Abstract
The management of melioidosis, caused by Burkholderia pseudomallei, presenting as an infected pseudoaneurysm requires radical debridement and prolonged antibiotics because the pathogen is resistant to host immunity. An extra-anatomical bypass might be a better treatment choice than in situ graft interposition or other methods.We report on a 76-year-oldman with an infected pseudoaneurysmlocated in the innominate artery and a method of extra-anatomical bypass that has not yet been reported in the literature. The patient recovered well without recurrence of infection after the surgical procedure.
Keywords: Burkholderia pseudomallei, Extra-anatomical bypass, Innominate artery, Melioidosis, Pseudoaneurysm
Pseudoaneurysms of the peripheral arteries can be induced by variable etiologies including trauma,1 iatrogenic insults,2,3 aortitis,4 and infections.5 However, pseudoaneurysms induced by infection rarely develop at the peripheral arteries, let alone the innominate artery.5 Moreover, Burkholderia pseudomallei, the pathogen of melioidosis, seldom affects the cardiovascular system. Very few articles in the literature have mentioned melioidosis associated with peripheral arterial aneurysms; furthermore, an infected pseudoaneurysm of the innominate artery (IPIA) caused by Burkholderia pseudomallei has never been reported. Therefore, the strategy and protocol in managing IPIA with melioidosis lack useful and referable precedents. Hereafter, we present the successful experience of treating a case of IPIA with melioidosis by performing an extra-anatomical bypass.
CASE REPORT
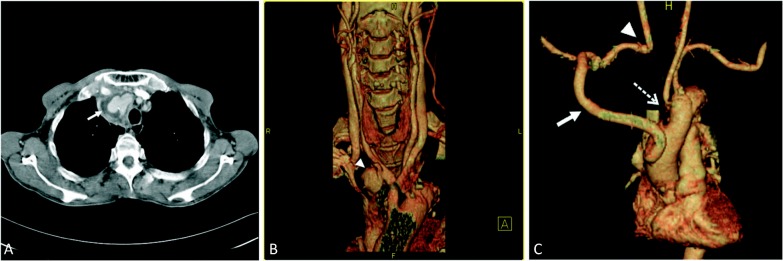
This patient was a 76-year-old male farmer who lived in a melioidosis-endemic area and also suffered from non-insulin dependent diabetes mellitus. He came to our emergency room with the principal complaint of intermittent spiking fever up to 40 °C for two weeks. Emergency room laboratory tests disclosed pyuria, leukocytosis, and elevated C-reactive protein. Therefore, he was admitted to the infectious ward with a suspected urinary tract infection. Empirical antibiotic therapy with quinolone was given initially. Later, when urine and blood cultures confirmed the pathogen to be Burkholderia pseudomallei, ceftazidime was administered in accordance with the results of drug sensitivity testing. Thereafter, his fever subsided after intensive antibiotic therapy. However, serial chest roentgenograms revealed a progressively enlarged mass which had developed over the right superior mediastinum. A subsequent computed tomography (CT) scan with contrast showed a saccular pouch (pseudoaneurysm) stemming from the distal part of the innominate artery. The size of this pseudoaneurysm was about 4.5 × 3 × 3 cm3 (Figure 1A and B). In light of image illustrations as well as culture results, the preliminary diagnosis was IPIA induced by melioidosis. To avoid the happening of severe septicemia and hemorrhagic shock, the patient was transferred to our cardiovascular surgical division for surgery.
Figure 1.
(A) CT axial view revealed an aneurysm protruding from the innominate artery (arrow). (B) CT angiography with three-dimensional reconstruction showed one pseudoaneurysm originating from the distal part of the innominate artery (arrowhead). (C) CT angiography with three-dimensional reconstruction at five months after surgery showed continuity of the extraanatomical bypass (arrow), the right subclavian artery connection to the common carotid artery (arrowhead), and the primary repair of the aortic arch (dashed arrow).
