Abstract
Background
The atheroprotective role of high-density lipoprotein (HDL-C) particles as measured by HDL-C level in coronary arterial disease (CAD) remains unsettled. The aim of our study was to ascertain whether HDL-C was associated with the development and severity of coronary artery disease in Chinese patients who underwent coronary angiogram with low background Low-density lipoprotein (LDL-C) levels, which has not been previously investigated.
Methods
Between March 1995 and May 2000, 566 consecutive patients (408 males, 66.7 ± 11.3 years of age) with background LDL-C less than 100 mg/dl who underwent coronary artery angiography at our cath lab for suspected CAD were retrospectively recruited into the study. The severity of coronary lesions was measured by conventional coronary angiography and modified Gensini scores.
Results
In those subjects with significant coronary lesions, there were more males and conventional CAD risk factors of diabetes mellitus, smoking, and chronic renal disease. They were also older compared to those in the control group. However, total cholesterol, LDL-C, HDL-C, triglyceride levels and use of statins were similar in both groups. In those subjects with significant coronary lesions, there was no difference in conventional coronary lesion severity or modified Gensini score between the quartered HDL-C subgroups. Furthermore, there was no significant correlation between serum HDL-C level and modified Gensini scores. In linear regression analysis, HDL-C was not an independent predictor for modified Gensini scores. Furthermore, HDL-C was also not an independent risk factor for the presence of significant coronary lesions in low LDL-C patients in logistic regression analysis.
Conclusions
In Chinese patients with low background LDL-C, serum HDL-C was not associated with development of CAD or lesion severity in patients with suspected CAD. Therefore, HDL-C did not appear to be atheroprotective in these patients.
Keywords: Coronary artery disease, Gensini score, High-density lipoprotein cholesterol
INTRODUCTION
Dyslipidemia is a well-known major risk factor for coronary artery disease (CAD). Low-density lipoprotein cholesterol (LDL-C) and non-high-density lipoprotein cholesterol (non-HDL-C) have long been regarded as key factors in atherogenesis and predictors of CAD.1-4 However, the role of high-density lipoprotein (HDL-C) particles, as measured by HDL-C levels in circulation, in atherogenesis and CAD remains controversial. HDL-C was inversely associated with the prevalence of CAD in some series,5-7 but low HDL-C was not shown to contribute to the prevalence of CAD in other studies.8-10 It was estimated that, for every 1 mg/dl increase in HDL-C, there is a 2-3% decrease in cardiovascular risk.3,9 The putative atheroprotective effect HDL-C has even been linked with a lower incidence of myocardial injury or hospitalization for ischemia in patients with low-LDL-C levels.11 However, several recent clinical studies failed to demonstrate additional benefits of raising HDL-C levels beyond those obtained by lowering LDL-C using standard statin therapy.
Although there is a substantial body of research which focused on the prognostic value of HDL-C for future cardiovascular events, to our knowledge, the possible independent association of HDL-C with the presence or absence of angiographically documented coronary lesion and lesion severity in patients with low background LDL-C levels has not been previously investigated. The present study was intended to investigate the possible correlation of HDL-C levels with the presence of and severity of coronary lesions in these subjects.
METHODS
A Windows 2000-based cardiac catheterization report databank which uses data in the hospital information system and contains all angiographic reports was established in 1994. Additionally, a blood databank which contains all blood specimens from patients who underwent different types of cardiac catheterization in our hospital and who were willing to give informed consent for research use was set up in 1995. This retrospective study was part of a research program that utilized these two datasets to investigate the effects of various risk factors in cardiovascular disease. Between March 1995 and May 2000, consecutive patients who underwent coronary angiography in our cath lab for suspected CAD and had background LDL-C less than 100 mg/dl were retrospectively queried from the databases. Patient characteristics including age, gender, coronary lesion severity (number of diseased coronary arteries), atherosclerotic risk factors (including hypertension, diabetes mellitus, smoking, dyslipidemia, chronic renal disease) and prescribed medications (including statins, fibrates, antiplatelet agents, beta blockers, calcium channel blockers, angiotensin-converting-enzyme inhibitors and angiotensin receptor blockers) were all collected and recorded. Patients who had a history of previous myocardial infarction, percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) were excluded from the study. Based on the findings of coronary angiography, these patients were divided into control and significant coronary lesion groups (diameter stenosis ≥ 50%). Coronary lesion severity was further measured by calculating the modified Gensini scores. Serum total cholesterol, LDL-C, triglyceride, and HDL-C were also queried from the database. These blood tests were performed within 24 hours before coronary angiography (CAG). HDL-C levels in this study were subdivided into high, normal, low, and very low HDL-C subgroups based on HDL-C levels of ≥ 60 mg/dl, 40 to 59 mg/dl, 30 to 39 mg/dl and < 30 mg/dl, respectively, as modified from the classification used in NECP ATP III.12 The patients’ clinical information upon admission was confirmed by thorough medical chart review.
