Abstract
Background
To determine the B-type natriuretic peptide (BNP) level in pediatric septic patients, and to investigate its association with cardiovascular dysfunction and clinical outcome.
Methods
Pediatric patients with sepsis or septic shock were prospectively enrolled in our pediatric intensive care unit (PICU). On day 1 of admission, plasma BNP levels were measured at the time-point of echocardiography. Myocardial dysfunction was defined as left ventricular fractional shortening (FS) < 30%. Inotropic support was quantified by inotropic scores and disease severity was assessed by Pediatric Risk of Mortality (PRISM) III scores. Therafter, associations between BNP levels and clinical parameters were analyzed.
Results
There were 94 patients (mean: 5.6 yr, range: 2 mo-17 yr) that were consecutively enrolled in this study. The median BNP level was 127 pg/ml (range: 5 to 4950 pg/ml). BNP levels were correlated with PRISM III (rho = 0.36, p = 0.001) and C-reactive protein level (r = 0.39, p = 0.001). The median BNP levels were not only higher in patients with septic shock (n = 34) than those with sepsis (n = 58) (213 vs. 54 pg/ml, p = 0.0004), but also higher in patients with myocardial dysfunction (n = 18) than those with preserved myocardial function (n = 66) (765 vs. 65 pg/ml, p < 0.001). We also found that BNP levels correlated negatively with FS (r = -0.56, p < 0.001) and positively with inotropic scores (r = 0.34, p = 0.04). Most importantly, the median BNP levels were higher in non-survivors (n = 13) than survivors (n = 81) (367 vs. 106 pg/ml, p = 0.003).
Conclusions
BNP levels are elevated in pediatric septic patients early in the disease course, and increased levels are associated with cardiovascular dysfunction and worse clinical outcome.
Keywords: B-type natriuretic peptide, Cardiac function, Pediatric, Sepsis, Septic shock
INTRODUCTION
Sepsis, defined as the systemic inflammatory response syndrome that occurs during infection,1 is a common cause of morbidity and mortality in children.2 Sepsis can proceed uncontrollably into septic shock, resulting in dysfunction of multiple organs, including the brain, kidney, liver, and the cardiovascular system. It has been found in adult septic patients where dysfunction of the cardiovascular system is associated with a significantly increased mortality rate of 70-90%, compared with 20% for those patients without cardiovascular impairment.3 Even though the exact mechanisms are not fully understood, sepsis-induced myocardial depression is a well-described phenomenon, which was observed within 2-3 days after the onset of sepsis, characterized by a marked deterioration of left ventricular ejection fraction.4,5
In children, sepsis can manifest with complex hemodynamic responses, including myocardial dysfunction, vasomotor dysfunction or a combination of both.6 In addition, it has been found that younger children are more likely to have alterations primarily in cardiac function whereas older children are more likely to have alterations in peripheral vascular tone.7,8 Given the varied nature of cardiac and vasomotor perturbation, timely identification and management of myocardial dysfunction in pediatric patients with sepsis is important but challenging.
There are several factors impeding timely recognition of sepsis-induced myocardial dysfunction in pediatric patients in current clinical settings. First, sympathetic activation observed in early sepsis stimulates the myocardium inotropically and chronotropically, and potentially masks myocardial depression. Second, thorough assessment of cardiac performance is often evaluated after initiation of hemodynamic support, including fluid resuscitations and inotropes. Third, the value of the Swan-Ganz catheter to evaluate myocardial performance has been doubted after emerging evidence showed a poor correlation between cardiac function evaluated by echocardiography,9,10 where such invasive monitoring is often not possible in younger children owing to size limitations. Finally, even though echocardiography is an established modality to evaluate cardiac function, its widespread use is limited due to lack of 24-hr availability of a bedside ultrasound and an experienced pediatric cardiologist in some PICUs. Therefore, an objective biomarker reflecting the cardiac function in septic children can be beneficial in the management of this life-threatening illness.
B-type natriuretic peptide (BNP), a 32 amino acid polypeptide hormone produced by cardiac ventricles in response to myocyte stretch, with diuretic, natriuretic and vasoactive properties,11-13 has been widely used as a diagnostic tool for congestive heart failure.14,15 In pediatric patients with congenital heart disease, we have previously shown the role for BNP as a biomarker for diagnosis, prognostication, and management.16-18 Given that cardiac dysfunction may develop in the context of sepsis, there is emerging evidence suggesting that BNP level is elevated in adult patients with sepsis and has prognostic implications.19-22 Unfortunately, substantially less data are available on its role in the intensive care of septic children.
In this pilot study, we hypothesized that elevated BNP levels would be associated with worse clinical outcome in a cohort of pediatric patients with sepsis and septic shock. Therefore, the objectives of this study were: (1) to determine BNP levels in infants and children with sepsis and septic shock, and (2) to investigate associations between BNP levels and cardiac function and clinical outcome.
