Abstract
Background
Traditionally, a radial or brachial arterial approach is unadvisable in hemodialysis patients. Consequently, coronary angiography or angioplasty is usually performed via a femoral artery approach in these patients, who carry a higher risk of vascular access complications. In hemodialysis patients, arteriovenous grafts (AVG) are created for repeated punctures; however, the feasibility and safety of a trans-AVG approach for coronary angiography or angioplasty remains unclear.
Methods
In our institution, cardiac catheterizations were attempted via AV grafts in hemodialysis patients with a U-shaped forearm AVG. We retrospectively identified coronary angiography or angioplasty procedures in hemodialysis patients from a computer-based database in our hospital. The procedure details and outcomes were obtained from review of the clinical, angiographic and hemodialysis records.
Results
From 2008 to 2013, 167 procedures in hemodialysis patients were identified from 2866 diagnostic or interventional coronary procedures in our institution. Out of these, 24 procedures in 17 patients were performed via a trans-AVG approach. In all AVG procedures, a 6F 16-cm or 7F 10-cm sheath was placed from the AVG into the brachial artery. All diagnostic procedures were successfully performed. In 14 procedures, the patients also underwent angioplasty and all of the angioplasty procedures were successful. There was no arterial spasm, arterial dissection, puncture site hematoma, or acute thrombosis of the AVG during or after the procedures.
Conclusions
A trans-AVG approach appears to be a feasible and safe route for coronary angiography or angioplasty in hemodialysis patients with a U-shaped forearm AVG. However, further studies with a larger patient number are necessary.
Keywords: Arteriovenous graft, Hemodialysis, Percutaneous coronary intervention
INTRODUCTION
Coronary artery disease is the primary cause of death in uremic patients on maintenance hemodialysis.1 However, performing coronary procedures in these patients presents unique risks, especially vascular access complications.2 Traditionally, coronary procedures have been performed via femoral, brachial, or radial arteries. The femoral artery has been the primary approach but is associated with a higher risk of vascular complications.2-5 After the report by Campeau, the radial approach has become increasingly popular with the advantage of better patient comfort and fewer vascular access complications.6-8 In certain circumstances, a radial artery approach is associated with fewer access site complications than a femoral artery approach.9-11 Nonetheless, creating vascular access via the upper-limb arteries is usually prohibited in hemodialysis patients because of the risk of arterial injury of existing access or worse outcomes when constructing new access. Therefore, coronary procedures are usually performed via a femoral artery approach in uremic patients, who carry a much higher risk of bleeding and vascular access complications.
In a substantial portion of hemodialysis patients, arteriovenous grafts (AVG) are created for repeated punctures in hemodialysis with the advantages of fewer vascular complications and ease of hemostasis.12 However, the feasibility and safety of a trans-AVG approach for coronary procedures has not been reported in the literature. The purpose of this study was to report our single-center experience of the use of a trans-AVG approach for coronary angiography and angioplasty in hemodialysis patients.
METHODS
Study design
We performed this retrospective study from January 2008 to January 2013 using an existing database in our institution. Written informed consent was not required from our institutional review board for this type of retrospective study, but written informed consent for the procedure was obtained from each patient after the nature of the procedure and the route of access was fully explained. We retrospectively identified coronary procedures in hemodialysis patients from a computer-based database. In our institution, both femoral artery and U-shape forearm AVGs are used as avenues for vascular access in coronary procedures in hemodialysis patients. The choice between a femoral artery and an AVG is determined by the individual operator. Demographic data, characteristics of vascular access, procedure details and follow-up data were obtained from medical records, angiography and angioplasty reports, and hemodialysis records.
Trans-AVG puncture
Before the intervention, an adequate “thrill” over the AVG was determined by physical examination of the vascular access by the operator; it was also confirmed that each patient underwent successful hemodialysis with adequate flow and pressure before this procedure. After local administration of 2% xylocaine, puncture of the AVG was attempted at the arterial limb of the U-shape graft using a 30 mm-20-G sheathed needle (Terumo, Tokyo, Japan) (Figure 1). The sheath was directed to the upper limb after leaving a distance of about 3-5 cm from the arterial anastomosis. After a puff of contrast to confirm the direction of the sheath, a 45-cm 0.025-inchhydrophilic guide wire was introduced into the brachial artery under fluoroscopic guidance. Subsequent to that, a 6-F or 7-F sheath (Terumo, Tokyo, Japan) was introduced into the brachial artery over the guide wire. To avoid arterial spasm or thrombosis of vascular access, verapamil 2.5 mg and heparin 3000 U was injected via the sheath. The sheath was introduced into the brachial artery above the arterial anastomosis and its location was confirmed by fluoroscopy.