Coronary artery angiographies
The angiographic measurements were made on a viewing workstation with software routinely used quantitative analysis of angiograms (Philips Inturis Suite, R2.2, Philips Medical Systems, USA; Medcon/Horizon/ TCS, Israel). The diagnostic coronary angiogram prior to coronary intervention was thoroughly reviewed. Significant CAD was defined as a greater than 50% reduction in diameter in a major epicardial artery. The number of vessels with CAD was defined as the number of the three major coronary vessels that had ≥ 50% diameter stenosis; the severity was further evaluated using the modified Gensini scoring system which has been well described elsewhere.13 Scoring was performed by two observers and the scores were averaged.
Statistical analysis
Continuous variables are presented as mean ± standard deviation (SD) and categorical variables frequencies and percentages. Differences in the means of continuous variables between groups were tested by independent t-test or analysis of variance as appropriate. Frequencies were compared by Chi-square test, and correlation analysis was tested by Pearson’s correlation coefficient. Logistic regression was used to estimate the odds of CAD risk factors and linear regression was used to estimate the independent predictors for coronary lesion severity as measured by modified Gensini score. The variables in univariate analysis included age, sex, body mass index (BMI), hypertension (HTN), diabetes mellitus (DM), chronic renal disease, smoking, C-reactive protein (CRP), HDL-C subgroups and statin, and variables which were statistically significant in univariate analysis were tested again in multivariate analysis. Differences were considered statistically significant if p < 0.05.
RESULTS
Patients’ characteristics
Between March 1995 and May 2000, a total of 566 consecutive patients with background LDL-C level less than 100 mg/dl and without any history of old myocardial infarction, previous PCI and CABG surgery underwent CAG for suspected CAD at our cath lab and were recruited into the current study. Of these patients, 165 (29.2%) had significant coronary lesions and the remaining 401 (70.8%) did not. The mean age of the entire population was 66.7 ± 11.3 years and 408 (72.1%) of them were males. In total, 285 (61.4%) of these patients had hypertension, 106 (26.9%) diabetes mellitus, and 134 (37.2%) smoking history. The mean body height was 162 ± 8 cm, body weight 64.7 ± 11.6 kg, and body mass index 24.6 ± 3.7 kg/m2. The mean total cholesterol of the entire group was 140 ± 26 mg/dl, LDL-C 73 ± 18 mg/dl, HDL-C 45 ± 15 mg/dl, and triglyceride level of 113 ± 68 mg/dl.
Comparison between patients with and without significant coronary lesions
The clinical characteristics of the patients with and without significant coronary lesions are presented in Table 1. The significant lesion group was older and had more males than the non-significant lesion group. In the significant coronary lesion group, there were more patients with diabetes mellitus and smoking history. CRP level and serum creatinine were also significantly higher in the coronary lesion group. However, there was no significant difference in total cholesterol, LDL-C, HDL-C, triglyceride levels, or quartered HDL-C subgroups between these two groups. The prescribed medications were also similar between these two groups. Furthermore, the modified Gensini scores were higher in the coronary lesion group as well.