METHODS
Patient population
A prospective cohort study enrolling consecutive children with sepsis or septic shock was conducted in the pediatric intensive care unit (PICU) at Kaohsiung Medical University Hospital. The Institutional Review Board at Kaohsiung Medical University Hospital approved this study (KMUH-IRB-970407) and informed parental consent was obtained for all participants.
Diagnosis of sepsis and septic shock was made according to the criteria defined by the International Pediatric Sepsis Consensus Conference.23 Using these consensus criteria, sepsis was defined as the systemic response to infection manifested by two or more of the following conditions as the result of suspected or confirmed infection: 1) temperature of > 38 °C or < 36 °C, 2) heart rate of > 90th percentile for age, 3) respiratory rate of > 90th percentile for age, apnea for > 15 seconds or mechanical ventilation, and 4) a white blood cell count of > 12,000 cells/mm3 or < 4000 cells/mm3 or the presence of > 10% bands. Septic shock was defined as sepsis with evidence of cardiovascular dysfunction. Cardiovascular dysfunction was defined as persistent hypotension, need for vasoactive drug to maintain blood pressure or perfusion abnormalities despite adequate fluid resuscitation (> 40 mL/kg within one hour). Examples of perfusion abnormalities include lactic acidosis, decreased peripheral pulses, mottled or cool extremities, prolonged capillary refill, oliguria (urine output < 1 mL/kg/hr), or an acute alteration in mental status. For this study, patients with septic shock were required to be receiving at least one vasoactive agent at the time of enrollment.
Patients were excluded if they were less than two weeks old or over 18 years of age upon admission, had clinical or echocardiographic evidence of pre-existing heart diseases, including congenital heart defects, cardiomyopathy, myocarditis, pericarditis, endocarditis, Kawasaki’s disease, cardiopulmonary resuscitation (cardioversion or defibrillation) within the previous 30 days or those who were with extracorporeal life support at admission.
Study protocol
Within 24 hrs after admission, after initial management for hemodynamic stabilization including fluid resuscitation and inotrope support, patients were classified as sepsis or septic shock. After consent was obtained, blood for BNP determinations was collected and echocardiograms were performed at the same time point. Echocardiographic studies were performed using a Phillips SONOS 7500 echocardiographic scanner (Phillips, Andover, MA, USA). All echocardiographic studies were performed by the same pediatric cardiologist to avoid inter-observer variability. He was also blinded to the BNP level during the study period. The echocardiogram was used to exclude previously undiagnosed cardiac defects and evaluate systolic function. Standard measures of left ventricular systolic function defined as fractional shortening (FS) calculated by standard M mode in the parasternal long-axis view.24 A FS < 30% was considered myocardial dysfunction.25 In addition to echocardiography, myocardial dysfunction was evaluated by the amount of maximal inotropic support within 24 hrs after admission. Inotrope support was quantified by a score adapted from Wernovsky et al.26 This technique uses inotrope requirement as an indirect index of cardiac dysfunction and has been used in our previous study and other investigations.18,27,28 The score was calculated from the level of inotropic support the patients were receiving (in mcg/kg/min), according to the following equation: dopamine + dobutamine + [(epinephrine + norepinephrine) × 100] + (milrinone × 20).
The management strategy for all of these patients followed standard institutional practices. The medical teams involved in the management of the patients were blinded to the BNP values.
Sample and data collection
Blood samples were obtained from patients within 24 hrs after admission. BNP levels were determined by the same method as we previously described.17 In brief, the samples were placed in EDTA tubes, and centrifuged at 3000 rpm for 15 minutes at 4 °C. Separated plasma was stored at minus 70 °C. For BNP determinations, the plasma was thawed to room temperature and BNP levels were measured using a commercially available fluorescence immunoassay (Triage® Meter Plus, Biosite® Diagnostics, San Diego, CA, USA). The measurable range of BNP on this device is between 5 to 5000 pg/ml, and the estimated coefficient of variation for the assay is 9.2-11.4%.
Clinical data were collected from patient medical records by an observer blinded to the BNP data and included in a relational access database. Clinical data included: age, weight, gender, intensive care unit (ICU) stay, primary source of infection, culture results, medications administered, white blood cell count and plasma level of C-reactive protein (CRP), and the presence of inotropic support. Severity of illness was measured using the adjusted Pediatric Risk of Mortality (PRISM) III score for the first day of ICU admission.29 All patients included in the analysis were followed-up until discharge from the PICU or death.
Data analysis
Descriptive statistics were computed for each of the variables, including means, medians, standard deviations, ranges and interquartile ranges. Values were expressed as mean ± standard deviation or median (range) depending on the presence of normal distribution. Spearman’s correlations were used to examine the association between each pair of continuous measures. Differences in the continuous variables between groups were tested with the Student’s t-test or the Mann Whitney U-test. A significance level of 0.05 was used for all statistical tests. Statistical analyses were performed with the use of Stata 6.0 (Stata Corp, College Station, Texas, USA), and graphs created with the use of Prism 6.0 (GraphPad Software, Inc, San Diego, California, USA). Multivariate analysis was performed using JMP software version 9.00 (SAS Institute, Cary, NC, USA).
