Abstract
Background
Patent ductus arteriosus (PDA) causes increased pulmonary blood flow, which can lead to pulmonary arterial hypertension (PAH). Rho-associated coiled-coil containing protein kinase (ROCK) may play an important pathophysiological role in PAH. We hypothesized that the increased pulmonary artery (PA) flow from PDA could activate ROCK.
Methods
Patients who received a PDA transcatheter closure in our hospital were consecutively enrolled in this study. Basic demographics and clinical hemodynamic data of the study participants were recorded. Then, ROCK activity was measured before and after the PDA occlusion procedure. ROCK activity was defined as the phosphorylation ratio of myosin-binding subunit by Western blot measurement. We also sub-divided patients into the coil group and occluder group based on the occlusion device used in each patient’s procedure.
Results
From January 2009 to December 2011, 25 patients with a median age of 2.3 years, ranging from 10 months to 72 years were enrolled. The mean PDA size was 0.31 ± 0.14 cm, the mean Qp/Qs shunt was 1.54 ± 0.41, and the mean systolic pulmonary artery pressure was 26.9 ± 10.3 mmHg. There were 10 patients (one boy and nine girls) in the coil group and 15 patients (four boys and eleven girls) in the occluder group. Following the closure of the PDA, ROCK activity significantly decreased (1.78 ± 2.25 vs. 0.77 ± 0.69, p < 0.01). There was a strong correlation between the leukocyte ROCK activity with the systolic PA pressure (y = 5.4608x + 22.54, R2 = 0.5539, p < 0.05), but not the Qp/Qs value. Both subgroups showed significant changes of ROCK activity after the procedure. Interestingly, when comparing the coil group with the occluder group, the decrease in ROCK activity was more apparent in the occluder group.
Conclusions
The findings of this study indicated that ROCK activity is higher in patients with PDA and correlates with PA pressure. The decrease in ROCK activity following the device closure suggests that ROCK may be an important biomarker for PDA patency.
Keywords: Patent ductus arteriosus (PDA), Pulmonary arterial pressure, Rho kinase, Transcatheter closure
INTRODUCTION
Patent ductus arteriosus (PDA) is a common congenital heart defect that is usually identified in childhood but sometimes remains unrecognized until late in life.1 The condition is characterized by a persistent connection between the aorta and the pulmonary artery. As a result, a continuous left to right shunting of blood occurs. This in turn leads to a volume and pressure overload and thus to pulmonary artery hypertension (PAH).1
Mounting evidence demonstrates that the RhoA/ Rho kinase pathway plays a pivotal role in various cellular functions, such as sensing and responding to mechanical changes in the environment, the contraction of smooth muscle cells (SMCs), actin cytoskeleton organization, cytokinesis, and gene expressions.2,3 Therefore, the RhoA/Rho kinase pathway participates in the pathogenesis of hypoxia and monocrotaline-induced PAH.4 Current evidence has also indicated that O2 activates Rho-associated coiled-coil containing protein kinase (ROCK) and increases ROCK expression in term ductus arteriosus (DA) smooth muscle cells by a redox-regulated, positive-feedback mechanism that promotes sustained vasoconstriction. Conversely, ROCK inhibitors may be useful in maintaining DA patency.5 According to these studies, understanding the ROCK mechanism will facilitate drug development for PAH with or without PDA.6
In recent years, percutaneous treatment of the persistent arterial duct in the catheterization laboratory has become the gold standard and is considered to be a safe therapy.7 Coils are generally used for small PDAs, as well as occluding devices for larger PDAs.8 In the following study, we hypothesized that PDA with increased pulmonary blood flow would chronically activate the systemic and local rho A/rho kinase pathway and thus contribute to the development or maintenance of PAH. We therefore expected that ROCK activity will be higher in patients with PDA, and the transcatheter closure of PDA flow by occlusion device would ameliorate the ROCK activity systemically.
PATIENTS AND METHODS
Patients
From January 2009 to September 2011, patients admitted to the National Cheng Kung University Hospital (Tainan, Taiwan) for a transcatheter closure of PDA were prospectively enrolled in this study. The indications for PDA closure were based on the symptoms/signs as described.1 Informed consent was obtained from the adult patients or from the parents of children. The study protocol was approved by our institutional review board (NCKUH-ER-97-056).
