Abstract
Background
For children with a history of Kawasaki disease (KD), low grade inflammation was generally reported to be associated with persistent coronary artery lesions (CAL). However, this association has not been clearly demonstrated to hold true in KD adolescents and young adults (10-25 years of age).
Methods
We enrolled 104 subjects into our study, who were separated into the following 3 groups and controls: 1): 22 KD patients with angiography-confirmed CAL which persisted for an average of 12.5 years after onset of KD; 2) 38 KD patients with regressed aneurysms; 3) 44 KD patients without any coronary complications from the disease onset; and 4) 31 age-matched (18.7 ± 1.88 years old) healthy controls. Plasma levels of high-sensitivity C reactive protein (hs-CRP) were measured for all participants.
Results
Plasma levels of hs-CRP were significantly higher in KD patients than in the controls, regardless of their coronary severity. However, there was no significant difference in hs-CRP levels among KD patients with different severities of CAL. Of the candidate risk factors of elevated hs-CRP such as body mass index, gender, coronary severity, and levels of high-density lipoprotein-cholesterol, linear regression analysis showed the only independent predictor of hs-CRP levels was BMI (β = 0.306, p = 0.01), rather than patient grouping (p = 0.091).
Conclusions
Our study found that levels of hs-CRP are significantly higher in adolescent and young adult patients with a history of KD, compared with age-matched controls. Low grade inflammation may play a minor role when KD patients enter into adulthood. body mass index (BMI), rather than coronary severity, was independently associated with the elevation of hs-CRP levels, one of biomarkers for further cardiovascular event. Therefore, ongoing control and management of BMI may be one of beneficial strategies that can be employed to help avoid elevation of hs-CRP levels in KD patients.
Keywords: Adolescents, High sensitivity-C reactive protein, Kawasaki disease, Young adult
INTRODUCTION
Kawasaki disease (KD) is an acute systemic vasculitis of unknown etiology, and is the most prevalent pediatric acquired cardiac disease in Taiwan. Coronary artery lesions (CALs) develop in 15-25% of untreated KD children, and may lead to myocardial infarction, sudden death, or ischemic heart disease.1,2 Since the introduction of intravenous immunoglobulin therapy, the incidence of CAL has declined but still occurs in about 5-10 % of KD patients.3,4 Previous studies have generally reported that persistent CALs were associated with low-grade inflammation in school-age children.5,6 However, whether the process of inflammation continues into adulthood in KD patients was infrequently investigated. The present study aimed to evaluate the profiles of inflammation biomarkers (hs-CRP) and elucidate the relationship between the bio-markers and severity of CAL in adult patients years after their KD onset.
METHODS
Study population
A total of 104 Taiwanese adolescent and young adult KD patients were enrolled in our study (male = 74, female = 30), with a mean age of KD onset at 2.9 years-old, and a mean patient age at the time of study of 18.7 year of age. The diagnosis of KD was according to the clinical criteria of the American Heart Association, Council on Cardiovascular Disease in the Young, Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease.7 The CALs were defined as a lumen diameter of at least 3 mm (> 5 y of age, 4 mm) or the internal diameter of a segment at least 1.5 times as large as that of an adjacent segment. The medical records of all study participants were reviewed. Additionally, there were 31 age-matched healthy participants enrolled as the control group.
Biochemical analysis
Venous blood samples were collected from the study subjects at the time of the clinical examination and stored at -80 °C until analysis. Serum total cholesterol (T-CHO), high and low density lipoprotein cholesterol (HDL-c and LDL-c), and triglyceride (TG) were determined using standard laboratory procedures. White blood cell count (WBC) data with percentage of segmented Neutrophils (Seg%) were also collected. hs-CRP levels were measured in plasma by use of a commercially available high-sensitivity mechanism (Immulite®, Siemens, USA).
Statistics
Statistical analysis was performed using PASW Statistics 18. Data are presented as the mean or median as appropriate, whereas frequencies and percentages summarize categorical variables. Comparisons among the four groups were conducted with non-parametric Kruskal-Wallis test (α = 0.05). In order to compare two groups of nonparametric variables, the Mann-Whitney U test was used. Additionally, differences in proportion were tested with the χ2 analysis. Statistical significance was set at p < 0.05.