Figure 1.
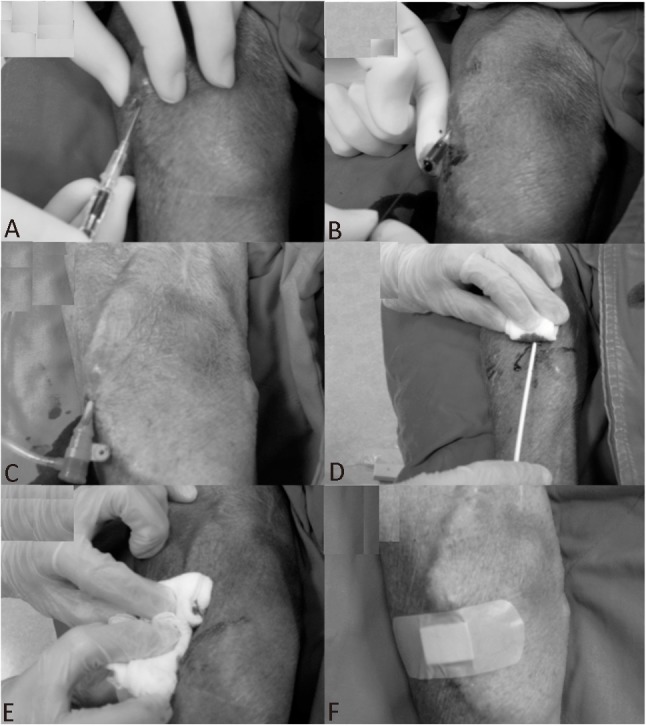
Trans-AVG puncture and removal of sheath. (A) After local anesthesia, puncture of the AVG was attempted at the arterial limb of the U-shape graft using a 30 mm-20-G sheathed needle (Terumo, Tokyo, Japan). (B) A 0.025” straight wire was introduced from the AVG to the brachial artery. (C) A sheath (6F 16 cm or 7F 10 cm) with a dilator was inserted into the brachial artery through the AVG-brachial artery junction. (D) The arterial sheath was removed. (E) Direct digital pressure was applied to the puncture hole and was released gradually to keep the puncture hole free from oozing but with a palpable thrill present. (F) After complete hemostasis, an elastic bandage was placed over the puncture hole.
Coronary angiography and angioplasty
A 6-F or 7-F diagnostic or guiding catheter was used depending on the size of the sheath. A 260-cm 0.035” guide wire (Terumo, Tokyo, Japan) was used for catheter exchange to facilitate the procedure and minimize catheter manipulation in the brachial artery, aortic arch and ascending aorta. For vascular access via a left-arm AVG, a Judkins left 4.0 catheter for the left coronary artery and a Judkins right 4.0 catheter for the right coronary artery were used at first; for vascular access via a right-arm AVG, a Judkins left 3.5 catheter for the left coronary artery was used.
If coronary angioplasty was attempted, a 6-F guiding catheter was used at first, and 7-F catheters were only used if devices or techniques required larger guiding catheters.
Hemostasis
At the end of the procedure, the arterial sheath was removed immediately if only 3,000 U heparin was given. If additional doses of heparin were administered, the arterial sheath was removed one hour later to ensure an activated clotting time of less than 150 seconds. After sheath removal, direct digital pressure was applied to the puncture hole and was released gradually to keep the puncture hole free from oozing but with a palpable thrill. After complete hemostasis, an elastic bandage was placed over the puncture hole (Figure 1). Further local compression was not routinely applied except for those patients in whom it was difficult to achieve hemostasis. All patients were monitored throughout their hospital stay and were re-evaluated prior to discharge and during follow-up outpatient clinic visits. The AVG function was confirmed by a palpable thrill immediately after the procedure and at least one successful hemodialysis session after that.