Catheterization procedure
We recorded the basic demographic data and the hemodynamic parameters of patients before and after PDA closure. The size of PDA was defined as the minimum diameter of the ductus as determined by angiography.9 The patients’ pulmonary artery pressure (PAP) were recorded during the cardiac catheterization which preceded the occlusion. The ratio of pulmonary blood flow to systemic blood flow (Qp/Qs) was calculated by use of the hemodynamic values obtained by cardiac catheterization. We followed the payment regulations of the Taiwan National Health Insurance Administration (NHI) to use the Gianturco coil (Cook Inc, Bloomington, IN, USA) to close the ducus size less than 2.5 mm and used the Amplatzer duct occluder (St. Jude Medical, Plymouth, MN, USA) if the ductus was larger than 2.5 mm.10 Therefore, we classified the patients into two groups: the coil group and the occluder group. The detail technique of device deployment was similar to that reported in the existing literature.7-9 Patients who received multiple device (coils) deployment were excluded in this study.
Samples for ROCK activity assay
We measured the ROCK activity of circulating leukocytes from patients’ venous blood before and 10 minutes after the PDA was closed. Previous studies reported that the circulating leukocyte ROCK activity was associated with a metabolic syndrome11 and acute coronary syndrome in humans.12 Leukocytes were isolated from 5 mL peripheral blood during the second and third visits, following a validated and standardized protocol.11,12 The leukocytes were frozen and stored at -80 °C until all samples were collected. The ROCK assays were performed on all leukocyte samples simultaneously. Thereafter, the samples were then analyzed by Western blotting for the phosphorylation of the myosin-binding subunit (MBS) of myosin light-chain phosphatase with an antibody that specifically recognizes phosphorylated Ser853 MBS (kindly provided by Liao JK). Inter-experimental results were standardized to lysophosphatidic acid–induced MBS phosphorylation (positive control).
Statistical analysis
We used IBM SPSS Statistics 19 (SPSS, Inc., Chicago, IL, USA) to analyze the database. We used an independent t-test to assess the difference of ROCK activity in the study and in the subgroups. We used a paired t-test to assess the difference of ROCK activity before and after PDA closure in the study group. Due to the small sample size in some subgroups, we tested the database using non-parametric analysis. We used the linear correlations model to test the correlation between ROCK activity and the parameters.
RESULTS
A total of 25 patients with a median age of 2.3 years (10 months to 72 years) were enrolled in this study. The mean PDA size was 0.31 ± 0.14 cm, the mean Qp/Qs shunt was 1.54 ± 0.41, and the mean systolic pulmonary artery (PA) pressure was 26.9 ± 10.3 mmHg. Their comparative initial clinical parameters in both groups were summarized in Table 1. There were 10 patients [90% (9/10) were female] in the coil group, and 15 patients [73.3% (11/15) were female] in the occluder group. Although the age, body weight, and pulmonary artery pressure were higher in the occluder group, the differences were not statistically significant. The PDA size and Qp/Qs of the occluder group were significantly larger than the coil group, indicating that patients in the occluder group had more pathophysiologically significant PDA shunts.
Table 1. Initial clinical parameters of children with patent ductus arteriosus .
| Coil group | Occluder group | |
| Patient number | 10 | 15 |
| Age (years) | 3.6 ± 2.8 | 14.5 ± 21.0 |
| Sex (male/female) | 1/9 | 4/11 |
| Body weights (kilogram) | 15.4 ± 8.9 | 30.2 ± 23.5 |
| PDA Size# (mm) | 1.9 ± 0.4 | 3.9 ± 1.2* |
| Mean systolic PAP (mmHg) | 22.7 ± 5.4 | 29.7 ± 11.9 |
| Qp/Qs shunt | 1.27 ± 0.26 | 1.72 ± 0.40* |
PAP, pulmonary artery pressure; PDA, patent ductus arteriosus; Qp/Qs, ratio of pulmonary blood flow to systemic blood flow.
* p < 0.05; # PDA size: the minimum diameter of the ductus as determined by angiography.
Following the closure of the PDA, both the coil and occluder groups showed a significantly decreased level of leukocyte ROCK activity after interventional procedure (1.78 ± 2.25 vs. 0.77 ± 0.69, p < 0.01). Interestingly, in comparison to the coil group, the ROCK activity decreased more obviously in the occluder group (Figure 1) & (Table 2).