RESULTS
Patient characteristics
A total of 94 patients were enrolled in our study, and patient characteristics for the entire cohort are shown in Table 1. The mean age was 5.6 (range 0.1-17) years. There were 36 (39%) patients diagnosed with sepsis and 58 (61%) patients diagnosed with septic shock. At day 1, the mean WBC count was 12005/μL and the mean CRP level was 138 mg/L (normal range: < 5 mg/L). The mean FS was 34.3%, with 18 patients (19%) with myocardial dysfunction (FS < 30%) and 76 patients (81%) without myocardial dysfunction (FS ≥ 30%).
Table 1. Characteristics of pediatric patients with sepsis and septic shock .
| Patient number | 94 |
| Age, year | 5.6 ± 4.8 |
| Weight, kg | 21.3 ± 16.0 |
| Male, n (%) | 43 (46%) |
| PRISM III score | 6.7 ± 6.7 |
| Classifications of patients | |
| Sepsis, n (%) | 58 (61%) |
| Septic shock, n (%) | 36 (39%) |
| Fractional shortening, % | 34.3 ± 5.3% |
| WBC, μL-1 | 12,005 ± 8,680 |
| CRP level, mg/L | 138 ± 122 |
| Primary sites of infection | |
| Lung, n (%) | 54 (58%) |
| Urinary tract, n (%) | 13 (14%) |
| Abdominal, n (%) | 8 (9%) |
| Blood, n (%) | 6 (6%) |
| Catheter-related, n (%) | 5 (5%) |
| Soft tissue, n (%) | 5 (5%) |
| Central nervous system, n (%) | 3 (3%) |
| PICU mortality, n (%) | 13 (14%) |
CRP, C-reactive protein; PICU, pediatric intensive care unit; PRISM, pediatric risk of mortality; WBC, white blood cell.
The mortality rate in this cohort was 14% (n = 13). The comparisons of characteristics of survivors and non-survivors are shown in the Table 2. As shown in this table, there were no significant differences of age, weight, gender, mean arterial pressure, heart rate, FS and laboratory data including white blood cell (WBC), CRP, blood urea nitrogen (BUN) and creatinine. However, there was a significant difference of disease severity and need of inotropic support at day 1 as defined by PRISM III and inotrope score, respectively.
Table 2. Comparisons of baseline characteristics between non-survivors and survivors .
| Non-survivor | Survivor | p | |
| Patient number | 13 | 81 | |
| Age, year | 6.4 ± 0.4 | 5.5 ± 0.5 | 0.41 |
| Weight, kg | 24.7 ± 12.5 | 20.6 ± 16.4 | 0.08 |
| Male, n (%) | 7 (54%) | 36 (44%) | 0.56 |
| PRISM III | 11.0 ± 7.4 | 5.9 ± 6.4 | 0.01 |
| WBC, μL-1 | 10,548 ± 9,382 | 12,223 ± 8,612 | 0.53 |
| CRP, mg/L | 150 ± 112 | 137 ± 124 | 0.71 |
| Inotropic score | 12.7 ± 18.0 | 4.2 ± 6.7 | 0.002 |
| Fractional shortening, % | 34 ± 9 | 35 ± 5 | 0.53 |
| Mean arterial pressure, mmHg | 71 ± 23 | 73 ± 13 | 0.76 |
| Heart rate, min-1 | 133 ± 15 | 125 ± 26 | 0.32 |
| Blood urea nitrogen | 14.9 ± 3.3 | 17.3 ± 2.5 | 0.70 |
| Creatinine | 0.46 ± 0.30 | 0.59 ± 0.38 | 0.26 |
CRP, C-reactive protein; PRISM, pediatric risk of mortality; WBC, white blood cell.
High BNP levels are associated with increased disease severity and worse clinical outcome
The BNP levels at day 1 ranged from 5-4950 pg/ml with a median value of 127 pg/ml. We found that BNP levels were positively correlated with PRISM III score calculated at day 1 (Spearman’s rho = 0.36, p = 0.001) (Figure 1A). In addition, BNP levels were positively correlated with serum CRP levels (Spearman’s rho = 0.39, p = 0.001) (Figure 1B), which is an important indicator of systemic inflammation. More importantly, we found BNP levels were higher in non-survivors than survivors [median (range): 367 (53-4,950) pg/ml vs. 106 (5-3,979) pg/ml, p = 0.003] (Figure 2). Of note, since PRISM III score and inotropic score are also significantly higher in non-survivors (Table 2), we did multivariate analysis to further evaluate the independent effects of BNP, PRISM III score and inotropic score on the mortality of children with sepsis. We found that only the PRISM III score remained significantly associated with mortality (p = 0.041, odds ratio = 1.087, 95% CI = 1.003-1.178).
Figure 1.