Definitions
Technical success of the trans-AVG procedure was defined as success in performing coronary angiography, or if intervention was required, in allowing an attempt at angioplasty. Coronary lesions were classified according to the 2011 ACCF/AHA/SCAI (American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions) guidelines for percutaneous coronary intervention.13 Non-access site complications included aortic dissection or coronary perforation, transient ischemic attack or cerebral vascular accident, and death during the index hospitalization. Bleeding complications were defined as access site bleeding, gastrointestinal bleeding, genitourinary bleeding, or other bleeding. All bleeding end points were further defined as requiring transfusion and/or prolonging the hospital stay, and/or causing a drop in hemoglobin > 3.0 g/dl. A hematoma more than 2 cm in size at the access site was also classified as access site bleeding. Vascular complications were defined as access site occlusion, peripheral embolization, arterial dissection, arterial pseudoaneurysm, or arterio-venous fistula.14
Statistical analysis
In the analysis, each endovascular procedure was treated as a separate event for patients receiving more than one procedure. Data are presented as mean ± standard deviation. Statistical analyses were performed using STATISTICA 8.0 software for Windows (StatSoft Inc., Tulsa, OK, USA).
RESULTS
Patient demographics
From January 2008 to January 2013, 167 coronary procedures in hemodialysis patients were identified from 2866 coronary procedures in our institution. Out of these, 24 procedures were performed via a trans-AVG approach in 17 patients. The mean age of patients was 68.7 ± 8.6 years old and the mean duration of hemodialysis was 34.8 ± 26.7 months. The indications for the coronary procedures were as follows: 13 for stable angina and 11 for acute coronary syndrome. No procedures were undertaken for primary angioplasty of ST elevation myocardial infarction. Unfractionated heparin was used before all of the 24 procedures. Low molecular weight heparin or glycoprotein IIb/IIIa inhibitors were not used in any procedures. Patient demographic characteristics are summarized in Table 1.
Table 1. Clinical characteristics of the study participants .
| N = 17 | |
| Age, y | 68.7 ± 8.6 |
| Gender, F/M | 7/10 |
| Body height (cm) | 158.2 ± 9.8 |
| Body weight (kg) | 63.5 ± 11.6 |
| BMI (kg/m2) | 25.2 ± 2.9 |
| Hemodialysis time (months) | 34.8 ± 26.7 |
| Cardiovascular risk factors | |
| Hypertension | 15 (88.2) |
| Diabetes mellitus | 10 (58.8) |
| Dyslipidemia | 7 (41.2) |
| Current smoker | 4 (23.5) |
| Previous cardiovascular diseases | |
| Myocardial infarction | 2 (11.8) |
| Cerebrovascular disease | 2 (11.8) |
| Previous PCI | 9 (52.9) |
| Previous CABG | 3 (17.6) |
BMI, body mass index; CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention.
AVG canalization
Trans-AVG puncture was attempted in 3 procedures through a right-arm AVG and in 21 procedures through a left-arm AVG. All the AVG punctures were successful without the need for femoral or other approaches. The average access time was 1.52 ± 2.40 minutes. In five of these procedures, 7F sheaths were used because angioplasty was intended.
Coronary angiography
Twenty-four coronary diagnostic procedures were performed and all of them were successful. One patient received diagnostic angiography via a left AVG for bypass vessels, including left internal mammary artery and saphenous vein grafts. The catheters used in coronary angiography are shown in Table 2. When using a right AVG, a Judkins left 3.5 catheter successfully engaged the left coronary artery in all procedures. When using a left AVG, a Judkins left 4.0 catheter successfully engaged the left coronary artery in 15 procedures, a Judkins left 3.5 catheter was successfully used in 5 procedures, and a Judkins left 3.0 catheter was successfully used in one procedure. All the right coronary arteries were successfully engaged by a Judkins right 4.0 catheter.