Figure 1.
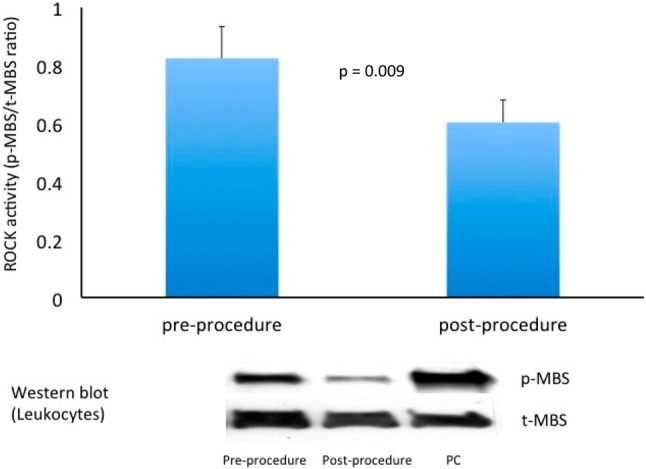
Leukocyte Rho-associated coiled-coil containing protein kinase (ROCK) activity was reduced after catheter intervention on PDA (defined as the phosphorylation ratio of myosin binding subunit by Western blot measurement). PC, positive control lane; p-MBS, phospho-myosin binding subunit; t-MBS, total-myosin binding subunit.
Table 2. Rho kinase (ROCK) activity before and after transcatheter occlusion .
| Before | After | |
| Total (n = 25) | 1.78 ± 2.25 | 0.77 ± 0.69# |
| Coil group (n = 10) | 0.61 ± 0.64 | 0.46 ± 0.40* |
| Occluder group (n = 15) | 2.40 ± 2.69 | 0.81 ± 0.68# |
* p < 0.05; # p < 0.01.
We found that the strongest association of the leukocyte ROCK activity and their clinical parameters was the correlation of the systolic PA pressure (y = 5.4608x + 22.54, R2 = 0.5539, p < 0.05). Interestingly, the association of the ROCK activity was not significant with the Qp/ Qs value in this PDA intervention cohort (Figure 2) & (Figure 3).
Figure 2.
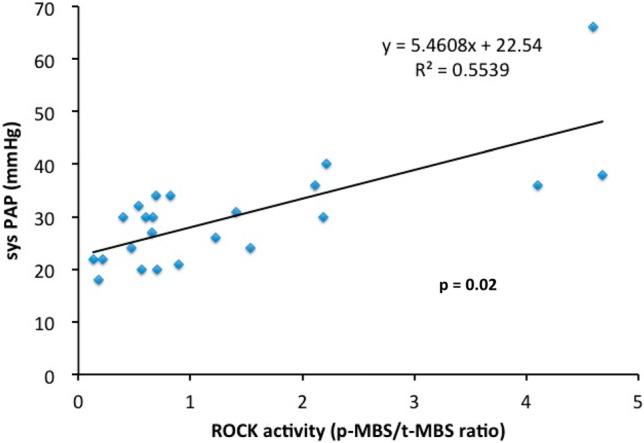
The correlation between the leukocyte Rho-associated coiled-coil containing protein kinase (ROCK) activity and the systolic pulmonary artery pressure. Sys PAP, systolic pulmonary arterial pressure; p-MBS, phospho-myosin binding subunit; t-MBS, total-myosin binding subunit.
Figure 3.
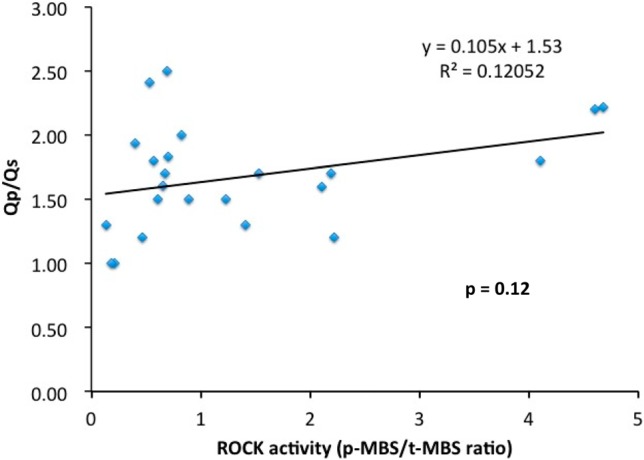
The correlation between the leukocyte Rho-associated coiled-coil containing protein kinase (ROCK) activity with the Qp/Qs value. p-MBS, phospho-myosin binding subunit; Qp/Qs, the ratio of pulmonary blood flow to systemic blood flow; t-MBS, total-myosin binding subunit.