Table 1. Demographic characteristics of low-LDL patients with and without significant coronary lesions .
| Non-significant lesion N = 401 | Significant lesion N = 165 | p value | |
| Age, years | 65 ± 12 | 71 ± 9 | < 0.001 |
| Male, N(%) | 274 (68.3) | 134 (81.2) | 0.002 |
| Hypertension, N(%) | 190 (47.4) | 95 (57.6) | 0.274 |
| Diabetes mellitus, N(%) | 56 (13.9) | 50 (30.3) | < 0.001 |
| Smoking, N(%) | 83 (20.7) | 51 (30.9) | 0.001 |
| CHF, N(%) | 35 (8.7) | 29 (17.6) | 0.003 |
| CKD, N (%) | 36 (9) | 39 (23.6) | < 0.001 |
| Body height, cm | 162 ± 8 | 163 ± 8 | 0.345 |
| Body weight, kg | 64.9 ± 11.6 | 64.4 ± 11.7 | 0.700 |
| Body mass index, kg/m2 | 24.7 ± 3.7 | 24.3 ± 3.7 | 0.226 |
| BP, systolic, mmHg | 125 ± 11 | 123 ± 11 | 0.211 |
| BP, diastolic, mmHg | 70 ± 10 | 69 ± 10 | 0.373 |
| Total cholesterol, mg/dl | 140 ± 26 | 141 ± 25 | 0.406 |
| LDL-cholesterol, mg/dl | 73 ± 19 | 73 ± 19 | 0.686 |
| HDL-cholesterol, mg/dl | 44 ± 14 | 46 ± 15 | 0.189 |
| HDL-cholesterol subgroup | 0.411 | ||
| Very low, <30 mg/dl | 54 (13.5) | 24 (14.5) | |
| Low, 30-39 mg/dl | 110 (27.4) | 35 (21.2) | |
| Normal, 40-59 mg/dl | 189 (47.1) | 81 (49.1) | |
| High, ≥ 60 mg/dl | 48 (12.0) | 25 (15.2) | |
| Triglyceride, mg/dl | 111 ± 67 | 116 ± 70 | 0.469 |
| C-reactive protein, mg/dl | 0.7 ± 1.3 | 1.4 ± 2.0 | < 0.001 |
| Serum creatinine, mg/dl | 1.1 ± 0.4 | 1.3 ± 0.6 | < 0.001 |
| LVEF, % | 39 ± 13 | 36 ± 11 | 0.116 |
| Modified Gensini score | 0.3 ± 1.0 | 22.7 ± 30.8 | < 0.001 |
| Medications | |||
| Antiplatelet agent (%) | 238 (59.3) | 105 (63.6) | 0.343 |
| ACE-I or ARB (%) | 110 (27.4) | 51 (30.9) | 0.405 |
| CCB (%) | 138 (34.4) | 57 (34.5) | 0.976 |
| Beta blocker (%) | 84 (20.9) | 36 (21.8) | 0.818 |
| Statin (%) | 93 (23.2) | 33 (20.0) | 0.407 |
| Fibrate (%) | 54 (13.5) | 18 (10.9) | 0.407 |
ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BP, blood pressure; CCB, calcium channel blocker; CHF, congestive heart failure; CKD, chronic kidney disease; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LVEF, left ventricular ejection fraction.
Comparisons among the quartered HDL-C subgroups in patients with CAD
The clinical characteristics of CAG-proven CAD patients in the quartered HDL-C subgroups are presented in Table 2. The left ventricular ejection fraction (LVEF) was higher in the high HDL-C subgroup than that in the very low and low HDL-C subgroups. Although the prevalence of diabetes mellitus was higher in the very low HDL-C subgroup than that in the other three subgroups, hypertension and history of smoking were similar in all three subgroups. There were significant differences in the total cholesterol levels among the four groups. However, there were no significant differences in modified Gensini scores (18.9 ± 22.5 vs. 21.9 ± 29.5 vs. 23.8 ± 33.9 vs. 23.9 ± 30.8, p = 0.942 among very low, low, normal, and high HDL-C subgroups, respectively) or the number of CAD vessels among the four subgroups.