Table 2. Characteristics of trans-AVG coronary procedures .
| Right AVG | Left AVG | |
| Coronary angiography (N = 24) | ||
| Sheath size | ||
| 6F | 3 | 16 |
| 7F | 0 | 5 |
| Left coronary artery | ||
| JL4.0 | 0 | 15 |
| JL3.0 | 0 | 1 |
| JL3.5 | 3 | 5 |
| Right coronary artery | ||
| JR4.0 | 3 | 21 |
| Total no. of vessels treated (N = 15) | ||
| LAD | 2 | 2 |
| LCX | 0 | 7 |
| RCA | 0 | 2 |
| Saphenous vein graft | 0 | 2 |
| ACC/AHA classification | ||
| A | 2 | 3 |
| B | 0 | 4 |
| C | 0 | 6 |
| SCAI classification | ||
| I | 2 | 7 |
| II | 0 | 5 |
| III | 0 | 0 |
| IV | 0 | 1 |
| Use of stenting | 1 | 5 |
ACC/AHA, American College of Cardiology and the American Heart Association; AVG, arteriovenous graft; JL, judkins left; JR, judkins right; LAD, left anterior descending; LCX, left circumflex artery; RCA, right coronary artery; SCAI, Society for Cardiovascular Angiography and Interventions; SVG, saphenous vein graft.
Coronary angioplasty
Fourteen procedures on 15 lesions involved angioplasty and the size of balloon catheters ranged from 1.0 mm to 4.0 mm. Stent implantation was performed in six of the procedures and the size of the stents ranged from 2.5 mm to 4.0 mm. The other procedures involving balloon angioplasty were only for in-stent restenosis with optimal results. A kissing balloon technique with 2.5 mm and 2.0 mm balloons was performed in one patient with bifurcation stenosis at the middle segment of the left anterior descending (LAD) artery and the orifice of the first diagonal branch. One patient underwent direct stenting of the middle segment of the saphenous vein graft. Angioplasty with stenting was performed in one patient with a chronic, totally occluded right coronary artery. All of the angioplasty and coronary stenting procedures were successful with an uneventful hospital course. Characteristics of the lesions and angioplasty procedures are summarized in Table 2.
Complications
There were no non-access site complications, such as aortic dissection, coronary perforation, transient ischemic attack, cerebral vascular accident, or death during hospitalization. There were also no access site complications following the procedures, such as puncture site hematomas, pseudoaneurysms, arterial dissection or acute thrombosis of the AVG. No patients developed ischemic arm complications. None of our patients required a blood transfusion or surgical repair of the vascular access after the procedure.
DISCUSSION
To the best of our knowledge, this is the first report of cardiac catheterization via a percutaneous AVG approach in hemodialysis patients. Our results demonstrate that coronary diagnostic and interventional procedures can be successful performed via an AVG approach. The cannulation of an AVG was not difficult for the interventionists to learn. The consequence is that patients are more comfortable because of easier hemostasis, earlier ambulation, and less pain during sheath manipulation. Based on the above results, this novel approach provides not only an alternative when a femoral approach fails, but also provides a better primary route of choice than the femoral artery.
The major advantage of an AVG approach is the ease of puncture. An AVG is usually 6-7 mm in diameter and is implanted just underneath the skin of the forearm. In consequence, it is much easier to puncture an AVG than the radial, brachial, or femoral arteries. The reported success rate for a radial approach ranges from 88-98%.7,15 In our study, all AVG punctures were successful and the access time was only 1.52 ± 2.40 minutes. In addition, all AVG cannulations were successful and the advancement of a guide wire or sheath was straightforward without difficulties. A learning curve like that required in a radial approach is not needed.6 In our practice, the tip of the sheath was usually advanced into the upstream brachial artery, so that manipulation of the wire or catheters in the brachial artery could be minimized to avoid vascular injury, spasm or thrombosis. If AVG has the history of stenosis and/or thrombosis status-post angioplasty and/or thrombectomy, trans-AVG approach was not contraindicated in our institution. But healthy AVGs may be more feasible to use.
Although all the procedures were successful in the study, the interpretation of successful rate is limited by the small number of patients. Theoretically, an AVG approach shares the same causes of failure as a radial or brachial approach. One of the causes of failure is the tortuosity of the brachial and subclavian arteries. Nonetheless, a femoral approach may also be difficult in these patients because of severe aorto-ileo-femoral obstructive disease or aneurysm of the aorta.