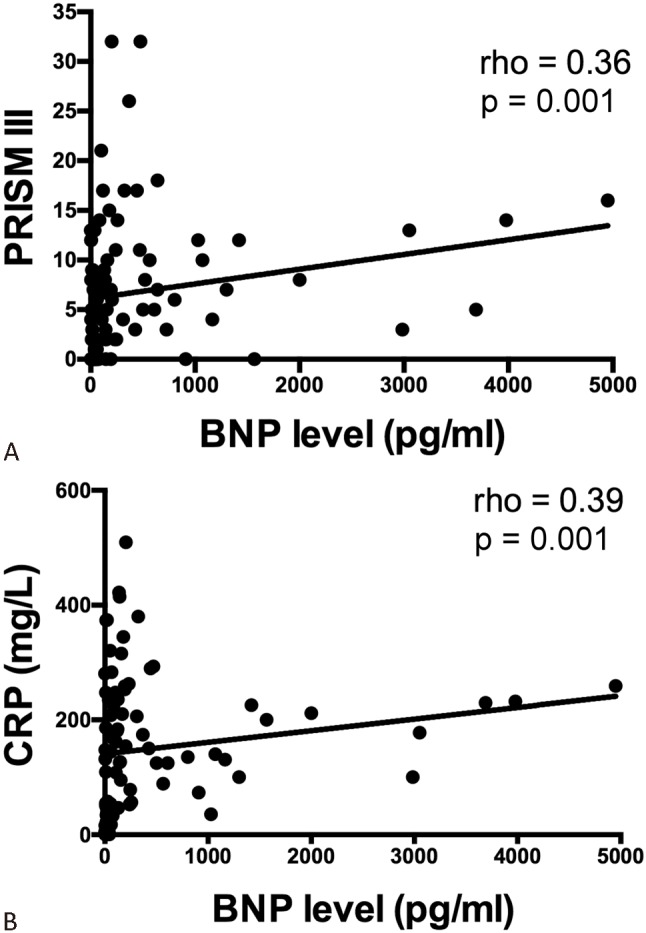
Scatter plots showing the relationship between BNP levels and PRISM III scores (A) and CRP (B). Higher BNP levels were associated with higher PRISM III and CRP. Abbreviations are in Table 1; BNP, B-type natriuretic peptide.
Figure 2.
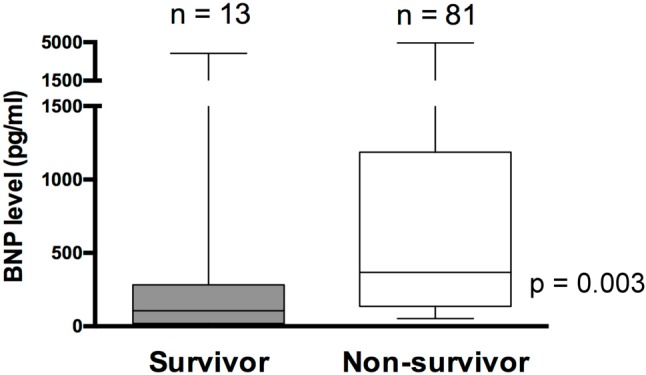
Box plots showing the relationship of BNP to PICU mortality. BNP levels were higher in non-survivors. Boxes show the interquartile range with the midline indicating the median level. I-bars represent the highest and lowest values. Abbreviations are in Table 1; BNP, B-type natriuretic peptide.
High BNP levels are associated with cardiac dysfunction
In patients with myocardial dysfunction (FS < 30%), the BNP levels were higher than those with preserved myocardial function (FS ≥ 30%) [765 (84-4,950) pg/ml vs. 65 (5-2,001) pg/ml, p < 0.001] (Figure 3A). Likewise, the BNP levels were significantly higher in patients with septic shock than those with sepsis [213 (11-4,950) pg/ml vs. 54 (5-2,001) pg/ml, p = 0.0004] (Figure 3B).
Figure 3.
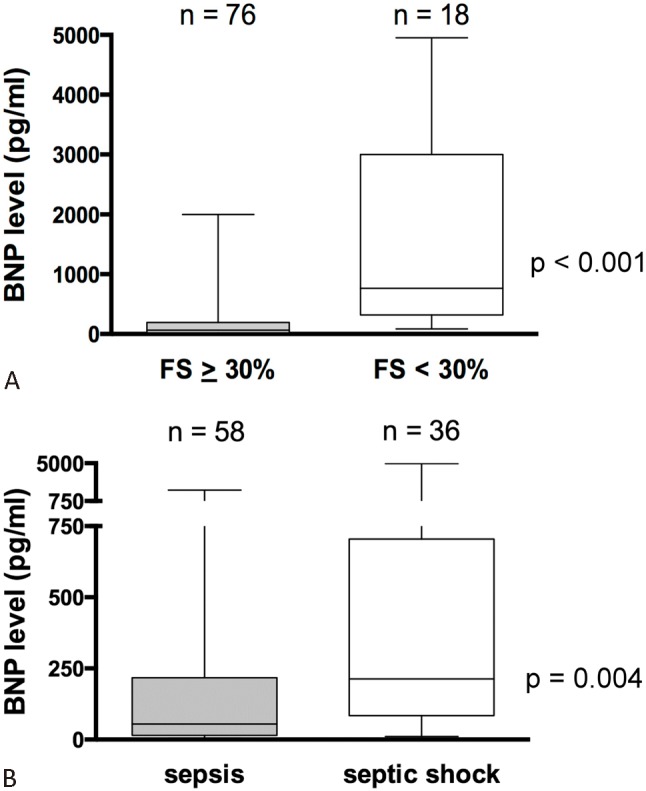
Box plots showing the relationship of BNP to presence of shock (A) and cardiac dysfunction (B). BNP levels were higher in patients with septic shock, and those with cardiac dysfunction. Boxes show the interquartile range with the midline indicating the median level. I-bars represent the highest and lowest values. BNP, B-type natriuretic peptide.
