocardium is actually composed of circumferential fibers in the mid-wall layer, with longitudinal- with oblique-oriented muscle fibers lining the endocardial and epicardial layers.32 The spatial orientation of these muscle fibers changes continuously from right-handed helix in subendocardium to lefthanded helix in subepicardium.33 Hence, cardiac systole is accomplished by a series of complicated myocardial contractions in a coordinated fashion leading to effective pumping from the LV with minimal individual sarcomere shortening.34 From a practical view point, strain can be classified into three different spatial components: longitudinal, circumferential, and radial (Figure 1).31 Myocardial deformation can thus be evaluated by assessing systolic strain and strain rate, though somewhat diverse in the reference values from different vendors and related algorithms, within a limited region, in a specific myocardial segment or as a global measure (Figure 2). These advanced techniques allow objective quantification of cardiac motion and deformation irrespective of echo beam direction, thus providing a detailed understanding of cardiac mechanics from all aspects, and further help to explore the full spectrum of a specific myocardial disorder not limited to the ventricle.35-37
Figure 1.
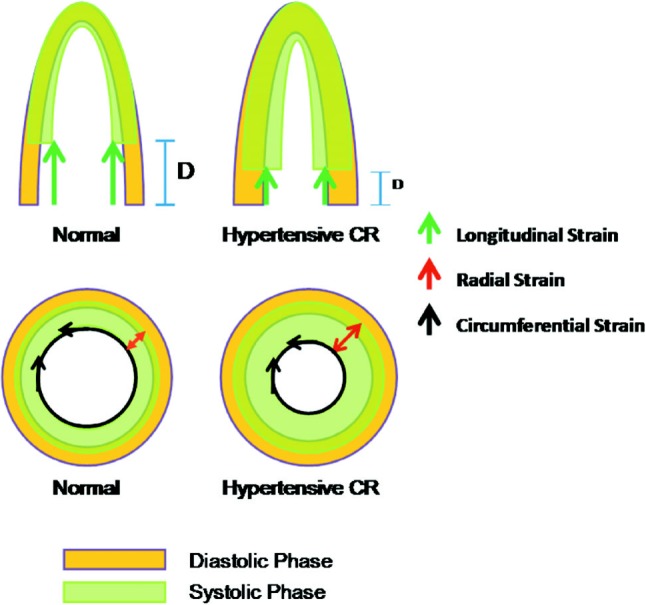
The commonly proposed or suggested three different spatial components of myocardial motion and related deformations as illustrated. The green arrow indicates longitudinal deformation with red and black arrows indicated radial and circumferential deformations, respectively. The differential longitudinal displacement (D) and corresponding short-axis function/motion in normal and hypertensive concentric remodeling (CR) subjects are demonstrated.
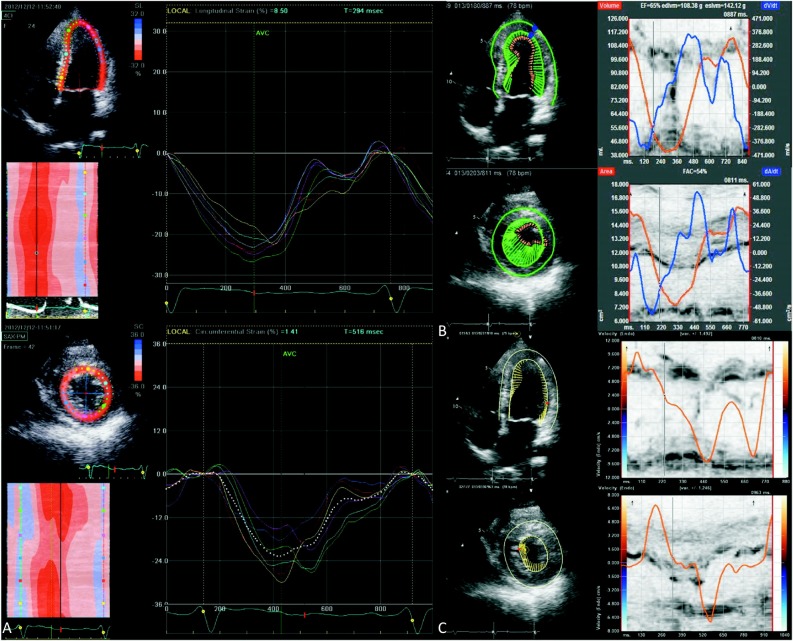
Figure 2.