A trans-AVG approach provides several advantages over a traditional radial or femoral artery approach in terms of patient comfort. In a radial artery approach, patients often complain of significant pain during insertion or withdrawal of the sheath. In contrast, less pain is experienced when the sheath is inserted through an AVG. Second, after withdrawal of the sheath, only slight digital compression on the puncture site of the AVG is required to stop the bleeding. In contrast, heavy and prolonged compression over the puncture hole of the femoral or brachial artery is usually needed, which causes more discomfort for patients. Third, an AVG approach allows earlier ambulation, which potentially decreases post-procedure nursing, hospital costs, and length of stay. In addition, this is an advantage for patients who have difficulty in lying down for prolonged periods.
Patients with complex coronary lesions may require a 7F or 8F system to provide better back support and flexibility in choice of devices, such as kissing stents, large-bone rotablators, and some distal protection devices. Compared to the radial artery, AVG and brachial arteries have larger inner dimensions (usually 6 mm to 7 mm), which allows for the application of 7F or 8F systems. In our study, 7F sheaths were used in five procedures successfully without vascular access complications. In contrast, a radial artery approach usually only allows the insertion of 6F systems. Compared to a femoral artery approach, an AVG approach has the advantage of easier hemostasis when 7F or 8F systems are used. However, this approach may not be suitable when an intra-aortic balloon pump is needed in emergencies. Thus, both groins should always be prepared in case an urgent femoral approach is necessary.
The risk of access complications was significantly lower compared with those previously reported with a femoral, brachial, or radial approach in Caucasian and Asian populations.2,15-17 After the procedure, the long term outcome of AVGs in our study was comparable to that suggested by the K-DOQI guidelines.12 The benefits of an AVG approach are plausible based on the theoretical advantages of the approach itself. First, the AVG has a superficial course and the overlying subcutaneous tissue is much thinner than that of the femoral artery. In consequence, an AVG is much easier both to puncture and to bring about hemostasis. Second, the absence of nerves or veins near the cannulation area is a safeguard against nerve injury or arteriovenous fistula. Third, the large diameter of the AVG and its location upstream of the brachial artery, usually by 6 mm to 7 mm, decrease the risk of arterial occlusion or spasm. Fourth, in hemodialysis patients, the incidence of ischemic damage to the hand is minimal despite repeated AVG puncture.12 Finally, hemostasis after removal of the sheath is usually much more straightforward than that for a femoral or brachial artery approach. The superficial AVG is readily compressible with manual compression or use of tourniquets designed for hemostasis after hemodialysis. Any subsequent bleeding or hematoma can easily be detected and resolved with immediate local compression by the patient.
There were some limitations to our study. First, the small sample size of our study may mean that it is underpowered to detect complications. It seems insufficient to prove the technical safety in only 17 patients collected retrospectively. The event might be free by change. Therefore, the actual complication rates should be clarified in larger cohorts. Second, this is a retrospective report, so the recorded data could not address the actual complications accurately. A small hematoma more than 2 cm as definition may not be recorded in the medical chart. Subclinical vascular dissection or spasm was also not confirmed by subsequent angiography. But major complications such as bleeding requiring transfusion or causing a drop in hemoglobin > 3.0 g/dl and acute thrombosis and AVG dysfunction would actually be reported and verified by subsequent successful hemodialysis. Third, only a U-shaped AVG is suitable as the vascular access because of the acute angle at the arterial-graft junction of a straight-shaped AVG. Finally, most AVGs were implanted in the non-dominant hand, usually the left hand, of hemodialysis patients. In consequence, a right hand approach, which may be more comfortable for the operator, is usually not possible.
CONCLUSIONS
A trans-AVG approach seems a feasible and safe route for coronary angiography or angioplasty in hemodialysis patients with a U-shaped AVG. Further studies with a larger patient number are needed.
Acknowledgments
This study was supported by grants from the National Taiwan University Hospital, Hsinchu Branch (No. 990004 and No. 100-026-F).
CONFLICT OF INTEREST
No conflicts of interest to declare.