Initially, we isolated the left common femoral vessels in preparation for a cardiopulmonary bypass. The third part of the right axillary artery was then exposed and reserved for further bypass grafting. A panoramic exploration of the superior mediastinum and neck root was facilitated by extending the median sternotomy toward the anterior border of the right sternocleidomastoid muscle. The pseudoaneurysm was found to be encapsulated by massive fibrotic tissue, which adhered firmly to the proximal aortic arch and the right subclavian and common carotid arteries. The innominate artery and vein were both embedded within the huge fibrotic mass. Any attempt to excise the pseudoaneurysm without cardiopulmonary bypass was clearly impossible. In situ reconstruction of the innominate artery around the infected region might not preclude later graft problems, even though there was no suppuration. Therefore, we decided to perform an aneurysmectomy with radical debridement under deep hypothermic circulatory arrest. A circuit of cardiopulmonary bypass was established with inflow cannulation via the left femoral artery and outflow drainage via the right atrium. Deep hypothermia to 18 °C was commenced. During the process of cooling, a 12 mm Hemashield woven graft (Hemashield Gold; Boston Scientific Medi-Tech, Wayne, NJ, USA), substituting for the innominate artery, was interposed between the mid-ascending aorta and the third part of the right axillary artery, through the apex of the right interpleural cavity and beneath the lower edge of the right first rib. Then, a direct end-to-end anastomosis between the distal right subclavian and common carotid arteries was performed to help maintain cerebral perfusion. Under deep hypothermic circulatory arrest, the pseudoaneurysm and innominate artery were excised and debrided. The bacterial vegetation noted within the proximal arch and the orifices of its great vessels was removed at the same time. A 3-cm defect at the proximal aortic arch resulting from radical debridement was repaired directly. The overall circulatory arrest time was 23 minutes. Tissue culture from the pseudoaneurysm showed Burkholderia pseudomallei. After the operation, the patient continued to receive intravenous injections of ceftazidime for six weeks, followed by a lifelong oral antibiotic regimen of cotrimoxazole and doxycycline, commencing at discharge.6
Evaluation of duplex ultrasonography showed equivalent flow velocities in the bilateral common carotid and ophthalmic arteries. The flow did not change while the patient’s right arm was exercised or after the exercise. CT angiographies performed one and five months postoperatively disclosed a smooth flow distribution along the graft to the branching vessels (Figure 1C). The patient has been doing well without any sequelae during follow-up for 5 years.
DISCUSSION
Melioidosis caused by Burkholderia pseudomallei, a Gram-negative aerobic saprophyte, presents with variable clinical manifestations, including localized abscess, severe pneumonia, and fatal septicemia.5 Melioidosis rarely involves the cardiovascular system, although it has been reported as a pseudoaneurysm in the English literature, which is associated with high morbidity and relapse rates without adequate management.5-7 According to the previous literature, most melioidosis-induced infected pseudoaneurysms are located in the abdominal or thoracic aorta. Few infected pseudoaneurysms appear at peripheral sites, such as the coronary, subclavian, renal, iliac, femoral, or intracranial arteries.6,8,9 To the best of our knowledge, IPIA derived from melioidosis has not been reported.
Because of the high risk of rupture and susceptibility to recrudescence, urgent repair combined with aggressive debridement and prolonged or even lifelong antibiotic therapy is mandatory for a pseudoaneurysm infected with Burkholderia pseudomallei.5,6 However, how to reconstruct the interrupted arterial flow-paths after eradication yet remains unsettled. Several methods have been proposed to restore blood flow after IPIA resection, including simple repair with autologous pericardium,3 carotid-carotid bypass with the internal jugular vein, in situ graft interposition, and endovascular stenting.2,4,7 Among these, extra-anatomical bypass that allows grafting away from the virulent lesion may be the preferable method in managing melioidosis-induced IPIA. This is because Burkholderia pseudomallei has mechanisms to evade host immunity and will remain latent until recurrence at a time of host immune insufficiency.6 Schindler et al. used an autogenous femoral vein graft for the in situ replacement of a subclavian artery pseudoaneurysm. To avoid a relapse due to residual organisms, they placed a pectoralis major muscle flap to cover the vein graft and the cavity after debridement. However, a secondary pseudoaneurysm still arose from the vein graft during intensive antibiotic treatment. Therefore, they discourage in situ graft replacement because of the character of Burkholderia pseudomallei. On the contrary, Luo et al. performed a femoral-femoral extra-anatomical bypass with a synthetic graft for a melioidosis-induced pseudoaneurysm in the left common iliac artery. Their patient was free from graft infection after a short-term follow-up with oral antibiotics. Considering the virulence of Burkholderia pseudomallei, extra-anatomical bypass grafting, which leaves no foreign body in the infected area, seems to be the optimal reconstructive procedure after IPIA resection.