Table 2. Demographic characteristics of low LDL-C CAD patients in quartered HDL-C groups (N=165) .
| HDL category (mg/dl) | p value | ||||
| Very low (< 30) N = 24 | Low (30-39) N = 35 | Normal (40-59) N = 81 | High (≥ 60) N = 25 | ||
| Age, years (mean ± SD) | 70 ± 9 | 72 ± 8 | 71 ± 9 | 75 ± 7 | 0.164 |
| Male, N(%) | 16 (66.7) | 32 (91.4) | 68 (83.9) | 18 (72) | 0.060 |
| Hypertension, N(%) | 12 (50) | 21 (60) | 47 (58.0) | 15 (60) | 0.905 |
| Diabetes mellitus, N(%) | 12 (50)* | 8 (22.9) | 23 (28.4) | 7 (28) | 0.030 |
| Smoking, N(%) | 3 (12.5) | 8 (22.9) | 31 (38.3) | 9 (36) | 0.102 |
| CHF, N(%) | 5 (20.8) | 6 (17.1) | 16 (19.8) | 2 (8) | 0.505 |
| Body height, cm | 161 ± 8 | 162 ± 8 | 164 ± 7 | 161 ± 10 | 0.279 |
| Body weight, kg | 65.2 ± 14.7 | 62.4 ± 8.6 | 65.8 ± 11.2 | 61.8 ± 13.4 | 0.434 |
| Body mass index, kg/m2 | 24.8 ± 4.3 | 24.0 ± 3.2 | 24.4 ± 3.7 | 23.6 ± 3.7 | 0.696 |
| BP, systolic, mmHg | 122 ± 11 | 123 ± 13 | 123 ± 10 | 126 ± 10 | 0.668 |
| BP, diastolic, mmHg | 67 ± 12 | 69 ± 8 | 70 ± 10 | 68 ± 12 | 0.613 |
| Total cholesterol, mg/dl | 122 ± 25* | 139 ± 21# | 144 ± 22‡ | 157 ± 24 | < 0.001 |
| LDL-cholesterol, mg/dl | 72 ± 24 | 80 ± 15 | 72 ± 17 | 66 ± 21 | 0.054 |
| Triglyceride, mg/dl | 137 ± 59 | 121 ± 80 | 112 ± 71 | 103 ± 58 | 0.326 |
| Serum creatinine, mg/dl | 1.3 ± 0.5 | 1.3 ± 0.7 | 1.2 ± 0.5 | 1.2 ± 0.6 | 0.850 |
| C-reactive protein, mg/dl | 1.6 ± 2.1 | 1.0 ± 1.6 | 1.3 ± 1.9 | 2.1 ± 2.3 | 0.139 |
| Modified Gensini score | 18.9 ± 22.5 | 21.9 ± 29.5 | 23.8 ± 33.9 | 23.9 ± 30.8 | 0.942 |
| Number of CAD | 0.123 | ||||
| One-vessel disease | 16 (66.7) | 23 (65.7) | 47 (58) | 16 (64) | |
| Two-vessel disease | 5 (20.8) | 2 (5.7) | 22 (27.2) | 5 (20) | |
| Three-vessel disease | 3 (12.5) | 10 (28.6) | 12 (14.8) | 4 (16) | |
| LVEF, % | 34 ± 11† | 32 ± 11# | 36 ± 11 | 42 ± 8 | 0.046 |
BP, blood pressure; CHF, congestive heart failure; CKD, chronic kidney disease; LDL, low-density lipoprotein; LVEF, left ventricular ejection fraction.
* p < 0.05, very low HDL-C group compared to low, normal and high HDL-C group. # p < 0.05, low HDL-C group compared to high HDL-C group. † p < 0.05, very low HDL-C group compared high HDL-C group. ‡ p < 0.05, normal HDL-C group compared high HDL-C group.
Predictors for the presence of significant coronary lesions
In the logistic regression analysis, age, male sex, DM, chronic kidney disease (CKD), CRP and smoking were all found to be independent predictors of CAD in univariate analysis. However, only age and DM were found to be independent risk factors for the presence of significant coronary lesions in multivariate analysis in our patients with low background LDL-C (Table 3).