We further analyzed the correlation between BNP level and myocardial function. We found that BNP levels were negatively correlated with left ventricular FS (Spearman’s rho = -0.56, p < 0.001) (Figure 4A) and positively correlated with inotropic scores (Spearman’s rho = 0.34, p = 0.04) (Figure 4B). To further analyze subgroups of patients with sepsis and septic shock, we found that BNP levels were negatively correlated with left ventricular FS within both subgroups (Spearman’s rho = -0.52, p < 0.01 in sepsis and Spearman’s rho = -0.36, p = 0.02 in septic shock).
Figure 4.
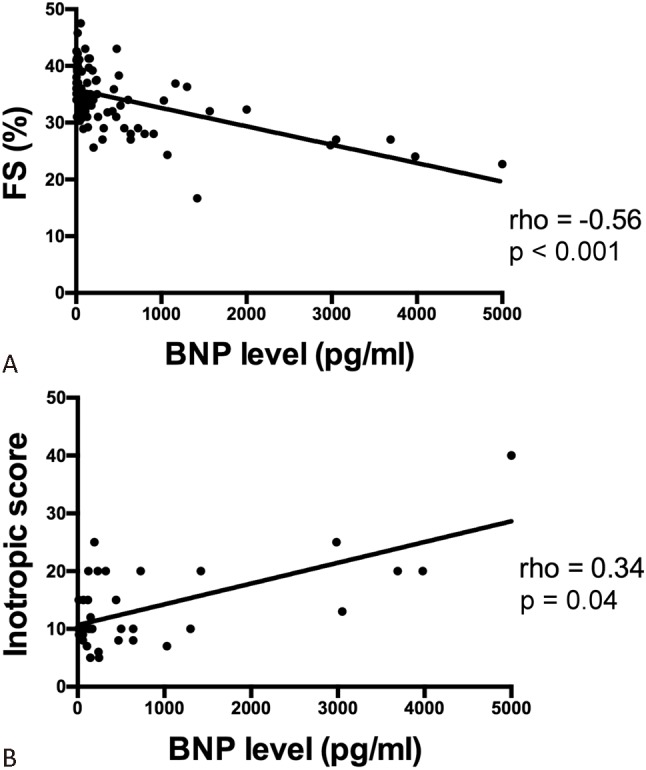
Scatter plots showing the relationship between FS (A) and inotropic scores (B). Higher BNP levels were associated with lower FS and higher inotropic scores. N = 36 in B because inotropes were only administered in patients with septic shock. BNP, B-type natriuretic peptide; FS, fractional shortening.
BNP levels were similar between the primary sites of infection. BNP levels were not correlated with age, gender, WBC count, BUN and creatinine.
DISCUSSION
In this cohort of children with sepsis and septic shock, elevated BNP levels were associated with cardiac dysfunction and worse clinical outcome. Specifically, we found that BNP levels obtained during the first day of diagnosis were inversely correlated with the left ventricular systolic function determined by echocardiography. In addition, we found that BNP levels were positively correlated with degree of disease severity and required inotropic support. Furthermore, BNP levels were higher in patients with septic shock, myocardial depression and in non-survivors. Taken together, these data indicate that cardiac function may impact outcome adversely in this septic children, and that BNP levels early in the disease course may provide useful prognostic information. To our knowledge, the present study is the first to examine associations between BNP and clinical outcomes in a pediatric cohort with sepsis and septic shock.
The exact mechanism of elevation of BNP in sepsis is not fully understood; however, we assume two possible explanations as observed in our study. First, we speculate that myocardial depression plays a major role to induce BNP elevations, since our results are consistent with previous studies in septic adults showing the association between BNP levels and cardiac index measured by Swan-Ganz catheters or left ventricular myocardial dysfunction assessed with echocardiography.21,22 Second, growing evidence suggests that BNP may also reflect the degree of inflammation. Recent in vitro studies demonstrated that pro-inflammatory cytokines and lipopolysaccharides can stimulate gene expression and secretion of BNP by cardiomyocytes,30,31 indicating that severe infection and inflammation may stimulate BNP generation by myocardium independent of hemodynamic changes. In the present study, we found the correlation between BNP levels and CRP, which may partly accounts for elevated BNP levels in some patients with sepsis but without myocardial depression. Nevertheless, irrespective of the cause for its elevation, our results indicate that BNP may have a role as a predictor of mortality for septic children.