RESULTS
Clinical characteristics
A total of 104 KD patients were enrolled in this study. From that number, 22 patients had angiography-confirmed CAL (KD-PCAL group), which persisted for an average of 12.5 years after onset of KD. 38 patients had regressed aneurysms (KD-RCAL group) and the remaining 44 patients had no coronary lesions from the time of disease onset (KD-NCAL group). There were also 31 age-matched healthy participants enrolled as the control group. Table 1 summarizes the demographic and clinical characteristics of KD and the control group. Between the subgroups, there was no significant difference in age at study, gender, body mass index (BMI), T-CHO, WBCs and percentage of segmented neutrophils (Seg%). However, compared with the control group, the TG was higher in the KD group (p = 0.038), and the level of HDL-c was lower in the KD group (p < 0.001). Table 2 summarizes the demographic and clinical characteristics of the three subgroups of KD patients. The three KD subgroups did not differ significantly with regard to age at study, BMI, TG, T-CHO, HDL-c and WBCs with percentage of segmented Neutrophils (Seg.).
Table 1. Clinical characteristics between KD and control groups .
| KD (N = 104) | Control (N = 31) | p value | |
| Age at study (year) | 17.5 ± 4.9 | 18.7 ± 1.88 | 0.125 |
| Gender, M | 74 (71.1%) | 22 (71%) | 0.984 |
| BMI (kg/m2) | 21.5 ± 3.7 | 21.6 ± 2.6 | 0.825 |
| TG (mmol/l) | 85.7 ± 49.9* | 62.3 ± 19.6 | 0.038 |
| HDL (mmol/l) | 46.2 ± 10.3* | 54.8 ± 9.1 | < 0.001 |
| T-CHO (mmol/l) | 165.7 ± 28.4 | 165.4 ± 31.6 | 0.873 |
| WBCs (k/μL) | 6826.6 ± 1771.2 | 6300.6 ± 1380.2 | 0.527 |
| Seg. (%) | 58.3 ± 11.2 | 54.7 ± 8.2 | 0.371 |
* p value < 0.05 compared with control.
BMI, body mass index; HDL, high-density lipoprotein; hs-CRP, high-sensitivity C-reactive protein; KD, Kawasaki disease; NCAL, non-coronary artery lesions; PCAL, persist coronary artery lesions; RCAL, regression coronary artery lesions; Seg.(%), segmented neutrophils; T-CHO, total cholesterol; TG, triglyceride; WBCs, white blood cell count.
Table 2. Clinical characteristics of the three subgroups in KD group .
| NCAL (N = 44) | RCAL (N = 38) | PCAL (N = 22) | p value | |
| Age at study (year) | 18.3 ± 5.34 | 16.3 ± 4.67 | 17.8 ± 4.25 | 0.180 |
| Gender, M | 31 (70.5%) | 27(71.1%) | 16 (72.7%) | 0.982 |
| BMI (kg/m2) | 21.1 ± 3.98 | 21.5 ± 2.82 | 22.2 ± 4.3 | 0.674 |
| TG (mg/dL) | 91.4 ± 52.2 | 77.0 ± 50.3 | 89.1 ± 44.7 | 0.199 |
| HDL (mg/dL) | 46.6 ± 11.3 | 46.3 ± 9.3 | 45.1 ± 10.4 | 0.863 |
| T-CHO (mg/dL) | 172.6 ± 27.4 | 158.5 ± 30 | 164.1 ± 25.5 | 0.108 |
| WBCs (k/μL) | 6630.6 ± 1819.4 | 7013.9 ± 1849.2 | 6951.4 ± 1770.9 | 0.628 |
| Seg. (%) | 54.6 ± 13.6 | 62.5 ± 10.3 | 56.9 ± 10.1 | 0.373 |
BMI, body mass index; HDL, high-density lipoprotein; hs-CRP, high-sensitivity C-reactive protein; KD, Kawasaki disease; NCAL, non-coronary artery lesions; PCAL, persist coronary artery lesions; RCAL, regression coronary artery lesions; Seg. (%), segmented Neutrophils; T-CHO, total cholesterol; TG, triglyceride; WBCs, white blood cell count.
Levels of hs-CRP of each study group
Kruskal-Wallis test followed by Mann-Whitney U test showed that patients in all KD subgroups (KD-NACL, KD-RCAL and KD-PCAL) had significantly higher hs-CRP levels than found in the controls. However, there was no significant difference in levels of hs-CRP among these three KD subgroups. Figure 1 shows the plasma levels of hs-CRP in each subgroup.