Myocardial longitudinal (upper panel in each demonstration) and short-axis (radial or circumferential displayed as lower panel in each demonstration) deformations are shown. Common encountered commercialized companies/software such as iE33 (QLab, Philips Medical Systems) (data not shown), GE Vingmed (A: GE Vingmed Ultrasound AS, Horten, Norway), TomTec (B: TomTec, Cardiac 2D Performance Analysis, Tomtec Imaging Systems) or Velocity Vector Imaging (C: Siemens Medical Solutions, Mountain View, CA, USA) and strain or related volume-velocity curve analysis in the same subjects are shown. The amplitude and direction from speckle-tracking analysis (B & C) represents the amplitude and direction of ongoing regional myocardial motions.
By examining the prior use of various techniques, earlier publications have shown that normal longitudinal strain can vary from 16% to 19%.38,39 Different image modalities have generated variable results regarding the uniformity of strain from base to apex. This regional variation might be related to angle-limitations caused by curvatures in the myocardial architecture. Therefore, segmental cutoff values might be more appropriate than a single normal cutoff value.
Effects of hypertensive heart remodeling on myocardial strain
Among the three layers of myocardium, longitudinal subendocardial fibers are most susceptible to adverse effects of ischemia, hypoperfusion and age-related interstitial fibrosis.33 Therefore, longitudinal dysfunction may serve as an early marker by accomplishing global longitudinal strain measurement at an early stage of myocardial damage40 before overt development of chamber-level failure, such as reduced EF.41 Instead, the mid-wall layer is less affected by these pathological insults as early as the longitudinal function, so strain analysis based on myocardial short-axis function may remain relatively preserved at this stage.
Indeed, previous studies comparing three-directional strain parameters from echocardiography studies had consistently shown that longitudinal function declines first with hypertension, in particular for those who had developed LV hypertrophy.42,43 Impaired longitudinal shortening is also observed in hypertensive remodeling without overt LV hypertrophy or clinical HF development while in short-axis function, especially for circumferential or radial mechanics, may remain preserved or even enhanced to maintain ventricular function (Table 1).41-44 In addition, increased matrix metal-loproteinase-1 (MMP-1) turnover, a biomarker of myocardial fibrosis, correlated well with decreased longitudinal strain in hypertension and hypertrophic cardiomyopathy.45 These findings again supported the concept that longitudinal contractile function as a sensitive marker is prone to early pathological changes of the myocardium, while radial and circumferential strain may be more resistant to such early myocardial alterations.46
Table 1. Speckle-tracking based analysis of different myocardial strain/deformations in studies relating to hypertension or hypertensive-remodeling .
| Modalities analyzed (Vendors) | Clinical settings | Longitudinal strain | Circumferential strain | Radial strain |
| Chen et al. (VVI)57 | LVH | Decreased | Decreased | - |
| Narayanan et al. (EchoPac)46* | HTN & LVH | Decreased† | Preserved | Preserved |
| Galderisi et al. (EchoPac)50 | HTN | Decreased | Preserved | Preserved |
| Kouzu et al. (EchoPac)43 | HTN/LVH | Preserved/Decreased | Preserved/Preserved | Increased or Preserved/Preserved |
| Imbalzano et al. (EchoPac)58 | HTN/LVH | Decreased/Decreased | Preserved/Increased | Preserved/Decreased |
| Kang et al. (EchoPac)45 | HTN | Decreased | Preserved | Preserved |
* Both longitudinal and circumferential strain correlated with LV mass; † Regional function.
HTN, hypertension; LVH, left ventricular hypertrophy.
In diastolic dysfunction, delayed relaxation can translate into increased end-diastolic pressure and limited ventricular filling, which may exacerbate hypertensive stress responses. Though it has been observed that both longitudinal and radial strains were decreased in diastolic heart failure,47,48 longitudinal functional decline may occur with concurrent diastolic dysfunction early in hypertensive subjects49 when short-axis function including radial or circumferential function remain unchanged.50 Subsequent impairment in short-axis contractile functional reserve may act as a contributing factor to the transition from subclinical myocardial dysfunction to heart failure development.