REFERENCES
- 1.Levin A, Foley RN. Cardiovascular disease in chronic renal insufficiency. Am J Kidney Dis. 2000;36:S24–S30. doi: 10.1053/ajkd.2000.19928. [DOI] [PubMed] [Google Scholar]
- 2.Applegate RJ, Sacrinty MT, Kutcher MA, et al. Trends in vascular complications after diagnostic cardiac catheterization and per-cutaneous coronary intervention via the femoral artery, 1998 to 2007. JACC Cardiovasc Interv. 2008;1:317–326. doi: 10.1016/j.jcin.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Kiemeneij F, Laarman GJ, Odekerken D, et al. A randomized comparison of percutaneous transluminal coronary angioplasty by the radial, brachial and femoral approaches: the access study. J Am Coll Cardiol. 1997;29:1269–1275. doi: 10.1016/s0735-1097(97)00064-8. [DOI] [PubMed] [Google Scholar]
- 4.Agostoni P, Biondi-Zoccai GG, de Benedictis ML, et al. Radial versus femoral approach for percutaneous coronary diagnostic and interventional procedures; systematic overview and meta-analysis of randomized trials. J Am Coll Cardiol. 2004;44:349–356. doi: 10.1016/j.jacc.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 5.Aziz EF, Pulimi S, Coleman C, et al. Increased vascular access complications in patients with renal dysfunction undergoing percutaneous coronary procedures using arteriotomy closure devices. J Invasive Cardiol. 2010;22:8–13. [PubMed] [Google Scholar]
- 6.Archbold RA, Robinson NM, Schilling RJ. Radial artery access for coronary angiography and percutaneous coronary intervention. BMJ. 2004;329:443–446. doi: 10.1136/bmj.329.7463.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campeau L. Percutaneous radial artery approach for coronary angiography. Cathet Cardiovasc Diagn. 1989;16:3–7. doi: 10.1002/ccd.1810160103. [DOI] [PubMed] [Google Scholar]
- 8.Kiemeneij F, Laarman GJ. Percutaneous transradial artery approach for coronary stent implantation. Cathet Cardiovasc Diagn. 1993;30:173–178. doi: 10.1002/ccd.1810300220. [DOI] [PubMed] [Google Scholar]
- 9.Hibbert B, Simard T, Wilson KR, et al. Transradial versus transfemoral artery approach for coronary angiography and percutaneous coronary intervention in the extremely obese. JACC Cardiovasc Interv. 2012;5:819–826. doi: 10.1016/j.jcin.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Valgimigli M, Saia F, Guastaroba P, et al. Transradial versus transfemoral intervention for acute myocardial infarction: a propensity score-adjusted and -matched analysis from the REAL (REgistro regionale AngiopLastiche dell’Emilia-Romagna) multicenter registry. JACC Cardiovasc Interv. 2012;5:23–35. doi: 10.1016/j.jcin.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 11.Hildick-Smith DJ, Walsh JT, Lowe MD, et al. Coronary angiography in the presence of peripheral vascular disease: femoral or brachial/radial approach? Catheter Cardiovasc Interv. 2000;49:32–37. doi: 10.1002/(sici)1522-726x(200001)49:1<32::aid-ccd6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 12.Clinical practice guidelines for vascular access. Am J Kidney Dis. 2006;48(Suppl 1):S176–S247. doi: 10.1053/j.ajkd.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 13.Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58:e44–e122. doi: 10.1016/j.jacc.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Rao SV, Ou FS, Wang TY, et al. Trends in the prevalence and outcomes of radial and femoral approaches to percutaneous coronary intervention: a report from the National Cardiovascular Data Registry. JACC Cardiovasc Interv. 2008;1:379–386. doi: 10.1016/j.jcin.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Wu CJ, Lo PH, Chang KC, et al. Transradial coronary angiography and angioplasty in Chinese patients. Cathet Cardiovasc Diagn. 1997;40:159–163. doi: 10.1002/(sici)1097-0304(199702)40:2<159::aid-ccd8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 16.Saito S, Miyake S, Hosokawa G, et al. Transradial coronary intervention in Japanese patients. Catheter Cardiovasc Interv . 1999;46:37–41; discussion 2. doi: 10.1002/(SICI)1522-726X(199901)46:1<37::AID-CCD10>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 17.Gan HW, Yip HK, Wu CJ. Brachial approach for coronary angiography and intervention: totally obsolete, or a feasible alternative when radial access is not possible? Ann Acad Med Singapore. 2010;39:368–373. [PubMed] [Google Scholar]