Figure 1.
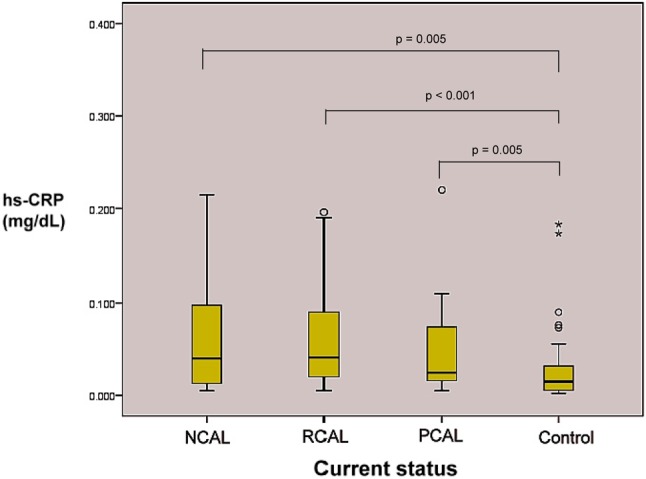
High sensitivity C-reactive protein (hs-CRP) in each KD subgroup and control. NCAL, non-coronary artery lesions; PCAL, persist coronary artery lesions; RCAL, regression coronary artery lesions.
Risk factors of elevated hs-CRP levels
Univariate analysis showed, among the candidate risk factors of elevated hs-CRP (including gender, patient grouping, BMI, TG, HDL and T-CHO), BMI (β = 0.33, p = 0.004), levels of TG (β = 0.340, p = 0.001) and HDL (β = -0.278, p = 0.007), rather than patient coronary grouping, were the only three factors associated with the elevation of hs-CRP levels.
Multiple linear regression analysis
To avoid confounding of risk factors and properly evaluate their independent effects on the levels of hs-CRP, we conducted multiple linear regression analysis. Because there was a significant correlation between levels of HDL and TG (r = -0.388, p < 0.001), we only used three risk factors in our analysis: BMI, levels of HDL and patient grouping based on coronary severity into the next-step multiple linear regression. We found BMI was the only independent risk factor for elevated hs-CRP (β = 0.31, p = 0.01). We found no significant association between hs-CRP and coronary severity (β = -0.191, p = 0.091) in this regression analysis.
DISCUSSION
Many earlier reports have recognized a significantly elevated serum CRP level in the acute phase of Kawasaki disease.8 It was later demonstrated in school-aged children that KD with persistent coronary artery lesion was independently associated with levels of high sensitivity C-reactive protein.6,9-11 However, serum CRP levels in KD patients without any earlier coronary arterial involvement showed inconsistent results in previous studies.6,9-11 The current study demonstrated, in adolescents and young adults after KD onset, that levels of hs-CRP were significantly higher than those of the control group; the presence of CAL did not affect these results.
Echocardiography and coronary angiography are recommended examinations to evaluate the long-term changes of coronary lesions in KD patients. However, intravascular ultrasound studies on the coronary arteries of KD patients with persistent or regressed coronary aneurysms showed marked thickening of the intima-media complex even in angiographically normal coronary segments.12,13 Furthermore, endothelial dysfunction may also persist for an extended period of time even after aneurysms regression.14,15 Previous studies have also demonstrated that CRP levels are inversely associated with endothelial synthesis of nitric oxide.16 Together, the thickening intima and the endothelial dysfunction might contribute to the persistent elevation of CRP levels in KD children when they grow into young adulthood.
Another interesting finding of our study is there was no significant difference of hs-CRP levels among KD patients with different coronary severities at very late disease stages. This result was different from those in two earlier studies,6,9 both of which showed increased concentrations of hs-CRP were associated with persistent CALs in KD patients. The mean intervals from diagnosis to time of study in these two studies were 7.8 and 10.8 years, respectively. In the current study, the enrolled KD patient ages were somewhat older (mean 17.5 years), and the intervals between the disease onset and date of study were relatively longer (mean 14.4 years). Other pathological studies of KD patients had also suggested that, in the late stage of KD, changes in the coronary aneurysm wall consisted of thickened intima and abundant smooth muscle cells, but only limited macrophages or fatty streaks.17,18 Therefore, we may infer that the role of inflammation in Kawasaki disease may decrease with age, especially when KD patients enter adulthood.