Table 3. Logistic regression analysis for predictors of significant coronary lesions in low LDL-cholesterol patients .
| Variables | OR | 95%CI | p value | OR | 95%CI | p value |
| Univariate logistic regression | Multivariate logistic regression | |||||
| Male | 2.0 | 1.29-3.12 | 0.002 | 1.76 | 0.79-3.90 | 0.166 |
| Age | 1.07 | 1.04-1.09 | < 0.001 | 1.06 | 1.02-1.09 | 0.001 |
| BMI | 0.97 | 0.91-1.02 | 0.226 | |||
| DM | 2.32 | 1.47-3.68 | < 0.001 | 2.90 | 1.46-5.77 | 0.002 |
| HTN | 1.26 | 0.84-1.89 | 0.275 | |||
| CKD | 3.14 | 1.91-5.15 | < 0.001 | 1.89 | 0.82-4.38 | 0.135 |
| CRP | 1.30 | 1.16-1.45 | < 0.001 | 1.12 | 0.91-1.36 | 0.288 |
| Smoking | 2.16 | 1.35-3.46 | 0.001 | 1.44 | 0.71-2.93 | 0.307 |
| Statin | 0.83 | 0.77-1.89 | 0.407 | |||
| HDL-C subgroup | ||||||
| 30-39 mg/dl | 0.726 | 0.39-1.32 | 0.285 | 0.64 | 0.21-1.98 | 0.438 |
| 40-59 mg/dl | 0.96 | 0.56-1.67 | 0.896 | 0.92 | 0.34-2.44 | 0.859 |
| ≥ 60 mg/dl | 1.17 | 0.59-2.32 | 0.648 | 1.45 | 0.45-4.64 | 0.535 |
Covariates included in the univariate logistic regression analysis were age, sex, BMI, HTN, DM, smoking, CRP, CKD and HDL-C subgroup and statin. Covariates included in the multivariate logistic regression analysis included sex, age, smoking, DM, CKD, CRP and HDL-C subgroup.
BMI, body mass index; CKD, chronic kidney disease; CRP, C-reactive protein; DM, diabetes mellitus; HDL, high-density lipoprotein; HTN, hypertension; OR, odds ratio; 95% CI, 95% confidence interval.
HDL-C and modified Gensini scores in patients with CAD
Pearson’s correlation analysis revealed that modified Gensini scores were not related to HDL-C level (Pearson’s r = 0.052, p = 0.557) (Figure 1). In the linear regression analysis model, neither age, gender, HTN, DM, CRP, BMI, smoking, statin or HDL-C subgroup were found to be independent predictors for CAD severity as measured by modified Gensini score in either univariate or multivariate analysis (Table 4).
Figure 1.
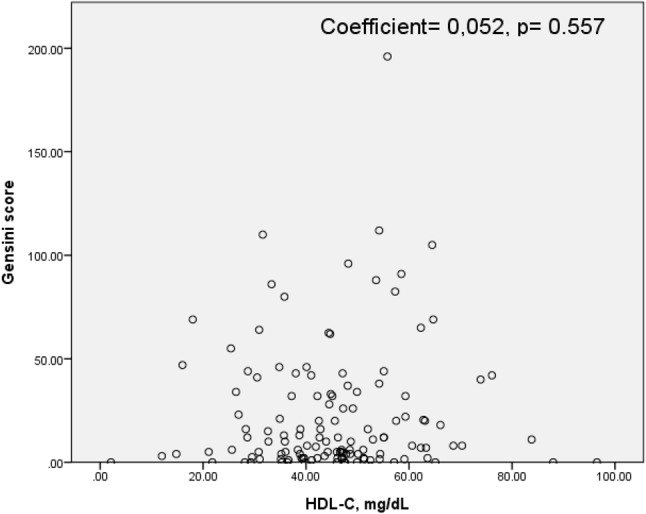
The relationship between modified Gensini scores and HDL-C level.