In our patient, the extra-anatomical bypass was constructed first by interposing a 12 mm Hemashield woven graft between the mid-ascending aorta and the third part of the right axillary artery. The noninfected proximal ends of the right common carotid and subclavian arteries were anastomosed to maintain the cerebral blood supply. No prosthetic material was placed in the infected region; nevertheless, the graft provided adequate flow towards the cerebral hemisphere and upper extremity with no steal phenomenon, according to the findings of a postoperative Doppler scan. However, disruption of the repaired proximal arch in the vicinity of the infected pseudoaneurysm is a major concern. Longterm antibiotics and continuous follow-up are still required.
CONCLUSIONS
Infected pseudoaneurysms caused by Burkholderia pseudomallei are uncommon. Moreover, cases involving the innominate artery have not yet been reported in the English literature. Our experience suggests that extraanatomic bypass via the right thoracic cavity could decrease the chance of recurrence when compared to in situ graft interposition.
REFERENCES
- 1.Halpin DP, Nicholson J. Innominate artery pseudoaneurysm presenting as a widened mediastinum. J Thorac Cardiovas Surg. 1995;109:390–391. doi: 10.1016/s0022-5223(95)70402-7. [DOI] [PubMed] [Google Scholar]
- 2.Bush RL, Hurt JE, Bianco CC. Endovascular management of a ruptured mycotic aneurysm of the innominate artery. Ann Thorac Surg. 2002;74:2184–2186. doi: 10.1016/s0003-4975(02)03976-0. [DOI] [PubMed] [Google Scholar]
- 3.Kaushal S, Shake JG, Yuh DD. Mycotic innominate artery pseudoaneurysm presenting as an embolic stroke. J Thorac Cardiovas Surg. 2005;129:945–946. doi: 10.1016/j.jtcvs.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 4.Kieffer E, Chiche L, Koskas F, Bahnini A. Aneurysms of the innominate artery: surgical treatment of 27 patients. J Vasc Surg. 2001;34:222–228. doi: 10.1067/mva.2001.115807. [DOI] [PubMed] [Google Scholar]
- 5.Luo CY, Ko WC, Lee HC, Yang YJ. Relapsing melioidosis as cause of iliacmycotic aneurysm: an indigenous case in Taiwan. J Vasc Surg. 2003;37:882–885. doi: 10.1067/mva.2003.164. [DOI] [PubMed] [Google Scholar]
- 6.Low JGH, Quek AML, Sin YK, Ang BSP. Mycotic aneurysm due to burkholderia pseudomallei infection:case reports and literature review. Clin Infect Dis. 2005;40:193–198. doi: 10.1086/426590. [DOI] [PubMed] [Google Scholar]
- 7.Schindler N, Calligaro KD, Dougherty MJ, et al. Melioidosis presenting as an infected intrathoracic subclavian artery pseudoaneurysmtreated with femoral vein interposition graft. J Vasc Surg. 2002;35:569–572. doi: 10.1067/mva.2002.118592. [DOI] [PubMed] [Google Scholar]
- 8.Tanyaowalak W, Sunthornyothin S, Luengtaviboon K, et al. Mycotic aneurysm caused by burkholderia pseudomallei with negative blood cultures. Scand J Infect Dis. 2004;36:68–70. doi: 10.1080/00365540310017465. [DOI] [PubMed] [Google Scholar]
- 9.Lin LS, Chen CP. Right coronary artery pseudoaneurysm treated by graft-stent deployment. Acta Cardiol Sin. 2011;27:259–262. [Google Scholar]