Other biological markers have been used to detect myocardial dysfunction in sepsis. The N-terminal pro-BNP, the inactive by-product of BNP production with similar utility as a biomarker in cardiac diseases, has also been found elevated in patients with sepsis. However, we believe that BNP levels may be better suited to follow dynamic alterations in myocardial performance given the shorter circulating half-life of BNP compared to NT-proBNP (20 minutes vs. 60-120 minutes).32 In addition, NT-proBNP is excreted by the kidney,33 while BNP is degraded by endocytosis through the trans-membrane natriuretic peptide receptor C (NPR-C) in many tissues, or cleaved by neutral endopeptidases found in vascular cells and renal tubules.34 Thus renal function, which is often impaired by sepsis, has a greater influence on NT-proBNP levels than BNP levels. Indeed, the BNP level was not correlated with renal function in the present study. Recently, another biomarker troponin I has also been shown correlated with cardiac function and disease severity in children with septic shock.35 However, since troponin is released after injury or death of cardiomyocytes, we speculate that BNP can reflects myocardial stress rather than direct cellular injury and would reveal myocardial dysfunction earlier than troponin I.
Our results are in line with recent studies examining BNP in adults with sepsis. Charpentier et al.22 reported that BNP was elevated in septic adults and found that BNP measured on day 1 was significantly higher in those patients with myocardial dysfunction when compared to those patients without cardiac compromise. Kandil et al.20 also demonstrated that in adults, the admission BNP levels were correlated with disease severity assessed by SOFA score and were higher in those with septic shock than those with sepsis. Furthermore, Post et al.19 found that in adults with septic shock, elevated BNP levels in days 3 and 5 were associated with cardiac dysfunction and mortality. To our knowledge, there has been only one pediatric report investigating the BNP levels in sepsis.36 In this study, 13 children with septic shock, 12 healthy controls and 5 PICU controls were enrolled, and Domico et al.36 found that admission BNP levels were higher in children with septic shock than healthy and PICU controls [median: 115 pg/mL (range: 26-2,960) vs. 9 pg/mL (5-30) and 10 pg/mL (5-30)]. The BNP levels were also correlated with PRISM III score and FS. However, there was no association between mortality and BNP levels found due to small sample size (2 non-survivors), and no patients with sepsis only (without shock) were included. Thus, to our knowledge, the present study is the first to report different BNP levels not only between survivors and non-survivors, but also between septic shock and sepsis in children. Such information would be helpful in risk stratification and identification of those potentially requiring inotropic support in septic children in the setting of critical care.
There are several limitations of this pilot study. We only measured BNP levels and performed echocardiography at day 1, while myocardial dysfunction or changes of BNP levels may develop later in the clinical course and may add more prognostic information as shown in some adult studies.19,22 We also did not perform more sophisticated echocardiographic analysis, such as wall stress to assess preload-independent systolic function or tissue Doppler to evaluate diastolic function. In addition, in the present study we could not rule out the possibility that BNP levels and myocardial function could be affected by fluid challenges and use of inotropes during initial management of sepsis. However, all our patients were studied while in stable conditions after aggressive resuscitations to minimize these confounding effects. Finally, as shown in the multivariate analysis that PRISM III score was the only independent risk factor of mortality, we believe that BNP cannot replace risk-stratification scale like PRISM III score derived from multi-organ system, but it may serve as a supplemental point-of-care biomarker promptly reflecting cardiovascular status with its unique advantage of short half-life (20 minutes).
Although this pilot study did not include BNP levels from a healthy group of children, several studies have documented BNP levels at different ages in normal subjects.37,38 In these studies, mean BNP levels were approximately 10 pg/ml in children > 2 weeks of age, significantly lower than the median level of 127 pg/ml for septic children in our study. However, it is unlikely that the primary utility of BNP levels will be in distinguishing normal patients from those with critically septic patients in PICU, but rather from identifying patients who might benefit from increased cardiac support. In addition, we found that in children with sepsis, there was a wide range of BNP levels (median: 54 pg/ml; range: 5-2,001 pg/ml), which were negatively correlated with left ventricular FS. Although this subgroup of patients did not require inotropic support in our study, these elevations could reflect subtle or transient myocardial depression in early sepsis. These data imply that intensivists may consider elevated BNP level as a reminder for further echocardiographic evaluation in children with sepsis.
In conclusion, we found BNP levels are elevated in pediatric patients with sepsis and septic shock early in the disease course, and increased levels are associated with a greater severity of illness, cardiac dysfunction and mortality. These data indicate BNP can be an important tool in patient risk stratification. Further studies are warranted to determine whether use of BNP for targeted therapy by optimizing cardiac support can improve outcomes in these critically ill children.
Acknowledgments
This research was supported by a grant from the National Science Council of Taiwan (NSC 98-2314-B-037-005-MY3), and by grants from the Kaohsiung Medical University Hospital, Taiwan (KMUH100-0R32 and KMUH 101-1R32).