Table 4. Linear regression analysis for predictors of coronary lesions severity measured by modified Gensini score in low LDL-cholesterol CAD patients .
| Variables | Univariate | |||
| Coefficients | p value | 95% CI | ||
| B | SE | |||
| Female | -6.037 | 7.211 | 0.404 | (-20.304, 8.231) |
| Age | -0.496 | 0.321 | 0.125 | (-1.131, 0.139) |
| BMI | 0.633 | 0.879 | 0.473 | (-1.111, 2.376) |
| HTN | 3.881 | 6.120 | 0.527 | (-8.239, 16.002) |
| DM | 5.983 | 6.525 | 0.361 | (-6.961, 18.927) |
| Smoking | 6.256 | 5.976 | 0.298 | (-5.643, 18.155) |
| Statin | -6.909 | 6.566 | 0.295 | (-19.902, 6.084) |
| CRP | -0.681 | 1.466 | 0.643 | (-3.582, 2.220) |
| HDL-C subgroup | 1.799 | 3.096 | 0.562 | (-4.326, 7.925) |
Covariates included in the model were age, sex, BMI, HTN, DM, smoking, CRP, HDL-C subgroup and statins.
BMI, body mass index; CRP, C-reactive protein; DM, diabetes mellitus; HDL, high-density lipoprotein; HTN, hypertension; 95% CI, 95% confidence interval.
DISCUSSION
In this study we found that in patients with low background LDL-C, HDL-C levels could not differentiate patients with significant coronary lesions from those without such lesions. Also, HDL-C was not correlated with coronary lesion severity as measured by modified Gensini scores, and not an independent risk factor for either significant CAD or coronary lesion severity.
Dyslipidemia is one of the major risk factors for CAD. Deposition of atherogenic lipoproteins in the vessel wall contributes to atherosclerosis, leading to progressive narrowing of coronary artery lumen or rupture of atherosclerotic plaques, which can ultimately result in cardiovascular events. LDL-C has conventionally been regarded as the key factor in atherogenesis and current guidelines recommend reducing LDL-C to below 70 mg/dl in patients with established CAD, PAD, or diabetes.14 However, there remains a risk of cardiovascular event of between 8.7% and 22.4%15-18 even after optimal control of LDL-C has been achieved. Numerous studies have recognized the existence of relationships between cardiovascular risk and other lipids, most notably HDL-C and TG.5-7,19
The anti-atherogenetic effects of HDL-C particles could be due to the combined effects of different mechanisms, including antioxidative, anti-inflammatory, antithrombotic, anti-apoptotic, and atheroprotective effects.20-25 HDL-C particles are involved in reverse cholesterol transport to remove excessive cholesterol from peripheral tissues, and this process is thought to be the major contribution of HDL-C in atheroprotection. The cholesterol efflux from peripheral tissues to HDL-C particles involves several pathways.26 However, the roles of HDL-C particles in atherogenesis and CAD are still controversial. In short, HDL-C levels are a measurement of cholesterol content of the HDL-C particles, and therefore could be used as an indirect measurement of the numbers of circulating HDL-C particles. HDL-C was inversely associated with the prevalence of CAD in some series,5-7 but was found to be correlated with the prevalence of CAD in others.8-10 Albert et al.5 reported that HDL-C was inversely associated with the prevalence of CAD. Hsia et al.6 found lower HDL-C levels in patients with CAD, but there were no significant differences in HDL-C among patients with one, two, or three diseased vessels. Notably, LDL-C levels in the aforementioned positive studies were higher than 100 mg/dl, which is currently recognized as the ideal target value in various guidelines, and was used as the exclusion criterion in the current study. In contrast, several recent clinical studies failed to demonstrate additional benefits of raising HDL-C levels beyond that achieved by lowering LDL-C with standard statin therapy.27-29 In the ILLUMINATE study,27 torcetrapib, a cholesteryl ester transfer protein inhibitor used to raise HDL-C levels, failed to reduce CV risk but actually caused an increase in CV deaths. The AIM-HIGH study28 also failed to demonstrate an incremental benefit of raising HDL-C by niacin among patients with atherosclerotic CVD and on-treatment LDL-C values of < 70 mg/dl. Furthermore, the ACCORD Lipid study29 concluded that the combination of fenofibrate and simvastatin did not reduce the rate of fatal cardiovascular events, nonfatal myocardial infarction, or nonfatal stroke by raising HDL-C, as compared with rates attained using simvastatin alone. In the TNT trial, the association between HDL-C and 5-year major adverse cardiac events rates was not linear with higher HDL-C, thus attesting to a lack of better protective effects of HDL-C under statin therapy.17,30 Furthermore, the cardioprotective effects of atorvastatin were also not proportional to the rise in HDL-C either.30 It is possible that the mixed findings could be attributed to the heterogeneous HDL-C particle functions, the different groups of participants studied,5-7,31 the different lipid-lowering agents used in the treatment studies,7,31 the delayed effects of raising HDL-C levels,28 or different functionalities of HDL-C particles as raised by pharmacologic or natural means. Concordant with these studies, our study also failed to demonstrate an association between HDL-C level and the presence of significant coronary lesions or lesion severity in low LDL-C patients who were suspected to have CAD. Our study findings suggest that the protective effect of HDL-C might be attenuated or unimportant when LDL-C levels were in or had been brought under optimal control, i.e., less than 100 mg/dl.