In this study, we observed that body mass index (BMI), rather than coronary severity, was an independent risk factor of elevated hs-CRP levels in KD patients. Several studies have demonstrated an association between BMI and CRP levels in apparently healthy populations.19,20 Furthermore, level of CRP was one of biomarkers for preclinical cardiovascular disease.21,22 Additionally, in patients with acute coronary syndrome, increased CRP levels were strongly associated with future poor outcomes. Furthermore, approximately 5% of young adults (< 40 years of age) who underwent coronary angiography to evaluate symptoms for myocardial ischemia may have a history of childhood KD.23 Therefore, in KD patients, BMI-related elevation of CRP levels may potentiate the risk of premature atherosclerosis when they grow up and reach adulthood. Appropriate intervention such as diet control and exercise to modify BMI may help to minimize the cardiovascular risk of KD patients.
LIMITATIONS
There were limitations in the current study. First, data upon which this study was based was derived from experiences at a single institution, which further involved a small study group sample size. Second, we didn’t explore the profiles of other potential inflammation biomarkers such as cytokines cellular adhesion molecules and myeloperoxidase. Therefore, it is difficult effectively recognize the interplay among hs-CRP and other inflammation biomarkers in the KD patients when they reach adulthood. Third, our study was limited by its cross-sectional rather than a longitudinal design. However, after multivariate analysis that considered possible factors, BMI was still significantly associated with elevated hs-CRP levels. However, further large scale studies involving more participants are needed.
CONCLUSIONS
Levels of hs-CRP are significantly higher in adolescent and young adult patients with a history of KD, compared to their age-matched controls. However, BMI, rather than coronary severity, was independently associated with the elevation of hs-CRP levels, and one of the biomarkers for further cardiovascular event. The results of our study indicate that low grade inflammation may play a minor role when KD patients enter into adulthood, and that BMI control maybe efficacious in any ongoing effort to avoid elevation of hs-CRP levels in KD patients.
REFERENCES
- 1. Dajani AS, Taubert KA, Gerber MA, et al. Diagnosis and therapy of Kawasaki disease in children. Circulation. 1993;87:1776–1780. doi: 10.1161/01.cir.87.5.1776. [DOI] [PubMed] [Google Scholar]
- 2. Kato H, Sugimura T, Akagi T, et al. Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation. 1996;94:1379–1385. doi: 10.1161/01.cir.94.6.1379. [DOI] [PubMed] [Google Scholar]
- 3. Dajani AS, Taubert KA, Takahashi M, et al. Guidelines for long-term management of patients with Kawasaki disease. Report from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 1994;89:916–922. doi: 10.1161/01.cir.89.2.916. [DOI] [PubMed] [Google Scholar]
- 4. Durongpisitkul K, Gururaj VJ, Park JM, Martin CF. The prevention of coronary artery aneurysm in Kawasaki disease: a meta-analysis on the efficacy of aspirin and immunoglobulin treatment. Pediatrics. 1995;96:1057–1061. [PubMed] [Google Scholar]
- 5. McConnell ME, Hannon DW, Steed RD, Gilliland MG. Fatal obliterative coronary vasculitis in Kawasaki disease. J Pediatr. 1998;133:259–261. doi: 10.1016/s0022-3476(98)70230-6. [DOI] [PubMed] [Google Scholar]
- 6. Cheung YF, Ho MH, Tam SC, Yung TC. Increased high sensitivity C reactive protein concentrations and increased arterial stiffness in children with a history of Kawasaki disease. Heart. 2004;90:1281–1285. doi: 10.1136/hrt.2003.018507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004;114:1708–1733. doi: 10.1542/peds.2004-2182. [DOI] [PubMed] [Google Scholar]