REFERENCES
- 1. Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 2. Kutko MC, Calarco MP, Flaherty MB, et al. Mortality rates in pediatric septic shock with and without multiple organ system failure. Pediatr Crit Care Med. 2003;4:333–337. doi: 10.1097/01.PCC.0000074266.10576.9B. [DOI] [PubMed] [Google Scholar]
- 3. Parrillo JE, Parker MM, Natanson C, et al. Septic shock in humans: advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med. 1990;113:227–242. doi: 10.7326/0003-4819-113-3-227. [DOI] [PubMed] [Google Scholar]
- 4. Hsu JH, Wu JR, Dai ZK, et al. Cardiac Arrhythmias - New Considerations, 1st ed. Croatia, InTech: In: Breijo-Marquez FR, Ed.; 2012. Approach to ventricular arrhythmias in the pediatric intensive care unit; p. DOI: 10.5772/25402. [Google Scholar]
- 5. Krishnagopalan S, Kumar A, Parrillo JE, Kumar A. Myocardial dysfunction in the patient with sepsis. Curr Opin Crit Care. 2002;8:376–388. doi: 10.1097/00075198-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 6. Ceneviva G, Paschall JA, Maffei F, Carcillo JA. Hemodynamic support in fluid refractory pediatric septic shock. Pediatrics. 1998;102:e19. doi: 10.1542/peds.102.2.e19. [DOI] [PubMed] [Google Scholar]
- 7. Pollack MM, Fields AI, Ruttiman UE. Distributions of cardiopulmonary variables in pediatric survivors and nonsurvivors of septic shock. Crit Care Med. 1985;13:454–459. doi: 10.1097/00003246-198506000-00002. [DOI] [PubMed] [Google Scholar]
- 8. Feltes TF, Pignatelli R, Kleinert S, Mariscalco MM. Quantitated left ventricular systolic mechanics in children with septic shock utilizing noninvasive wall stress analysis. Crit Care Med. 1994;22:1647–1658. [PubMed] [Google Scholar]
- 9. Jardin F, Valtier B, Beauchet A, et al. Invasive monitoring combined with two-dimensional echocardiographic study in septic shock. Intensive Care Med. 1994;20:550–554. doi: 10.1007/BF01705720. [DOI] [PubMed] [Google Scholar]
- 10. Vieillard Baron A, Schmitt JM, Beauchet A, et al. Early preload adaptation in septic shock? A transesophageal echocardiographic study. Anesthesiology. 2001;94:400–406. doi: 10.1097/00000542-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 11. Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med. 1998;339:321–328. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- 12. McGrath MF, de Bold ML, de Bold AJ. The endocrine function of the heart. Trends Endocrinol Metab. 2005;16:469–477. doi: 10.1016/j.tem.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 13. Hsu JH, Liou SF, Yang SN, et al. B-type natriuretic peptide inhibits angiotensin II-induced proliferation and migration of pulmonary arterial smooth muscle cells. Pediatr Pulmonol. 2014;49:734–744. doi: 10.1002/ppul.22904. [DOI] [PubMed] [Google Scholar]
- 14. Silver MA, Maisel A, Yancy CW, et al. BNP Consensus Panel 2004: A clinical approach for the diagnostic, prognostic, screening, treatment monitoring, and therapeutic roles of natriuretic peptides in cardiovascular diseases. Congest Heart Fail. 2004;10:1–30. doi: 10.1111/j.1527-5299.2004.03271.x. [DOI] [PubMed] [Google Scholar]
- 15. McCullough PA, Nowak RM, McCord J, et al. B-type natriuretic peptide and clinical judgment in emergency diagnosis of heart failure: analysis from Breathing Not Properly (BNP) Multinational Study. Circulation. 2002;106:416–422. doi: 10.1161/01.cir.0000025242.79963.4c. [DOI] [PubMed] [Google Scholar]
- 16. Hsu JH, Keller RL, Chikovani O, et al. B-type natriuretic peptide levels predict outcome after neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2007;134:939–945. doi: 10.1016/j.jtcvs.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 17. Hsu JH, Yang SN, Chen HL, et al. B-type natriuretic peptide predicts responses to indomethacin in premature neonates with patent ductus arteriosus. J Pediatr. 2010;157:79–84. doi: 10.1016/j.jpeds.2009.12.045. [DOI] [PubMed] [Google Scholar]
- 18. Hsu JH, Oishi PE, Keller RL, et al. Perioperative B-type natriuretic peptide levels predict outcome after bidirectional cavopulmonary anastomosis and total cavopulmonary connection. J Thorac Cardiovasc Surg. 2008;135:746–753. doi: 10.1016/j.jtcvs.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 19. Post F, Weilemann LS, Messow CM, et al. B-type natriuretic peptide as a marker for sepsis-induced myocardial depression in intensive care patients. Crit Care Med. 2008;36:3030–3037. doi: 10.1097/CCM.0b013e31818b9153. [DOI] [PubMed] [Google Scholar]
- 20. Kandil E, Burack J, Sawas A, et al. B-type natriuretic peptide: a biomarker for the diagnosis and risk stratification of patients with septic shock. Arch Surg. 2008;143:242–246. doi: 10.1001/archsurg.2007.69. [DOI] [PubMed] [Google Scholar]