DISCUSSION
For the first time in an empirical study, we have demonstrated that ROCK activity was higher in patients with PDA and significantly correlates with their PA pressure. The decrease in ROCK activity following the transcatheter closure of PDA also suggests that ROCK can be an important biomarker for PDA patency.
Accumulating evidence suggests an inverse relationship between endothelial nitric oxide synthase (eNOS) and small GTPase RhoA/Rho-kinase.13 The actions of the Rho family of GTPases (Rho, Rac, and CDC42) are mediated by their specific downstream effector ROCK, a serine-threonine kinase.14-16 Rho-kinase might negatively regulate eNOS expression and activation, as well as nitric oxide (NO) bioavailability,17 and it mediates the hypoxia-induced downregulation of endothelial NO synthase.18 The smooth muscle-selective RhoGAP GRAF3 is a critical regulator of vascular tone and hypertension.19 The active Rho family protein has been found to promote the formation of fiber tension in vascular smooth muscle cells by functioning with serotonin-threonine kinase.20 Besides, up-regulation of the ROCK activity can cause endothelial dysfunction.21 Initially, the Rho system is up-regulated selectively in the ductus arteriosus (DA) at birth.5,22,23 Hong Z et al. had shown that Rho-kinase inhibitors relax endothelium-denuded rabbit DA,22 Costa et al. also demonstrated that RhoB gene expression was increased with the maturation in a DA model among rats.23 The hypothesized mechanism showed that oxygen (O2) activates ROCK and increases ROCK expression in term DA smooths muscle cells by a redox-regulated, positive-feedback mechanism that promotes sustained vasoconstriction.5 As expected, Rho-kinase activation is a universal, distal step in both O2- and agonist-induced DA constrictions.5 Hence, the potential usefulness of the ROCK studies among PDA does not derive from a general vessel constriction property, but rather from an interference with a mechanism specifically linked to birth. Rho-kinase is specifically inhibited by Y-2763217 or fasudil (HA1077).24 Momma et al. had showed the in vivo dilation of the DA by Rho kinase inhibition in the rat.25 Recently, ROCK inhibitor Y-27632 perturbs endothelial functions variable under shear stress in a concentration-dependent manner.26 Therefore, the manipulation of ROCK had strong influences in PDA. In this study, we also showed that the transcatheter closure of PDA could ameliorate leukocyte rho kinase activities. Importantly, the ROCK activity decreased more obviously in the occluder group, which implied that intervention for a larger shunt would produce even greater effects.
PAH is an important issue in adult heart disease. Because PAH is a progressive and fatal disease, it is crucial to better understand the basic concepts of the initiation and progression of PAH.27 There were many scientific reports which studied the roles of the Rho family in the PAH mechanism.3,6,28-30 Some physicians used Rho kinase inhibitors to treat patients with PAH.29,31 PDA could induce PAH through a high flow mechanism.1 Over time, augmented pulmonary flow may also induce increased shear stress and circumferential stretch.32 The role of ROCK was also critical in the flow-induced PAH model.29,33 Shear stress regulates endothelial cell (EC) alignment and remodeling through the activation of Rho family GTPases (Cdc42, Rho, and Rac) that enhance the formation of stress fibers and focal adhesions and regulates cytoskeletal reorganization.34,35 In this study, we clearly demonstrated a strong correlation between the leukocyte ROCK activity with the severity of PAH. This connection is represented by the degree of PA pressure but not the Qp/Qs value. Several possibilities explain this reason. One is that the exact calculation of Qp/Qs value might be influenced by individual oxygen consumption. In addition, Qp/Qs value might be also influenced by the pulmonary vascular resistance among some PDA patients, Therefore, the Qp/Qs value might be able to represent the true shunt flow condition.