Study limitations
There were several limitations in our study. First of all, this was a retrospective and nonrandomized study and subject to all the limitations inherent in the study design. Secondly, although we found that the HDL-C was not associated with the development of CAD or its severity in patients with LDL-C level less than 100 mg/dl, the mechanism of this lack of association was not really understood or addressed here. This suggests that more studies are necessary in the future to help understand the real mechanisms.
CONCLUSIONS
In summary, in this study we found that in Chinese patients with low background LDL-C and suspected CAD, serum HDL-C was not associated with development of CAD, number of diseased coronary vessels, or coronary lesion severity in those subjects who had significant coronary lesions. The atheroprotective role of HDL-C in CAD did not appear to be significant in those patients with low treated or non-treated LDL levels.
Acknowledgments
This work was supported in part by the Yen Tjing-Ling Medical Foundation (CI-100) and grants from Taichung Veterans General Hospital (TCVGH-996301C and TCVGH-993106C).
DISCLOSURES
The authors state that they have no conflict of interest to disclose.
REFERENCES
- 1. Arsenault BJ, Rana JS, Stroes ES, et al. Beyond low-density lipoprotein cholesterol: respective contributions of non-high-density lipoprotein cholesterol levels, triglycerides, and the total cholesterol/high-density lipoprotein cholesterol ratio to coronary heart disease risk in apparently healthy men and women. .. J Am Coll Cardiol. 2009;55:35–41. doi: 10.1016/j.jacc.2009.07.057. [DOI] [PubMed] [Google Scholar]
- 2. Miller M, Ginsberg HN, Schaefer EJ. Relative atherogenicity and predictive value of non-high-density lipoprotein cholesterol for coronary heart disease. Am J Cardiol. 2008;101:1003–1008. doi: 10.1016/j.amjcard.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 3. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 4. Okamura T, Kokubo Y, Watanabe M, et al. Low-density lipoprotein cholesterol and non-high-density lipoprotein cholesterol and the incidence of cardiovascular disease in an urban Japanese cohort study: The Suita study. Atherosclerosis. 2009;203:587–592. doi: 10.1016/j.atherosclerosis.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 5. Alber HF, Wanitschek MM, de Waha S, et al. High-density lipoprotein cholesterol, C-reactive protein, and prevalence and severity of coronary artery disease in 5641 consecutive patients undergoing coronary angiography. Eur J Clin Invest. 2008;38:372–380. doi: 10.1111/j.1365-2362.2008.01954.x. [DOI] [PubMed] [Google Scholar]
- 6. Lan Hsia S, Duncan R, Schob AH, et al. Serum levels of high-density lipoprotein phospholipids correlate inversely with severity of angiographically defined coronary artery disease. Atherosclerosis. 2000;152:469–473. doi: 10.1016/s0021-9150(99)00499-2. [DOI] [PubMed] [Google Scholar]
- 7. Scott R, O'Brien R, Fulcher G, et al. Effects of fenofibrate treatment on cardiovascular disease risk in 9,795 individuals with type 2 diabetes and various components of the metabolic syndrome: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetes Care. 2009;32:493–498. doi: 10.2337/dc08-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Naito HK, Greenstreet RL, David JA, et al. HDL-cholesterol concentration and severity of coronary atherosclerosis determined by cine-angiography. Artery. 1980;8:101–112. [PubMed] [Google Scholar]
- 9. Gordon DJ, Probstfield JL, Garrison RJ, et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79:8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- 10. Jin Z, Zhang Y, Chen J, et al. Study of the correlation between blood lipid levels and the severity of coronary atherosclerosis in a Chinese population sample. Acta Cardiol. 2006;61:603–606. doi: 10.2143/AC.61.6.2017958. [DOI] [PubMed] [Google Scholar]