The exact mechanisms underlying the maintenance of ventricular short-axis function in the context of early stages of hypertension were believed to come from the cross-fiber shortening phenomenon from hypertensionrelated ventricular remodeling and increased systolic wall thickening,51 partially due to multiplied myofiber units and extensive extra-cellular matrix deposition.52,53
However, such compensation of short-axis function under clinical scenarios of hypertensive heart diseases does not happen without limits. It has been observed that both longitudinal and short-axis based strain measures may actually decrease in heart failure subjects even at a stage when LV EF does not decrease.54,55 Since deteriorated longitudinal function already exists in the early stages of hypertensive heart disease, subsequent loss of short-axis reserve may theoretically contribute to the transition from hypertension to heart failure (Table 2).40,53,56
Table 2. Comparisons and changes of diastolic function and three-direction myocardial deformations in normal subjects with subjects with preserved (HFpEF) or reduced (HFrEF) ejection fraction heart failure.
| Clinical scenarios | Global EF | Diastolic filling pressures | Longitudinal mechanics | Circumferential mechanics |
| Normal | Normal | Normal | Normal | Normal |
| HFpEF | Preserved or minimally impaired | Elevated | Markedly impaired | Preserved |
| HFrEF | Markedly impaired | Elevated | Markedly impaired | Markedly impaired |
CONCLUSIONS
Hypertension and heart failure are closely related. Recent advances in noninvasive cardiovascular imaging modalities have contributed new insights into the understanding of underlying cardiac mechanics, and further provided accurate and objective measures on global/regional contractile function. The early detection of subclinical systolic dysfunction by deformation imaging may also help in identifying hypertensive subjects at risk for subsequent adverse events, and in selecting individuals for preventive treatment delivery.
REFERENCES
- 1.Miura K, Dyer AR, Greenland P, et al. Pulse pressure compared with other blood pressure indexes in the prediction of 25-year cardiovascular and all-cause mortality rates: the Chicago Heart Association Detection Project in Industry Study. Hypertension. 2001;38:232–237. doi: 10.1161/01.hyp.38.2.232. [DOI] [PubMed] [Google Scholar]
- 2.Haider AW, Larson MG, Franklin SS, et al. Systolic blood pressure, diastolic blood pressure, and pulse pressure as predictors of risk for congestive heart failure in the Framingham Heart Study. Ann Intern Med. 2003;138:10–16. doi: 10.7326/0003-4819-138-1-200301070-00006. [DOI] [PubMed] [Google Scholar]
- 3.Kass DA. Ventricular arterial stiffening: integrating the pathophysiology. Hypertension. 2005;46:185–193. doi: 10.1161/01.HYP.0000168053.34306.d4. [DOI] [PubMed] [Google Scholar]
- 4.Chen CH, Nakayama M, Nevo E, et al. Coupled systolic-ventricular and vascular stiffening with age: implications for pressure regulation and cardiac reserve in the elderly. J Am Coll Cardiol. 1998;32:1221–1227. doi: 10.1016/s0735-1097(98)00374-x. [DOI] [PubMed] [Google Scholar]
- 5.Querejeta R, López B, González A, et al. Increased collagen type I synthesis in patients with heart failure of hypertensive origin: relation to myocardial fibrosis. Circulation. 2004;110:1263–1268. doi: 10.1161/01.CIR.0000140973.60992.9A. [DOI] [PubMed] [Google Scholar]
- 6.Gradman AH, Wilson JT. Hypertension and diastolic heart failure. Curr Cardiol Rep. 2009;11:422–429. doi: 10.1007/s11886-009-0061-5. [DOI] [PubMed] [Google Scholar]
- 7.Shah SJ, Gheorghiade M. Heart failure with preserved ejection fraction: treat now by treating comorbidities. JAMA. 2008;300:431–433. doi: 10.1001/jama.300.4.431. [DOI] [PubMed] [Google Scholar]
- 8.He J, Ogden LG, Bazzano LA, et al. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. 2001;161:996–1002. doi: 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 9.Muiesan ML, Salvetti M, Monteduro C, et al. Left ventricular concentric geometry during treatment adversely affects cardiovascular prognosis in hypertensive patients. Hypertension. 2004;43:731–738. doi: 10.1161/01.HYP.0000121223.44837.de. [DOI] [PubMed] [Google Scholar]
- 10.Levy D, Larson MG, Vasan RS, et al. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557–1562. [PubMed] [Google Scholar]