In recent years, the transcatheter treatment of PDA has become the gold standard and a safe therapy.7 Compared with the traditional surgical method, transcatheter intervention is less invasive and most of the shunts were immediately diminished when the device was successfully deployed. This makes the negative impact of the intervention on PDA patients very minimal and helps us to clarify the role of ROCK in PDA patients. These immediate physiological changes after PDA closure were originally from the cessation of pulmonary blood flow, as well as a reduction in the pulmonary arterial oxygen content. A previous in vivo study showed that bovine endothelial cell ROCK activity could be induced by flow-mediated shear stress.36 Recent studies also showed that Rho-kinase activity was not augmented in yak pulmonary arteries compared with those of bulls, suggesting a reason for the relatively low pulmonary vascular tone of yaks despite living in hypoxic circumstances.37 We therefore hypothesized that the ROCK activity changes in our study resulted from flow-mediated shear stress because the rapid characteristic of Rho kinase in regulating cell physiological changes and function.
However, there were some major limitations in this study. First, the patient number was still limited. Second, the age, body weight, and pulmonary artery pressure were higher in the occluder group, although not statistically significant. Third, we did not repeat measurement of pulmonary artery pressure after PDA occlusion, therefore, we did not compare the association of decrease of RCOK activity and the decreased pulmonary pressure. Fourth, we measured the ROCK activity of circulating leukocytes from patients’ venous blood, could this ROCK activity in the peripheral leukocyte correlate with the in situ pulmonary artery tissue? This question was difficult to confirm in a human study, so it may be necessary to further pursue the animal model study.
CONCLUSIONS
The results in this study demonstrated that ROCK activity is higher in patients with PDA and also correlated with PAH severity, represented by the measured PA pressure. The decrease in ROCK activity following device closure suggests that ROCK may be an important biomarker in PDA.
Acknowledgments
This project was in part supported by a grant from National Cheng Kung University Hospital (NCKUH-9905 001). We also thank Dr. Wen-Lan Yen (Chia-Yi Christian Hospital) for the clinical sample collection.
DISCLOSURES
The authors have declared no potential conflicts of interest.
REFERENCES
- 1. Schneider DJ, Moore JW. Patent ductus arteriosus. Circulation. 2006;114:1873–1882. doi: 10.1161/CIRCULATIONAHA.105.592063. [DOI] [PubMed] [Google Scholar]
- 2. Loirand G, Gue´rin P, Pacaud P. Rho kinases in cardiovascular physiology and pathophysiology. Circ Res. 2006;98:322–334. doi: 10.1161/01.RES.0000201960.04223.3c. [DOI] [PubMed] [Google Scholar]
- 3. Li FH, Xia W, Li AW, et al. Inhibition of rho kinase attenuates high flow induced pulmonary hypertension in rats. Chin Med J (Engl) 2007;120:22–29. [PubMed] [Google Scholar]
- 4. Fagan KA, Oka M, Bauer NR, et al. Attenuation of acute hypoxic pulmonary vasoconstriction and hypoxic pulmonary hypertension in mice by inhibition of Rho-kinase. Am J Physiol Lung Cell Mol Physiol. 2004;287:L656–L664. doi: 10.1152/ajplung.00090.2003. [DOI] [PubMed] [Google Scholar]
- 5. Kajimoto H, Hashimoto K, Bonnet SN, et al. Oxygen activates the Rho/Rho-kinase pathway and induces RhoB and ROCK-1 expression in human and rabbit ductus arteriosus by increasing mitochondria-derived reactive oxygen species: a newly recognized mechanism for sustaining ductal constriction. Circulation. 2007;115:1777–1788. doi: 10.1161/CIRCULATIONAHA.106.649566. [DOI] [PubMed] [Google Scholar]