- 11. deGoma EM, Leeper NJ, Heidenreich PA. Clinical significance of high-density lipoprotein cholesterol in patients with low low-density lipoprotein cholesterol. J Am Coll Cardiol. 2008;51:49–55. doi: 10.1016/j.jacc.2007.07.086. [DOI] [PubMed] [Google Scholar]
- 12. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 13. Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51:606. doi: 10.1016/s0002-9149(83)80105-2. [DOI] [PubMed] [Google Scholar]
- 14. Fraker TD, Jr., Fihn SD, Gibbons RJ, et al. 2007 chronic angina focused update of the ACC/AHA 2002 Guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines Writing Group to develop the focused update of the 2002 Guidelines for the management of patients with chronic stable angina. Circulation. 2007;116:2762–2772. doi: 10.1161/CIRCULATIONAHA.107.187930. [DOI] [PubMed] [Google Scholar]
- 15. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 16. Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 17. LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–1435. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
- 18. Pedersen TR, Faergeman O, Kastelein JJ, et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA. 2005;294:2437–2445. doi: 10.1001/jama.294.19.2437. [DOI] [PubMed] [Google Scholar]
- 19. Sarwar N, Danesh J, Eiriksdottir G, et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007;115:450–458. doi: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]
- 20. Calabresi L, Gomaraschi M, Franceschini G. Endothelial protection by high-density lipoproteins: from bench to bedside. Arterioscler Thromb Vasc Biol. 2003;23:1724–1731. doi: 10.1161/01.ATV.0000094961.74697.54. [DOI] [PubMed] [Google Scholar]
- 21. Barter PJ, Nicholls S, Rye KA, et al. Antiinflammatory properties of HDL. Circ Res. 2004;95:764–772. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 22. Assmann G, Gotto AM., Jr. HDL cholesterol and protective factors in atherosclerosis. Circulation. 2004;109: III8– III14. doi: 10.1161/01.CIR.0000131512.50667.46. [DOI] [PubMed] [Google Scholar]
- 23. Shah PK, Kaul S, Nilsson J, Cercek B. Exploiting the vascular protective effects of high-density lipoprotein and its apolipoproteins: an idea whose time for testing is coming, part I. Circulation. 2001;104:2376–2383. doi: 10.1161/hc4401.098467. [DOI] [PubMed] [Google Scholar]
- 24. Shah PK, Kaul S, Nilsson J, Cercek B. Exploiting the vascular protective effects of high-density lipoprotein and its apolipoproteins: an idea whose time for testing is coming, part II. Circulation. 2001;104:2498–2502. doi: 10.1161/hc4501.098468. [DOI] [PubMed] [Google Scholar]
- 25. Toth PP. Reverse cholesterol transport: high-density lipoprotein’s magnificent mile. Curr Atheroscler Rep. 2003;5:386–393. doi: 10.1007/s11883-003-0010-5. [DOI] [PubMed] [Google Scholar]
- 26. Shah PK. Inhibition of CETP as a novel therapeutic strategy for reducing the risk of atherosclerotic disease. Eur Heart J. 2007;28:5–12. doi: 10.1093/eurheartj/ehl392. [DOI] [PubMed] [Google Scholar]
- 27. Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 28. Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 29. Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barter P, Gotto AM, LaRosa JC, et al. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007;357:1301–1310. doi: 10.1056/NEJMoa064278. [DOI] [PubMed] [Google Scholar]
- 31. Summary of the second report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II) JAMA. 1993;269:3015–3023. [PubMed] [Google Scholar]