- 11.Kehat I, Molkentin JD. Molecular pathways underlying cardiac remodeling during pathophysiological stimulation. Circulation. 2010;122:2727–2735. doi: 10.1161/CIRCULATIONAHA.110.942268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamdani N, Bishu KG, von Frieling-Salewsky M, et al. Deranged myofilament phosphorylation and function in experimental heart failure with preserved ejection fraction. Cardiovasc Res. 2012 Dec 4 (Accepted in press) doi: 10.1093/cvr/cvs353. [DOI] [PubMed] [Google Scholar]
- 13.Maeder MT, Kaye DM. Heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol. 2009;53:905–918. doi: 10.1016/j.jacc.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Cottrell C, Kirkpatrick JN. Echocardiographic strain imaging and its use in the clinical setting. Expert Rev Cardiovasc Ther. 2010;8:93–102. doi: 10.1586/erc.09.165. [DOI] [PubMed] [Google Scholar]
- 15.Phan TT, Abozguia K, Nallur Shivu G, et al. Heart failure with preserved ejection fraction is characterized by dynamic impairment of active relaxation and contraction of the left ventricle on exercise and associated with myocardial energy deficiency. J Am Coll Cardiol. 2009;54:402–409. doi: 10.1016/j.jacc.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 16.Paulus WJ, Tschöpe C, Sanderson JE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 17.Jessup M, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 18.Paulus WJ, van Ballegoij JJ. Treatment of heart failure with normal ejection fraction: an inconvenient truth! J Am Coll Cardiol. 2010;55:526–537. doi: 10.1016/j.jacc.2009.06.067. [DOI] [PubMed] [Google Scholar]
- 19.Zile MR, Gottdiener JS, Hetzel SJ, et al. I-PRESERVE Investigators. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation. 2011;124:2491–2501. doi: 10.1161/CIRCULATIONAHA.110.011031. [DOI] [PubMed] [Google Scholar]
- 20.Mayet J, Hughes A. Cardiac and vascular pathophysiology in hypertension. Heart. 2003;89:1104–1109. doi: 10.1136/heart.89.9.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verma A, Medris A, Hicham S, et al. Prognostic implications of left ventricular mass and geometry following myocardial infarction. JACC Cardiovasc Imag. 2008;1:582–591. doi: 10.1016/j.jcmg.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 22.De Simone G, Pasanisi F, Contaldo F. Link of nonhemodynamic factors to hemodynamic determinants of left ventricular hypertrophy. Hypertension. 2001;38:13–18. doi: 10.1161/01.hyp.38.1.13. [DOI] [PubMed] [Google Scholar]
- 23.Fortuno MA, Ravassa S, Fortuno A, et al. Cardiomyocyte apoptotic cell dath in arterial hypertension:mechanisms and potential management. Hypertension. 2001;38:1406–1412. doi: 10.1161/hy1201.099615. [DOI] [PubMed] [Google Scholar]
- 24.Edvardsen T, Gerber BL, Garot J, et al. Quantitative assessment of intrinsic regional myocardial deformation by Doppler strain rate echocardiography in humans: validation against three-dimensional tagged magnetic resonance imaging. Circulation. 2002;106:50–56. doi: 10.1161/01.cir.0000019907.77526.75. [DOI] [PubMed] [Google Scholar]
- 25.Pela G, Bruschi G, Cavatorta A, et al. Doppler tissue echocardiography: myocardial wall motion velocities in essential hypertension. Eur J Echocardiogr. 2001;2:108–117. doi: 10.1053/euje.2000.0057. [DOI] [PubMed] [Google Scholar]
- 26.Thomas JD, Popovi ZB. Assessment of left ventricular function by cardiac ultrasound. J Am Coll Cardiol. 2006;48:2012–2025. doi: 10.1016/j.jacc.2006.06.071. [DOI] [PubMed] [Google Scholar]
- 27.Sohn DW, Chai IH, Lee DJ, et al. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol. 1997;30:474–480. doi: 10.1016/s0735-1097(97)88335-0. [DOI] [PubMed] [Google Scholar]
- 28.Rakowski H, Appleton C, Chan KL, et al. Canadian consensus recommendations for the measurement and reporting of diastolic dysfunction by echocardiography: from the Investigators of Consensus on Diastolic Dysfunction by Echocardiography. J Am Soc Echocardiogr. 1996;9:736–760. doi: 10.1016/s0894-7317(96)90076-0. [DOI] [PubMed] [Google Scholar]
- 29.Abraham TP, Dimaano VL, Liang HY. Role of tissue Doppler and strain echocardiography in current clinical practice. Circulation. 2007;116:2597–2609. doi: 10.1161/CIRCULATIONAHA.106.647172. [DOI] [PubMed] [Google Scholar]