- 8. Anderson MS, Burns J, Treadwell TA, et al. Erythrocyte sedimentation rate and C-reactive protein discrepancy and high prevalence of coronary artery abnormalities in Kawasaki disease. Pediatr Infect Dis J. 2001;20:698–702. doi: 10.1097/00006454-200107000-00011. [DOI] [PubMed] [Google Scholar]
- 9. Mitani Y, Sawada H, Hayakawa H, et al. Elevated levels of high-sensitivity C-reactive protein and serum amyloid-A late after Kawasaki disease: association between inflammation and late coronary sequelae in Kawasaki disease. Circulation. 2005;111:38–43. doi: 10.1161/01.CIR.0000151311.38708.29. [DOI] [PubMed] [Google Scholar]
- 10. Borzutzky A, Gutierrez M, Talesnik E, et al. High sensitivity C-reactive protein and endothelial function in Chilean patients with history of Kawasaki disease. Clin Rheumatol. 2008;27:845–850. doi: 10.1007/s10067-007-0808-6. [DOI] [PubMed] [Google Scholar]
- 11. Ou CY, Tseng YF, Lee CL, et al. Significant relationship between serum high-sensitivity C-reactive protein, high-density lipoprotein cholesterol levels and children with Kawasaki disease and coronary artery lesions. J Formos Med Assoc. 2009;108:719–724. doi: 10.1016/S0929-6646(09)60395-8. [DOI] [PubMed] [Google Scholar]
- 12. Sugimura T, Kato H, Inoue O, et al. Intravascular ultrasound of coronary arteries in children. Assessment of the wall morphology and the lumen after Kawasaki disease. Circulation. 1994;89:258–265. doi: 10.1161/01.cir.89.1.258. [DOI] [PubMed] [Google Scholar]
- 13. Suzuki A, Yamagishi M, Kimura K, et al. Functional behavior and morphology of the coronary artery wall in patients with Kawasaki disease assessed by intravascular ultrasound. J Am Coll Cardiol. 1996;27:291–296. doi: 10.1016/0735-1097(95)00447-5. [DOI] [PubMed] [Google Scholar]
- 14. Yamakawa R, Ishii M, Sugimura T, et al. Coronary endothelial dysfunction after Kawasaki disease: evaluation by intracoronary injection of acetylcholine. J Am Coll Cardiol. 1998;31:1074–1080. doi: 10.1016/s0735-1097(98)00033-3. [DOI] [PubMed] [Google Scholar]
- 15. Iemura M, Ishii M, Sugimura T, et al. Long term consequences of regressed coronary aneurysms after Kawasaki disease: vascular wall morphology and function. Heart. 2000;83:307–311. doi: 10.1136/heart.83.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cleland SJ, Sattar N, Petrie JR, et al. Endothelial dysfunction as a possible link between C-reactive protein levels and cardiovascular disease. Clin Sci (Lond) 2000;98:531–535. [PubMed] [Google Scholar]
- 17. Liu AM, Ghazizadeh M, Onouchi Z, Asano G. Ultrastructural characteristics of myocardial and coronary microvascular lesions in Kawasaki disease. Microvasc Res. 1999;58:10–27. doi: 10.1006/mvre.1999.2155. [DOI] [PubMed] [Google Scholar]
- 18. Suzuki A, Miyagawa-Tomita S, Nakazawa M, Yutani C. Remodeling of coronary artery lesions due to Kawasaki disease: comparison of arteriographic and immunohistochemical findings. Jpn Heart J. 2000;41:245–256. doi: 10.1536/jhj.41.245. [DOI] [PubMed] [Google Scholar]
- 19. Acevedo M, Arnaiz P, Barja S, et al. Relationship of C-reactive protein to adiposity, cardiovascular risk factors and subclinical atherosclerosis in healthy children. Rev Esp Cardiol. 2007;60:1051–1058. doi: 10.1157/13111237. [DOI] [PubMed] [Google Scholar]
- 20. Brooks GC, Blaha MJ, Blumenthal RS. Relation of C-reactive protein to abdominal adiposity. Am J Cardiol. 2010;106:56–61. doi: 10.1016/j.amjcard.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 21. Rohde LE, Hennekens CH, Ridker PM. Survey of C-reactive protein and cardiovascular risk factors in apparently healthy men. Am J Cardiol. 1999;84:1018–1022. doi: 10.1016/s0002-9149(99)00491-9. [DOI] [PubMed] [Google Scholar]
- 22. Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 23. Daniels LB, Tjajadi MS, Walford HH, et al. Prevalence of Kawasaki disease in young adults with suspected myocardial ischemia. Circulation. 2012;125:2447–2453. doi: 10.1161/CIRCULATIONAHA.111.082107. [DOI] [PMC free article] [PubMed] [Google Scholar]