- 21. Witthaut R, Busch C, Fraunberger P, et al. Plasma atrial natriuretic peptide and brain natriuretic peptide are increased in septic shock: impact of interleukin-6 and sepsis associated left ventricular dysfunction. Intensive Care Med. 2003;29:1696–1702. doi: 10.1007/s00134-003-1910-0. [DOI] [PubMed] [Google Scholar]
- 22. Charpentier J, Luyt CE, Fulla Y, et al. Brain natriuretic peptide: a marker of myocardial dysfunction and prognosis during severe sepsis. Crit Care Med. 2004;32:660–665. doi: 10.1097/01.ccm.0000114827.93410.d8. [DOI] [PubMed] [Google Scholar]
- 23. Goldstein B, Giroir B, Randolph A. International pediatric consensus conference: definitions for sepsis and organ dysfunction on pediatrics. Pediatr Crit Care Med. 2005;6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 24. Bu’Lock FA, Mott MG, Oakhill A, Martin RP. Early identification of anthracycline cardiomyopathy: possibilities and implications. Arch Dis Child. 1996;75:416–422. doi: 10.1136/adc.75.5.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schiller NB, Shah PM, Crawford M, et al. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. Recommendations for the quantification of the left ventricle by two-dimensional echocardiography. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 26. Wernovsky G, Wypij D, Jonas RA, et al. Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants. A comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation. 1995;92:2226–2235. doi: 10.1161/01.cir.92.8.2226. [DOI] [PubMed] [Google Scholar]
- 27. Thiru Y, Pathan N, Bignall S, et al. A myocardial cytotoxic process is involved in the cardiac dysfunction of meningococcal septic shock. Crit Care Med. 2000;28:2979–2983. doi: 10.1097/00003246-200008000-00049. [DOI] [PubMed] [Google Scholar]
- 28. ver Elst KM, Spapen HD, Nguyen DN, et al. Cardiac troponin I and T are biological markers of left ventricular dysfunction in septic shock. Clin Chem. 2000;46:650–657. [PubMed] [Google Scholar]
- 29. Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated pediatric risk of mortality score. Crit Care Med. 1996;24:743–753. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 30. Ma KK, Ogawa T, de Bold AJ. Selective upregulation of cardiac brain natriuretic peptide at the transcriptional and translational levels by pro-inflammatory cytokines and by conditioned medium derived from mixed lymphocyte reactions via p38 MAP kinase. J Mol Cell Cardiol. 2004;36:505–513. doi: 10.1016/j.yjmcc.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 31. Tomaru Ki K, Arai M, Yokoyama T, et al. Transcriptional activation of the BNP gene by lipopolysaccharide is mediated through GATA elements in neonatal rat cardiac myocytes. J Mol Cell Cardiol. 2002;34:649–659. doi: 10.1006/jmcc.2002.2005. [DOI] [PubMed] [Google Scholar]
- 32. Costello JM, Goodman DM, Green TP. A review of the natriuretic hormone system’s diagnostic and therapeutic potential in critically ill children. Pediatr Crit Care Med. 2006;7:308–318. doi: 10.1097/01.PCC.0000224998.97784.A3. [DOI] [PubMed] [Google Scholar]
- 33. McLean AS, Huang SJ, Nalos M, et al. The confounding effects of age, gender, serum creatinine, and electrolyte concentrations on plasma B-type natriuretic peptide concentrations in critically ill patients. Crit Care Med. 2003;31:2611–2618. doi: 10.1097/01.CCM.0000094225.18237.20. [DOI] [PubMed] [Google Scholar]
- 34. Matsukawa N, Grzesik WJ, Takahashi N, et al. The natriuretic peptide clearance receptor locally modulates the physiological effects of the natriuretic peptide system. Proc Natl Acad Sci USA. 1999;96:7403–7408. doi: 10.1073/pnas.96.13.7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fenton KE, Sable CA, Bell MJ, et al. Increases in serum levels of troponin I are associated with cardiac dysfunction and disease severity in pediatric patients with septic shock. Pediatr Crit Care Med. 2004;5:533–538. doi: 10.1097/01.PCC.0000144711.97646.0C. [DOI] [PubMed] [Google Scholar]
- 36. Domico M, Liao P, Anas N, Mink RB. Elevation of brain natriuretic peptide levels in children with septic shock. Pediatr Crit Care Med. 2008;9:478–483. doi: 10.1097/PCC.0b013e3181849b99. [DOI] [PubMed] [Google Scholar]
- 37. Koch A, Singer H. Normal values of B type natriuretic peptide in infants, children, and adolescents. Heart. 2003;89:875–878. doi: 10.1136/heart.89.8.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yoshibayashi M, Kamiya T, Saito Y, et al. Plasma brain natriuretic peptide concentrations in healthy children from birth to adolescence: marked and rapid increase after birth. Eur J Endocrinol. 1995;133:207–209. doi: 10.1530/eje.0.1330207. [DOI] [PubMed] [Google Scholar]