- 30.Weidemann F, Breunig F, Beer M, et al. The variation of morphological and functional cardiac manifestation in Fabry disease: potential implications for the time course of the disease. Eur Heart J. 2005;26:1221–1227. doi: 10.1093/eurheartj/ehi143. [DOI] [PubMed] [Google Scholar]
- 31.Gorcsan J, 3rd, Tanaka H. Echocardiographic assessment of myocardial strain. J Am Coll Cardiol. 2011;58:1401–1413. doi: 10.1016/j.jacc.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 32.Torrent-Guasp F, Buckberg GD, Clemente C, et al. The structure and function of the helical heart and its buttress wrapping. I. The normal macroscopic structure of the heart. Semin Thorac Cardiovasc Surg. 2001;13:301–319. doi: 10.1053/stcs.2001.29953. [DOI] [PubMed] [Google Scholar]
- 33.Sengupta PP, Krishnamoorthy VK, Korineck J, et al. Left ventricular form and function revisited: applied translational science to cardiovascular ultrasound imaging. J Am Soc Echocardiogr. 2007;20:539–551. doi: 10.1016/j.echo.2006.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalogeropoulos AP, Georgiopoulou VV, Gheorghiade M, et al. Echocardiographic evaluation of left ventricular structure and function: new modalities and potential applications in clinical trials. J Card Fail. 2012;18:159–172. doi: 10.1016/j.cardfail.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 35.Langeland S, D’hooge J, Wouters PF, et al. Experimental validation of a new ultrasound method for the simultaneous assessment of radial and longitudinal myocardial deformation independent of insonation angle. Circulation. 2005;112:2157–2162. doi: 10.1161/CIRCULATIONAHA.105.554006. [DOI] [PubMed] [Google Scholar]
- 36.Leitman M, Lysyansky P, Sidenko S, et al. Two-dimensional strain-a novel software for real-time quantitative echocardiographic assessment of myocardial function. J Am Soc Echocardiogr. 2004;17:1021–1029. doi: 10.1016/j.echo.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 37.Amundsen BH, Helle-Valle T, Edvardsen T, et al. Noninvasive myocardial strain measurement by speckle tracking echocardiography: validation against sonomicrometry and tagged magnetic resonance imaging. J Am Coll Cardiol. 2006;47:789–793. doi: 10.1016/j.jacc.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 38.Sun JP, Popovic ZB, Greenberg NL, et al. Noninvasive quantification of regional myocardial function using Doppler-derived velocity, displacement, strain rate, and strain in healthy volunteers: effects of aging. J Am Soc Echocardiogr. 2004;17:132–138. doi: 10.1016/j.echo.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Edvardsen T, Gerber BL, Garot J, et al. Quantitative assessment of intrinsic regional myocardial deformation by Doppler strain rate echocardiography in humans: validation against three-dimensional tagged magnetic resonance imaging. Circulation. 2002;106:50–56. doi: 10.1161/01.cir.0000019907.77526.75. [DOI] [PubMed] [Google Scholar]
- 40.Kuznetsova T, Herbots L, Richart T, et al. Left ventricular strain and strain rate in a general population. Eur Heart J. 2008;29:2014–2023. doi: 10.1093/eurheartj/ehn280. [DOI] [PubMed] [Google Scholar]
- 41.Koulouris SN, Kostopoulos KG, Triantafyllou KA, et al. Impaired systolic dysfunction of left ventricular longitudinal fibers: a sign of early hypertensive cardiomyopathy. Clin Cardiol. 2005;28:282–286. doi: 10.1002/clc.4960280605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mizuguchi Y, Oishi Y, Miyoshi H, et al. The functional role of longitudinal, circumferential, and radial myocardial deformation for regulating the early impairment of left ventricular contraction and relaxation in patients with cardiovascular risk factors: a study with two-dimensional strain imaging. J Am Soc Echocardiogr. 2008;21:1138–1144. doi: 10.1016/j.echo.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 43.Kouzu H, Yuda S, Muranaka A, et al. Left ventricular hypertrophy causes different changes in longitudinal, radial, and circumferential mechanics in patients with hypertension: a two-dimensional speckle tracking study. J Am Soc Echocardiogr. 2011;24:192–199. doi: 10.1016/j.echo.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 44.Wang J, Khoury DS, Yue Y, et al. Preserved left ventricular twist and circumferential deformation, but depressed longitudinal and radial deformation in patients with diastolic heart failure. Eur Heart J. 2008;29:1283–1289. doi: 10.1093/eurheartj/ehn141. [DOI] [PubMed] [Google Scholar]