- 6. Weir EK, Obreztchikova M, Vargese A, et al. Mechanisms of oxygen sensing: a key to therapy of pulmonary hypertension and patent ductus arteriosus. Br J Pharmacol. 2008;155:300–307. doi: 10.1038/bjp.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang JK, Wu MH, Hwang JJ, et al. Transcatheter closure of moderate to large patent ductus arteriosus with the Amplatzer duct occluder. Catheter Cardiovasc Interv. 2007;69:572–578. doi: 10.1002/ccd.20701. [DOI] [PubMed] [Google Scholar]
- 8. Brunetti MA, Ringel R, Owada C, et al. Percutaneous closure of patent ductus arteriosus: a multi-institutional registry comparing multiple devices. Catheter Cardiovasc Interv. 2010;76:696–702. doi: 10.1002/ccd.22538. [DOI] [PubMed] [Google Scholar]
- 9. Lloyd TR, Fedderly R, Mendelsohn AM, et al. Transcatheter occlusion of patent ductus arteriosus with Gianturco coils. Circulation. 1993;88:1412–1420. doi: 10.1161/01.cir.88.4.1412. [DOI] [PubMed] [Google Scholar]
- 10. National Health Insurance Administration, Ministry of Health and Welfare. Program and medical services payment standard. www.nhi.gov.tw/Resource/bulletin/609_bbs920411.pdf [Google Scholar]
- 11. Liu PY, Chen JH, Lin LJ, Liao JK. Increased Rho kinase activity in a Taiwanese population with metabolic syndrome. J Am Coll Cardiol. 2007;49:1619–1624. doi: 10.1016/j.jacc.2006.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hou HW, Li XG, Yan M, et al. Increased leukocyte Rho-kinase activity in a population with acute coronary syndrome. Mol Med Rep. 2013;8:250–254. doi: 10.3892/mmr.2013.1463. [DOI] [PubMed] [Google Scholar]
- 13. Ming XF, Viswambharan H, Barandier C, et al. Rho GTPase/Rho kinase negatively regulates endothelial nitric oxide synthase phosphorylation through the inhibition of protein kinase B/Akt in human endothelial cells. Mol Cell Biol. 2002;22:8467–8477. doi: 10.1128/MCB.22.24.8467-8477.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matsumoto Y, Tanaka K, Harimaya K, et al. Small GTP-binding protein, Rho, both increased and decreased cellular motility, activation of matrix metalloproteinase 2 and invasion of human osteosarcoma cells. Jpn J Cancer Res. 2001;92:429–438. doi: 10.1111/j.1349-7006.2001.tb01113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maruta H, Nheu TV, He H, Hirokawa Y. Rho family-associated kinases PAK1 and rock. Prog Cell Cycle Res. 2003;5:203–210. [PubMed] [Google Scholar]
- 16. Amano M, Fukata Y, Kaibuchi K. Regulation and functions of Rho associated kinase. Exp Cell Res. 2000;261:44–51. doi: 10.1006/excr.2000.5046. [DOI] [PubMed] [Google Scholar]
- 17. Rikitake Y, Liao JK. Rho GTPases, statins, and nitric oxide. Circ Res. 2005;97:1232–1235. doi: 10.1161/01.RES.0000196564.18314.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Takemoto M, Sun J, Hiroki J, et al. Rho-kinase mediates hypoxia-induced downregulation of endothelial nitric oxide synthase. Circulation. 2002;106:57–62. doi: 10.1161/01.cir.0000020682.73694.ab. [DOI] [PubMed] [Google Scholar]
- 19. Peng G, Ivanovska J, Kantores C, et al. Sustained therapeutic hypercapnia attenuates pulmonary arterial Rho-kinase activity and ameliorates chronic hypoxic pulmonary hypertension in juvenile rats. Am J Physiol Heart Circ Physiol. 2012;302:H2599–H2611. doi: 10.1152/ajpheart.01180.2011. [DOI] [PubMed] [Google Scholar]
- 20. Owczarek J, Jasinska M, Michalak DO. Rho-Kinase – a new trend in cardiovascular disease pharmacotherapy: the role of the vascular smooth muscle spasm. Med Sci Tech. 2010;51:163–166. [Google Scholar]