- 45.Kang SJ, Lim HS, Choi BJ, et al. Longitudinal strain and torsion assessed by two-dimensional speckle tracking correlate with the serum level of tissue inhibitor of matrix metalloproteinase-1, a marker of myocardial fibrosis, in patients with hypertension. J Am Soc Echocardiogr. 2008;21:907–911. doi: 10.1016/j.echo.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 46.Narayanan A, Aurigemma GP, Chinali M, et al. Cardiac mechanics in mild hypertensive heart disease: a speckle-strain imaging study. Circ Cardiovasc Imaging. 2009;2:382–390. doi: 10.1161/CIRCIMAGING.108.811620. [DOI] [PubMed] [Google Scholar]
- 47.Tan YT, Wenzelburger F, Lee E, et al. The pathophysiology of heart failure with normal ejection fraction: exercise echocardiography reveals complex abnormalities of both systolic and diastolic ventricular function involving torsion, untwist, and longitudinal motion. J Am Coll Cardiol. 2009;54:36–46. doi: 10.1016/j.jacc.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 48.Wang J, Khoury DS, Yue Y, et al. Preserved left ventricular twist and circumferential deformation, but depressed longitudinal and radial deformation in patients with diastolic heart failure. Eur Heart J. 2008;29:1283–1289. doi: 10.1093/eurheartj/ehn141. [DOI] [PubMed] [Google Scholar]
- 49.Lumens J, Delhaas T, Arts T, et al. Impaired subendocardial contractile myofiber function in asymptomatic aged humans, as detected using MRI. Am J Physiol Heart Circ Physiol. 2006;291:H1573–H1579. doi: 10.1152/ajpheart.00074.2006. [DOI] [PubMed] [Google Scholar]
- 50.Galderisi M, Lomoriello VS, Santoro A, et al. Differences of myocardial systolic deformation and correlates of diastolic function in competitive rowers and young hypertensives: a speckle-tracking echocardiography study. J Am Soc Echocardiogr. 2010;23:1190–1198. doi: 10.1016/j.echo.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 51.Kosmala W, Plaksej R, Strotmann JM, et al. Progression of left ventricular functional abnormalities in hypertensive patients with heart failure: an ultrasonic two-dimensional speckle tracking study. J Am Soc Echocardiogr. 2008;21:1309–1317. doi: 10.1016/j.echo.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 52.LeGrice IJ, Smaill BH, Chai LZ, et al. Laminar structure of the heart: ventricular myocyte arrangement and connective tissue architecture in the dog. Am J Physiol. 1995;269:H571–H582. doi: 10.1152/ajpheart.1995.269.2.H571. [DOI] [PubMed] [Google Scholar]
- 53.Aurigemma GP, Zile MR, Gaasch WH. Contractile behavior of the left ventricle in diastolic heart failure: with emphasis on regional systolic function. Circulation. 2006;113:296–304. doi: 10.1161/CIRCULATIONAHA.104.481465. [DOI] [PubMed] [Google Scholar]
- 54.Tan YT, Wenzelburger F, Lee E, et al. The pathophysiology of heart failure with normal ejection fraction: exercise echocardiography reveals complex abnormalities of both systolic and diastolic ventricular function involving torsion, untwist, and longitudinal motion. J Am Coll Cardiol. 2009;54:36–46. doi: 10.1016/j.jacc.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 55.Wang J, Khoury DS, Yue Y, et al. Preserved left ventricular twist and circumferential deformation, but depressed longitudinal and radial deformation in patients with diastolic heart failure. Eur Heart J. 2008;29:1283–1289. doi: 10.1093/eurheartj/ehn141. [DOI] [PubMed] [Google Scholar]
- 56.Borlaug BA, Lam CS, Roger VL, et al. Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol. 2009;54:410–418. doi: 10.1016/j.jacc.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen J, Cao T, Duan Y, et al. Velocity vector imaging in assessing myocardial systolic function of hypertensive patients with left ventricular hypertrophy. Can J Cardiol. 2007;23:957–961. doi: 10.1016/s0828-282x(07)70857-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Imbalzano E, Zito C, Carerj S, et al. Left ventricular function in hypertension:new insight by speckle tracking echocardiography. Echocardiography. 2011;28:649–657. doi: 10.1111/j.1540-8175.2011.01410.x. [DOI] [PubMed] [Google Scholar]
- 59.Geyer H, Caracciolo G, Abe H, et al. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr. 2010;23:351–369. doi: 10.1016/j.echo.2010.02.015. [DOI] [PubMed] [Google Scholar]