- 21. Noma K, Kihara Y, Higashi Y. Striking crosstalk of ROCK signaling with endothelial function. J Cardiol. 2012;60:1–6. doi: 10.1016/j.jjcc.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 22. Hong Z, Hong F, Olschewski A, et al. Role of store-operated calcium channels and calcium sensitization in normoxic contraction of the ductus arteriosus. Circulation. 2006;114:1372–1379. doi: 10.1161/CIRCULATIONAHA.106.641126. [DOI] [PubMed] [Google Scholar]
- 23. Costa M, Barogi S, Socci ND, et al. Gene expression in ductus arteriosus and aorta: comparison of birth and oxygen effects. Physiol Genomics. 2006;25:250–262. doi: 10.1152/physiolgenomics.00231.2005. [DOI] [PubMed] [Google Scholar]
- 24. Asano T, Ikegaki I, Satoh S, et al. Mechanism of action of a novel antivasospasm drug, HA1077. J Pharmacol Exp Ther. 1987;241:1033–1040. [PubMed] [Google Scholar]
- 25. Momma K, Toyoshima K, Sun F, Nakanishi T. In vivo dilatation of the ductus arteriosus by Rho kinase inhibition in the rat. Neonatology. 2009;95:324–331. doi: 10.1159/000181162. [DOI] [PubMed] [Google Scholar]
- 26. Kolluru GK, Majumder S, Chatterjee S. Rho-kinase as a therapeutic target in vascular diseases: striking nitric oxide signaling. Nitric Oxide. 2014;43:45–54. doi: 10.1016/j.niox.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 27. Archer SL, Weir EK, Wilkins MR. Basic science of pulmonary arterial hypertension for clinicians: new concepts and experimental therapies. Circulation. 2010;121:2045–2066. doi: 10.1161/CIRCULATIONAHA.108.847707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Do EZ, Fukumoto Y, Takaki A, et al. Evidence for Rho-kinase activation in patients with pulmonary arterial hypertension. Circ J. 2009;73:1731–1739. doi: 10.1253/circj.cj-09-0135. [DOI] [PubMed] [Google Scholar]
- 29. Li F, Xia W, Yuan S, Sun R. Acute inhibition of Rho-kinase attenuates pulmonary hypertension in patients with congenital heart disease. Pediatr Cardiol. 2009;30:363–366. doi: 10.1007/s00246-008-9315-z. [DOI] [PubMed] [Google Scholar]
- 30. Guilluy C, Eddahibi S, Agard C, et al. RhoA and Rho kinase activation in human pulmonary hypertension: role of 5-HT signaling. Am J Respir Crit Care Med. 2009;179:1151–1158. doi: 10.1164/rccm.200805-691OC. [DOI] [PubMed] [Google Scholar]
- 31. Fujita H, Fukumoto Y, Saji K, et al. Acute vasodilator effects of inhaled fasudil, a specific Rho-kinase inhibitor, in patients with pulmonary arterial hypertension. Heart Vessels. 2010;25:144–149. doi: 10.1007/s00380-009-1176-8. [DOI] [PubMed] [Google Scholar]
- 32. Diller GP, Gatzoulis MA. Pulmonary vascular disease in adults with congenital heart disease. Circulation. 2007;115:1039–1050. doi: 10.1161/CIRCULATIONAHA.105.592386. [DOI] [PubMed] [Google Scholar]
- 33. Li F, Xia W, Li A, et al. Long-term inhibition of Rho kinase with fasudil attenuates high flow induced pulmonary artery remodeling in rats. Pharmacol Res. 2007;55:64–71. doi: 10.1016/j.phrs.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 34. Kuchan MJ, Jo H, Frangos JA. Role of G proteins in shear stress-mediated nitric oxide production by endothelial cells. Am J Physiol. 1994;267:C753–C758. doi: 10.1152/ajpcell.1994.267.3.C753. [DOI] [PubMed] [Google Scholar]
- 35. Tzima E. Role of small GTPases in endothelial cytoskeletal dynamics and the shear stress response. Circ Res. 2006;98:176–185. doi: 10.1161/01.RES.0000200162.94463.d7. [DOI] [PubMed] [Google Scholar]
- 36. Lin T, Zeng L, Liu Y, et al. Rho-ROCK-LIMK-cofilin pathway regulates shear stress activation of sterol regulatory element bnding proteins. Circ Res. 2003;92:1296–1304. doi: 10.1161/01.RES.0000078780.65824.8B. [DOI] [PubMed] [Google Scholar]
- 37. Ishizaki T, Mizuno S, Sakai A, et al. Blunted activation of rho-kinase in yak pulmonary circulation. Biomed Res Int. 2015;2015:720250. doi: 10.1155/2015/720250. [DOI] [PMC free article] [PubMed] [Google Scholar]


